新型烷氧基嘧啶拼接3-吡咯螺环氧化吲哚类化合物的合成及其抗肿瘤活性
陈 爽, 肖 乐, 陈智勇, 杨 超, 巩 艺, 余章彪*, 周 英, 刘雄利*
(1. 贵州医科大学 生物与工程学院,贵州 贵阳 550025; 2. 郴州市第一中学 化学组,湖南 郴州 423000;3. 贵州大学 贵州省中药民族药创制工程中心,贵州 贵阳 550025)
·研究论文·
新型烷氧基嘧啶拼接3-吡咯螺环氧化吲哚类化合物的合成及其抗肿瘤活性
陈 爽1, 肖 乐2, 3, 陈智勇3, 杨 超3, 巩 艺3, 余章彪3*, 周 英3, 刘雄利3*
(1. 贵州医科大学 生物与工程学院,贵州 贵阳 550025; 2. 郴州市第一中学 化学组,湖南 郴州 423000;3. 贵州大学 贵州省中药民族药创制工程中心,贵州 贵阳 550025)
以取代烷氧基嘧啶3-烯键氧化吲哚衍生物为原料,与肌氨酸及多聚甲醛在甲苯中回流经1,3-偶极子3+2环加成反应,合成了8个新型烷氧基嘧啶拼接3-吡咯螺环氧化吲哚类化合物(3a~3h),产率67%~82%,d/r值6/1~20/1, 其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。采用MTT法研究了3a~3h对人肺癌细胞(A549)、人前列腺癌细胞(PC-3)和人白血病细胞(K562)的体外抗肿瘤活性。结果表明:3d和3g对人白血病细胞K562具有明显的活性,IC50分别为24.1 mol·L-1和32.4 μmol·L-1,接近阳性对照药顺铂。
肌氨酸; 甲醛; 烷氧基嘧啶3-烯键氧化吲哚; 烷氧基嘧啶拼接3-吡咯螺环氧化吲哚; 1,3-偶极环加成反应; 合成; 抗肿瘤活性
3-吡咯螺环氧化吲哚具有广泛的生物活性[1-2],吸引了许多化学工作者及医药化学团队的广泛关注,如Spirotryprostatins B可完全抑制tsFT210细胞,阻断细胞分裂的G2/M期[3]。天然产物台钩藤碱具有调节大脑皮层M受体亚型和5-羟色胺受体的功能[4]。Alstonisine等也有类似功效[5]。3-吡咯螺环氧化吲哚的合成方法目前已有一些文献进行了报道[1-7]。
目前已上市或即将上市的抗肿瘤或抗病毒感染的药物大多由烷氧基嘧啶衍生而成[8-12],如齐多夫定、双脱氧胞苷及拉米夫定等是HIV逆转录酶抑制剂;烷氧基嘧啶化合物V-X为药物分子或具有广泛的生物活性分子。
鉴于3-吡咯螺环氧化吲哚和烷氧基嘧啶[13]具有良好的生物活性,根据药效团和骨架迁越原理,将烷氧基嘧啶骨架拼合到3-吡咯螺环氧化吲哚骨架中,合成新型的烷氧基嘧啶拼接3-吡咯螺环氧化吲哚,可为生物活性筛选提供化合物源,对药物筛选有一定参考意义。
本文以取代烷氧基嘧啶3-烯键氧化吲哚衍生物(1a~1h)为原料,与肌氨酸(2)和多聚甲醛在甲苯中回流经1,3-偶极子3+2环加成反应,合成了8个新型烷氧基嘧啶拼接3-吡咯螺环氧化吲哚类化合物(3a~3h, Scheme 1),产率67%~82%,d/r值6/1~20/1,其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。采用MTT法研究了3a~3h对人肺癌细胞(A549)、人前列腺癌细胞(PC-3)和人白血病细胞(K562)的体外抗肿瘤活性。
1 实验部分
1.1 仪器与试剂
WRS-1B型数字熔点仪;Bruker-400 MHz型核磁共振仪(CD3Cl为溶剂,TMS为内标); MicroTMQ-TOF型高分辨质谱仪。
所用试剂均为分析纯。
1.2 3a~3h的合成(以3a为例)
在反应管中依次加入双甲氧基嘧啶拼接3-烯键氧化吲哚(1a)93.3 mg(0.3 mmol), 2 53.4 mg(0.6 mmol),多聚甲醛27.0 mg(0.9 mmol)和甲苯8.0 mL,搅拌下回流反应8 h。经硅胶柱层析[洗脱剂:V(石油醚)∶V(乙酸乙酯)=3 ∶1]纯化得3a 87.3 mg。
用类似的方法合成3b~3h。
3a: 黄色固体, m.p. 124.3~126.1 ℃;1H NMRδ: 2.46(s, 3H), 3.02~3.10(m, 2H), 3.13(s, 3H), 3.55(s, 3H), 3.66(t,J=9.2 Hz, 1H), 3.80(s, 6H), 4.10~4.18(m, 2H), 6.62~6.68(m, 1H), 6.93~7.00(m, 2H), 8.07(s, 1H);13C NMRδ: 21.2, 26.4, 29.8, 42.3, 45.3, 54.0, 58.1, 59.5, 67.1, 102.1, 106.8, 114.6, 122.4, 122.8, 125.1, 130.1, 134.3, 139.3, 141.1, 154.9, 168.2, 180.6; HR-MS(ESI-TOF)m/z: Calcd for C20H24N4O3Na{[M+Na]+}391.174 6, found 391.174 9。
3b: 白色固体, m.p. 162.7~164.0 ℃;1H NMRδ: 1.35(t,J=7.1 Hz, 6H), 2.49(s, 3H), 2.64(d,J=9.2 Hz, 1H), 3.02(t,J=8.6 Hz, 1H), 3.13(t,J=8.9 Hz, 1H), 3.22(d,J=9.5 Hz, 1H), 3.72~3.82(m, 1H), 4.21~4.32(m, 4H), 6.73~6.77(m, 2H), 6.97~7.04(m, 1H), 7.13(d,J=6.8 Hz, 1H), 7.25(s, 1H), 8.03(s, 1H);13C NMRδ: 14.5, 42.2, 44.5, 47.7, 59.0, 59.6, 62.7, 63.8, 100.0, 109.2, 117.6, 121.7, 124.5, 127.9, 130.7, 140.9, 154.8, 160.5, 168.1, 182.5; HR-MS(ESI-TOF)m/z: Calcd for C20H24N4O3Na{[M+Na]+} 391.174 6, found 391.174 2。
3c: 黄色固体, m.p. 148.2~150.1 ℃;1H NMRδ: 1.38(t,J=7.1 Hz, 6H), 2.52(s, 3H), 2.94(d,J=9.2 Hz, 1H), 3.03(s, 1H), 3.09(d,J=9.2 Hz, 1H), 3.13(s, 3H), 3.15~3.19(m, 1H), 3.19~3.24(m, 1H), 4.24~4.31(m, 4H), 6.48~6.52(m, 1H), 6.74~6.79(m, 1H), 7.15~7.18(m, 1H), 8.04(s, 1H);13C NMRδ: 14.5, 22.8, 26.5, 29.8, 32.0, 42.1, 44.4, 58.4, 62.8, 107.4, 113.0, 139.3, 139.4, 135.8, 155.0, 157.5, 159.8, 166.8, 168.0; HR-MS(ESI-TOF)m/z: Calcd for C21H25N4O3FNa {[M+Na]+}423.180 8, found 423.180 8。
3d: 淡黄色固体, m.p. 117.1~118.7 ℃;1H NMRδ: 1.34(t,J=7.1 Hz, 6H), 2.50(s, 3H), 2.79(d,J=9.2 Hz, 1H), 2.91(d,J=8.8 Hz, 1H), 3.14(d,J=9.2 Hz, 1H), 3.81~3.87(m, 1H), 4.18~4.30(m, 4H), 4.39~4.45(m, 1H), 4.77(d,J=15.9 Hz, 1H), 4.95(d,J=15.9 Hz, 1H), 6.31~6.34(m, 1H), 6.61~6.67(m, 1H), 7.08~7.11(m, 1H), 7.19~7.22(m, 1H), 7.23~7.25(m, 2H), 7.26(d,J=1.3 Hz, 1H), 7.29(d,J=2.8 Hz, 1H), 8.10(s, 1H);13C NMRδ: 14.5, 29.8, 42.1, 43.8, 44.1, 58.3, 59.2, 62.8, 66.8, 101.5, 108.6, 113.1, 113.4, 114.0, 126.9, 127.8, 128.8, 133.3, 133.4, 135.8, 138.5, 155.1, 168.1, 179.2; HR-MS(ESI-TOF)m/z: Calcd for C27H29N4O3FNa{[M+Na]+}499.212 1, found 499.212 1。
3e: 苍黄色固体, m.p. 216.5~217.9 ℃;1H NMRδ: 0.97(t,J=7.4 Hz, 6H), 1.71~1.81(m, 4H), 2.48(s, 3H), 2.65(d,J=9.5 Hz, 1H), 3.14(t,J=8.3 Hz, 1H), 3.21(d,J=10.5 Hz, 2H), 3.74(t,J=9.1 Hz, 1H), 4.09~4.19(m, 4H), 6.72~6.77(m, 2H), 6.97~7.01(m, 1H), 7.12(d,J=7.4 Hz, 1H), 8.02(s, 1H), 9.16(br s, 1H);13C NMRδ: 10.8, 22.3, 29.8, 42.2, 44.4, 51.9, 58.7, 68.6, 101.9, 103.0, 109.4, 121.6, 124.4, 127.9, 136.9, 141.0, 154.7, 157.8, 161.8, 168.2, 183.5; HR-MS(ESI-TOF)m/z: Calcd for C22H28N4O3Na{[M+Na]+}419.205 9, found 419.206 2。
3f: 黄色油状液体;1H NMRδ: 2.47(s, 3H), 2.51~2.58(m, 1H), 3.17(t,J=8.3 Hz, 1H), 3.25(d,J=9.6 Hz, 1H), 3.49~3.55(m, 1H), 3.78(s, 6H), 3.80(s, 3H), 4.08~4.12(m, 1H), 6.72~6.78(m, 2H), 6.98~7.04(m, 2H), 8.25(br s, 1H);13C NMRδ: 29.8, 42.4, 44.6, 53.7, 54.5, 58.5, 67.3, 94.4, 109.0, 121.7, 124.6, 127.8, 131.8, 162.5, 169.9; HR-MS(ESI-TOF)m/z: Calcd for C19H22N4O4Na{[M+Na]+}393.153 9, found 393.153 5。
3g: 黄色固体, m.p. 79.5~82.6 ℃;1H NMRδ: 2.46(s, 3H), 2.65(d,J=9.4 Hz, 1H), 2.92~2.98(m, 1H), 3.06~3.11(m, 1H), 3.13(s, 3H), 3.62~3.67(m, 1H), 3.79(s, 6H), 3.80(s, 3H), 4.12~4.16(m, 1H), 6.49~6.52(m, 1H), 6.73~6.78(m, 1H), 7.01~7.04(m, 1H);13C NMRδ: 26.4, 29.9, 36.0, 42.2, 44.6, 47.5, 52.2, 53.9, 59.0, 66.0, 93.9, 107.5, 113.8, 114.0, 133.3, 139.3, 157.4, 159.8, 162.5, 163.7, 169.7, 179.9; HR-MS(ESI-TOF)m/z: Calcd for C20H23N4O4FNa{[M+Na]+} 425.160 1, found 425.160 3。
3h: 黄色油状液体;1H NMRδ: 1.36~1.23(m, 9H), 2.47(s, 3H), 3.35~3.39(m, 1H), 3.48~3.52(m, 1H), 3.72~3.76(m, 1H), 3.77~3.82(m, 2H), 4.05~4.34(m, 6H), 6.70~6.78(m, 1H), 6.96~7.05(m, 1H), 7.16(t,J=8.2 Hz, 1H), 7.25(s, 1H), 8.25(br s, 1H);13C NMRδ: 14.4, 29.9, 33.3, 35.9, 42.3, 44.3, 47.6, 52.3, 58.7, 60.1, 62.6, 93.8, 103.0, 109.3, 121.6, 124.6, 127.8, 141.2, 162.1, 163.8, 169.4, 183.4; HR-MS(ESI-TOF)m/z: Calcd for C22H28N4O4Na{[M+Na]+}435.200 8, found 435.201 1。
1.3 体外抗肿瘤活性测试
采用MTT法[14-15]测试了3a~3h对人肺癌细胞(A549)、人前列腺癌细胞(PC-3)和人白血病细胞(K562)的体外抗肿瘤活性,以顺铂为阳性对照药。
2 结果与讨论
2.1 合成
通过底物扩展,我们发现该反应的活性普遍较高,12 h内基本反应完全(TLC检测)。TLC显示反应有少量的副产物产生,核磁和质谱不能对其进行结构鉴定,可能为分解产物或聚合物。其中,底物1中嘧啶取代基位阻较大时产率较低,但非对映选择性较高(3e,产率67%,d/r值20/1)。
2.2 抗肿瘤活性
表1为3a~3h对K562、 PC-3和A549的体外抗肿瘤活性。虽然仅仅从以上几例体外活性数据很难总结其构效关系, 但由表1可见,化合物3d和3g对人白血病细胞K562具有明显的抑制活性,IC50分别为24.1 μmol·L-1和32.4 μmol·L-1,接近阳性对照药顺铂。其他测试化合物对A549、 PC-3和K562的抑制活性弱于顺铂。以上结果表明化合物3a~3h可作为先导化合物的骨架进一步研究。
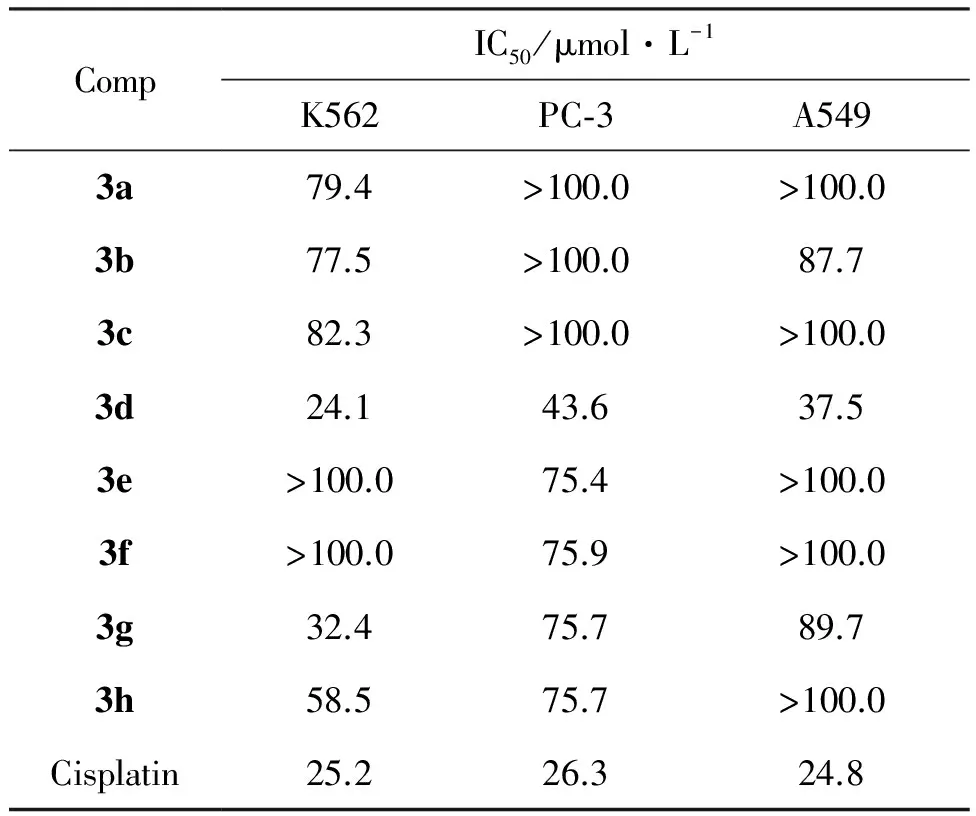
表1 3a~3h的体外抗肿瘤活性
以取代烷氧基嘧啶3-烯键氧化吲哚衍生物为原料,与肌氨酸及多聚甲醛经1,3-偶极子3+2环加成反应合成了8个新型烷氧基嘧啶拼接3-吡咯螺环氧化吲哚类化合物(3a~3h),产率67%~82%,d/r值6/1~20/1。并用MTT法研究了3a~3h对人肺癌细胞(A549)、人前列腺癌细胞(PC-3)和人白血病细胞(K562)的体外抗肿瘤活性。结果表明:化合物3d和3g对人白血病细胞K562具有明显的抑制活性,接近阳性对照药顺铂。以上结果表明化合物3可作为先导化合物的骨架进一步研究,其他相关药理活性的研究正在进行中。
[1] Galliford C V, Scheidt K A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents[J].Angew Chem Int Ed,2007,46:8748-8758.
[2] Han W Y, Zhao J Q, Zuo J,etal. Recent advances ofa-isothiocyanato compounds in the catalytic asymmetric reaction[J].Adv Synth Catal,2015,357:3007-3031.
[3] Cui C B, Kakeya H, Osada H,etal. Novel mammalian cell cycle inhibitors,spirotryprostatins A and B,produced byAspergillusfumigatus,which inhibit mammalian cell cycle at G2/M phase[J].Tetrahedron 1996,52:12651-12666.
[4] Ding K, Lu Y, Coleska N Z,etal. Structure-based design of potent non-peptide MDM2 Inhibitors[J].J Am Chem Soc,2005,127:10130-10131.
[5] Wong W H, Lim P B, Chuah C H,etal. Oxindole alkaloids fromAlstoniamacrophylla[J].Phytochemistry 1996,41:313-315.
[6] 彭礼军,周根,韩朔楠,等. 通过1,3-偶极环加成反应合成3-吡咯螺环氧化吲哚的研究进展[J].山地农业生物学报,2015,34(2):009-013.
[7] 刘雄伟,周根,姚震,等. 异噁唑拼接吡咯螺环氧化吲哚化合物的合成及其抗肿瘤活性[J].合成化学,2016,24(5):389-392.
[8] Takama H, Tanaka H, Sudo T,etal. Population pharmacokinetic modeling and model validation of a spicamycin derivative,KRN5500,in phase 1 study[J].Cancer Chemother Pharmacol, 2001,47(5):404-410.
[9] Gadgeel S M, Boinpally R R, Heilbrun L K,etal. A phase I clinical trial of Spicamycin derivative KRN5500(NSC 650426) using a phase I accelerated titration“2B”design[J]. Invest New Drugs,2003,21(1):63-74.
[10] Dordoni P L, Frassanito L, Bruno M F,etal.Invivoandinvitroeffects of different anaesthetics on platelet function[J].Br J Haematol,2004,125(1):79-82.
[11] Kazimierczuk Z, Cottam H B, Revankar G R,etal. Synthesis of 2′-deoxytubercidin, 2′-deoxyadenosine, and related 2′-deoxynucleosidesviaa novel direct stereospecific sodium salt glycosylation procedure[J].J Am Chem Soc,1984,106(21):6379-6382.
[12] Supko J G, Ryan D P, Seiden M V,etal. Phase I clinical trial and pharmacokinetic study of the spicamycin analog KRN5500 administered as a 1-hour intravenous infusion for five consecutive days to patients with refractory solid tumors[J].Clin Cancer Res,2003,9(14):5178-5186.
[13] 彭礼军,周根,韩朔楠,等. 新型芳姜黄酮拼合吡咯螺环氧化吲哚类化合物的合成及其抗肿瘤活性[J].合成化学,2016,24(8):669-672.
[14] Mosman T J. Rapid colorimetric assay for eellulair growth and survival:Application and cytotxicity assays[J].Immunol Methods,1983,65:55-63.
[15] Alley M C, Scudiero D A, Monks A,etal. Feasibility of drug screening with panals of human tumor cell lines using a mycroculture tetrazolium assay[J].Cancer Res,1988,48:589-601.
2017年第25卷合 成 化 学Vol.25, 2017 第1期, 26~31Chinese Journal of Synthetic ChemistryNo.1, 26~31
Synthesis and Antitumor Activities of Novel Pyrimidine-fused Spiropyrrolidine Oxindoles
CHEN Shuang1, XIAO Le2,3, CHEN Zhi-yong3, YANG Chao3, GONG Yi3, YU Zhang-biao3*, ZHOU Ying3, LIU Xiong-li3*
(1. Institute of Biology and Engineering, Guizhou University of Medical Sciences, Guiyang 550025, China; 2. Chemical Group, Chenzhou City First Middle School, Chenzhou 423000, China; 3. Guizhou Engineering Center for Innovative Traditional Chinese Medicine and Ethnic Medicine, Guizhou University, Guiyang 550025, China)
Eight novel pyrimidine-fused spiropyrrolidine oxindoles(3a~3h)were synthesized by 1,3-dipolar reaction of pyrimidine-fused 3-alkenyloxindoles with azomethine ylides(thermally generatedinsitufrom sarcosine and formaldehyde). The yields andd/rof 3a~3h were 67%~82% and 6/1~20/1, respectively. The structures were characterized by1H NMR,13C NMR and HR-MS(ESI-TOF). Theinvitroantitumor activities against human lung cancer cells(A549), human prostate cancer cells PC-3 and human leukemia cells(K562) were demonstrated by MTT assays. The results showed that 3d and 3g exhibited certaininvitroinhibitory activities against K562 with IC50of 24.1 mol·L-1and 32.4 mol·L-1, respectively, equipotent to the positive control of Cisplatin.
sarcosine; formaldehyde; pyrimidine-fused 3-alkenyloxindole; pyrimidine-fused 3-alkenyloxindole; 1,3-dipolar cycloaddition reaction; synthesis; antitumor activity
2016-09-21
国家自然科学基金地区基金资助项目(81560563); 贵州省教学改革创新项目(SJJG201423);贵州省制药工程专业学位研究生工作站(黔教研合JYSZ字【2014】002); 贵州省研究生教改课题(黔教研合JG字[2016]06)
陈爽(1995-),女,仡佬族,贵州安顺人,本科生,主要从事天然产物活性成分研究。 E-mail: 2568715818@qq.com
刘雄利,博士,副教授,硕士生导师, E-mail: xlliu1@gzu.edu.cn; 余章彪,教授,硕士生导师, E-mail: gym.zbyu@gzu.edu.cn
O626.13; O623.7
A
10.15952/j.cnki.cjsc.1005-1511.2017.01.16243

