Ru/Ce(OH)CO3纳米复合材料催化氨硼烷水解产氢
陈健民 卢章辉 熊丽华
(江西师范大学化学化工学院无机膜材料工程技术研究中心,南昌330022)
Ru/Ce(OH)CO3纳米复合材料催化氨硼烷水解产氢
陈健民 卢章辉*熊丽华
(江西师范大学化学化工学院无机膜材料工程技术研究中心,南昌330022)
采用一种简单的方法快速合成了Ru/Ce(OH)CO3纳米复合材料。基于TG,XRD,TEM,EDX,XPS和ICP等方法详细表征了所制备的催化剂,并用于催化氨硼烷水解制氢。表征结果表明尺寸大约为4.8 nm的Ru纳米粒子高度分散在Ce(OH)CO3纳米棒上。该催化剂对于氨硼烷水解制氢表现出优异的催化性能,在室温下其转化频率(TOF)达到389.6molH2·molRu-1·min-1。而且该催化剂循环使用11次之后依然能够对氨硼烷催化产氢保持很高的活性。
能源材料;氨硼烷;稀土;催化水解;制氢
0 Introduction
Ammonia borane(NH3BH3,AB)complex has been identified as one of the most attractive candidates for on-board portable hydrogen applications among all other practical hydrogen storage materials due to its high hydrogen content(19.6%,w/w),low molecular weight,high stability under ambient condition,and environmentally friendly nature[1-7].More importantly, hydrogen stored in AB can be released at an appreciable rate via hydrolysis in the presence of a suitable catalyst,which appears to be the most
convenient one for portable hydrogen storage application[8-10].The catalytic hydrolysis reaction can be briefly expressed as follows:

To date,a number of catalyst systems have been tested in the hydrolysis of AB,such as noble metals Au[11],Pt[12-14],Ru[15-17],Rh[18-20],Pd[21,22],Ag[23],and nonnoblemetals Cu[24-27],Fe[28-31],Ni[32-34],Co[35-38].Although metal in nano-size show a higher catalytic activity, they easily aggregate into clumps during the catalytic processes due to their high surface energy,resulting in a large reduction of their catalytic activity or inactivation[12].For the development of heterogeneous catalysts,decreasing the size of the catalyst and preventing aggregation are the keys to obtaining high catalytic activity.One strategy to achieve this goal is to immobilize the catalyst onto specific supports[18].In recent years,rare earth compounds have been widely applied in catalysis,fuel cells,optical materials,gas sensors,and chemical materials due to their specific property.In particularly,cerium oxides have been successfully used as carriers to support noble metal nanoparticles(NPs)[39].To our knowledge,there are few reports about using the cerium hydroxide carbonate(Ce(OH)CO3)as the support for immobilize themetal NPs.
In this work,we report a facile synthesis of Ru/ Ce(OH)CO3nanocomposites(NCs)under mild condition.Neither additional functional molecules nor stabilizing molecules are employed during the whole preparation.The as-synthesized catalysts exert a higher catalytic activity than most of reported metal NPs in the hydrolytic dehydrogenation of AB.The high catalytic activity and good durability of the Ru/ Ce(OH)CO3NCsmake them a very promising candidate to be used as catalyst in the practical application hydrogen generation systems using AB as solid hydrogen storagematerial.
1 Experimental
1.1 Materials
Ammonia borane(NH3BH3,Aldrich,90%),hexaammine ruthenium(II)chloride(Ru(NH3)6Cl3,J&K Scientific Ltd.,97.5%),cerium(II)nitrate hexahydrate, (Ce(NO3)3·6H2O,J&K Scientific Ltd.,99.5%),urea (CO(NH2)2,Aladdin,99%),sodium borohydride(NaBH4, Aldrich,99.9%)were all used without further purification.Ultrapure water with the specific resistance of 18.3 MΩ·cm was obtained by reversed osmosis followed by ion exchange and filtration.
1.2 Synthesis of Ru/Ce(OH)CO3NCs
Ce(OH)CO3nanorods was prepared by a slight modification of a recipe described previously[40-42].In a typical synthesis,calculated amounts of aqueous solutions of Ru(NH3)6Cl3(6 mL,3.26 mg)corresponding to 6.0%(w/w,the same below)Ru loading were added to 16.8 mg of as-prepared Ce(OH)CO3nanorods under stirring.Themixture was kept at 90℃for 3 h, followed by washing with water and then reduced by freshly aqueous solution of NaBH4(3 mg,5mL).After a few minute,a grey suspension of Ru/Ce(OH)CO3NCs were obtained and then used as the catalyst for the catalytic reaction.The other four samples with different Ru loadings(2%,4%,8%and 10%)were also prepared using the abovemethod by changing the amountof Ce(OH)CO3.
For comparison,Ru NPs with different supports (such as C,Al2O3,SiO2,CeO2,and Ce(OH)CO3)were also prepared by the impregnationmethod(IMP).2.6 mg of RuCl3and 16.8 mg of different supports were add into the 5 mL of water solution,and then the solution was stirred and sonicated at room temperature for 30min.Afterward,3 mg of NaBH4was added into the above obtained solution with vigorous stirring. After 5 min,the black product were obtained,and then used as catalyst for the catalytic reaction.The Ru loading is 6.0%in order to obtain similar active particles.
1.3 M aterial characterization
The powder X-ray diffraction(XRD)was performed on a Rigaku RINT-2200 X-ray diffractometer with a Cu Kα(λ=0.154 05 nm)source(40 kV,20mA) to determine the structure of these catalysts.Scanning electron microscope(SEM,Hitachi,SU8020)image was taken on a cold field-emission instrument to survey themorphology of catalyst(5 kV).Transmission
electron microscope(TEM),energy-dispersive X-ray spectroscopy(EDX),were observed using transmission electron microscope(TEM,JEM-2100)operating at 200 kV.The TEM samples were dispersed in ethanol by sonication 30 min,and then one droplet of the nanoparticle suspension was dropped onto a carboncoated copper grid and dried in air.X-ray photoelectron spectroscopy(XPS)was carried out on an ESCALABMKLL X-ray photoelectron spectrometer with Al Kαradiation.The weight contentof Ru in Ru/ Ce(OH)CO3NCs was analyzed bymeans of a 725-ES inductively coupled plasma(ICP)spectrophotometer (Varian Corp).Thermogravimetric analyses(TG)was carried outwith a Netzsch-409 STA simultaneous TGDTA apparatus with a heating rate of 10℃·min-1under flowing air.
1.4 Catalytic activity
Catalytic reactions were carried out at room temperature using a two-necked round bottom flask with one of the flask openings connected to a gas burette and the other for the introduction of AB. During the reaction,the flask was placed in a thermostat that was equipped with a water circulating system to maintain the reaction temperature(25℃), usually within±0.5℃.Then 1 mmol AB(34.30 mg) was added into the reaction vessel and the reactant was quickly stirred with amagnetic stirrer during the reaction.The volume of hydrogen gas was evaluated in a typicalwater-filled graduated burette system.
1.5 K inetic studiesof hydrolytic dehydrogenation of AB catalyzed by Ru/Ce(OH)CO3NCs
Sets of experiments were carried out to establish the rate law for catalytic hydrolysis of AB in aqueous solution using Ru/Ce(OH)CO3NCs as catalyst.In the first set of experiments,different concentrations of Ru (0.35,0.69,1.04,and 1.38 mmol·L-1)were performed at room temperature while the AB concentration was kept the same(200 mmol·L-1).In the second set of experiments,different concentrations of AB(100,200, 300,and 400 mmol·L-1)were performed at room temperature while the Ru concentration was kept the same(1.38mmol·L-1).Finally,the reaction temperature was varied at 20,25,30,and 35℃while the molar ratio of nRu/nABwas kept constantof 0.006 9.
1.6 Durability test
For durability test,catalytic reactions were repeated 11 times at room temperature by adding another equivalent of AB(1 mmol)into the reactor after the previous cycle.Themolar ratio of Ru to AB was keptat0.006 9 during each cycle test.
2 Result and discussion
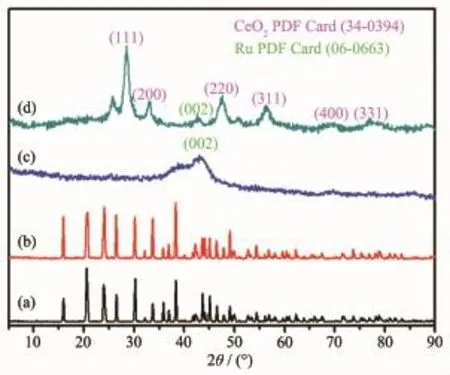
Fig.1 XRD patterns of the samples:(a)Ce(OH)CO3nanorods;(b)Ru/Ce(OH)CO3NCs;(c)Ru NPs; (d)Ru/Ce(OH)CO3NCs after heat treatmentat 450℃for 2 h in argon atmosphere
2.1 Catalyst characterization
According to the TG/DTA analysis,the Ce(OH)CO3material is stable under the temperature less than 280℃(Fig.S1).Therefore,the synthesized Ce(OH)CO3can be used as the support for nanocatalysts.The assynthesized Ru/Ce(OH)CO3NCswere characterized by ICP,TG,XRD,TEM,HRTEM,EDX,and XPS.The contents of Ru in the Ru/Ce(OH)CO3NCs were determined to be 6.0%by ICPmeasurements(Table S1). The XRD patterns of the as-synthesized Ce(OH)CO3, Ru NPs,Ru/Ce(OH)CO3NCs before and after calcination are presented in Fig.1,respectively.As shown in Fig.1a,all peaks in this pattern can be well-indexed to a pure phase of Ce(OH)CO3,which is in good agreementwith the PDF file for Ce(OH)CO3(PDF No. 41-0013).Similar diffraction peaks are also observed in Ru/Ce(OH)CO3NCs,indicating that hostmaterials almost remain intact after the loading of Ru NPs.For free Ru NPs(Fig.1c),the diffraction peak 2θ=44.0°
attributed to Ru was observed.No distinct diffraction peaks attributed to Ru were detected from the XRD pattern of Ru/Ce(OH)CO3NCs(Fig.1b),which might be due to the fact that the loading of Ru is too small and the diffraction peaks of Ru NPs are covered by Ce(OH)CO3.To get the XRD information of Ru specie in the NCs,we have tested Ru/Ce(OH)CO3NCswith a highmetal loading of 60.0%[43-44](Fig.S2c).It is found that obvious diffraction peak of Ru show up, indicating the existence of Ru in the NCs.As for Ru/ Ce(OH)CO3NCs with a Ru loading of 6.0%,after heated treatment at 450℃for 2 h in argon atmosphere,Ru and CeO2with pure face-centered cubic (fcc)structure are observed(Fig.1d).
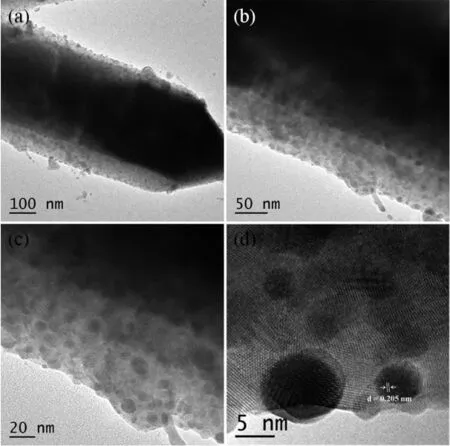
Fig.2 TEM(a~c)and HR-TEM(d)images of Ru/Ce(OH)CO3NCs
The microstructure of Ru/Ce(OH)CO3NCs were fully analyzed by SEM,TEM,HRTEM,and EDX observations.The TEM and SEM imagesof Ce(OH)CO3are shown in Fig.S3 and S4,respectively.The rod-like Ce(OH)CO3support with 150~350 nm in diameter, tens of nanometers in length,and smooth surface are observed.As shown in Fig.S5,the SEM images of the as-prepared Ru/Ce(OH)CO3NCs display the rougher surface of Ce(OH)CO3,indicating that Ru NPs are immobilized on the surface of Ce(OH)CO3.When the Ru loading increases from 2%to 10%,the size of the metal NPs supported on Ce(OH)CO3grows gradually. Especially,the SEM images of Ru/Ce(OH)CO3NCs with a high Ru loading 8%and 10%show a large particle aggregation on the surface of Ce(OH)CO3.The TEM images of 6%Ru/Ce(OH)CO3NCs are shown in Fig.2a~c.It can be found that the round and dark points of Ru NPs are embedded and highly dispersed
on the gray rods of Ce(OH)CO3,which can help to prevent the agglomeration of Ru NPs.The lattice fringes can be observed in the high-resolution TEM (HRTEM)image(Fig.2d).Fig.2d shows the d spacing of 0.205 nm,which is consistentwith the(101)plane of Ru.Furthermore,the HRTEM image shows that the as-prepared Ru NPs with the size of about 4.8 nm. The TEM-EDX spectrum was shown in Fig.S6, indicating that ruthenium is the only element detected in the sample in addition to the framework elements of Ce(OH)CO3(Ce,C,O).Ru/Ce(OH)CO3-IMP can be prepared by impregnationmethod with the Ce(OH)CO3and RuCl3.The representative SEM image of Ru/Ce (OH)CO3-IMP isshown in Fig.S7.A similarmorphology was observed as for Ru/Ce(OH)CO3NCs(Fig.S5).The compositions of Ru and Ce in the Ru/Ce(OH)CO3-IMP is 0.006 9 and 0.073 6mmol,which are quite close to the initial amountof Ru and Ce,respectively.
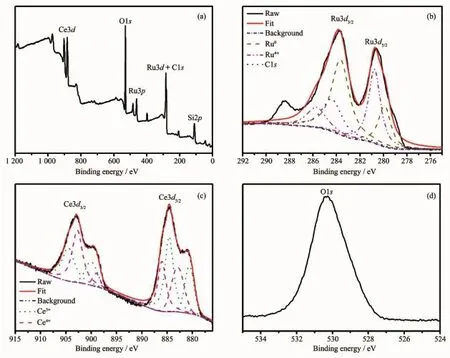
Fig.3 Survey XPSspectrum of Ru/Ce(OH)CO3NCs(a)and the high resolution XPSspectra of Ru3d,Ce3d,and O1s of Ru/Ce(OH)CO3NCs(b~d)
Additionally,XPS is applied to confirm the presence ofmetallic ruthenium,which reveal that the Ru is the only element detected in addition to the Ce(OH)CO3framework elements(C,O,Ce)(Fig.3a). The results are well agreed with the EDX results.As shown in Fig.3b,the XPS spectrum consists of C1s peaksand Ru3d doublets.Thepeak-fittingofRu3d core level reveals four peaks,the peaks at 279.9(Ru3d5/2) and 283.6 eV(Ru3d3/2)are attributed to Ru0[45-46].While the other two peaks at 280.7 and 285.7 eV stand for oxidized Ru[47-48],whichmay originate from the oxidation of the Ru NPs upon exposure to air.The former peak at284.4 eV,covered by the signal ofC1s,is consistent with binding energy values related to Ru3d3/2[46]. Additionally,C1s peak at288.4 eV(C=O)is observed in the XPS.In summary,the nanocatalysts appeared to consist of Ru0and RuO2embedded on Ce(OH)CO3. It should be noted that the partial oxidation of Ru should not exert significant influence on the catalytic activity of the Ru/Ce(OH)CO3NCs catalyst because of the inactive RuO2might be reduced back to Ru0by AB or NaBH4[49].Furthermore,XPS analysis is applied
for the sample with Ce(Fig.3c)and O(Fig.3d).It can be seen that,in this sample,Ce has the oxidized states of Ce3+and Ce4+.The peak of O1s located at about 530.5 eV is due to the chemisorbed oxygen caused by Ce(OH)CO3.It is reported that the oxygen defect structure of cerium oxide is dynamic and may change spontaneously due to physical parameters such as temperature,presence of other ions,and partial pressure of oxygen[50-51].Therefore,the existences of Ce3+and Ce4+in the sample are reasonable.
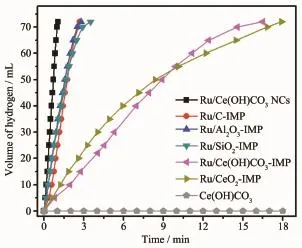
Fig.4 Plots of the volume of hydrogen generation from AB hydrolysis as a function of time catalyzed by Ru/Ce(OH)CO3NCs,Ru/C-IMP,Ru/Al2O3-IMP, Ru/SiO2-IMP,Ru/Ce(OH)CO3-IMP,Ru/CeO2-IMP, and Ce(OH)CO3at25℃,respectively(nRu/nAB= 0.006 9)
2.2 Catalytic activities for hydrolysis of AB
Catalytic activity of the prepared Ru/Ce(OH)CO3NCs together with Ru/Ce(OH)CO3-IMP,Ru/C-IMP, Ru/Al2O3-IMP,Ru/SiO2-IMP,and Ru/CeO2-IMP have been investigated for the hydrolysis of AB aqueous solution at25℃(nRu/nAB=0.006 9).As shown in Fig.4, the H2generation rate from aqueous AB significantly depends on different catalysts.No hydrogen generation was observed for Ce(OH)CO3,indicating that Ce(OH) CO3is inactive for hydrolysis of AB.The Ru/C-IMP, Ru/Al2O3-IMP,Ru/SiO2-IMP,Ru/CeO2-IMP and Ru/Ce (OH)CO3-IMP catalysts show a lower catalytic activity, as compared to the activity of Ru/Ce(OH)CO3NCs. Evidently,the as-synthesized Ru/Ce(OH)CO3NCsshow an excellent catalytic activity(1.1 min)with a total TOF value as high as 389.6 molH2·molRu-1·min-1,relatively high values for hydrolytic dehydrogenation of AB(Table S2)[12,15,17-19,21,25-26,52-60].The highly catalytic activity of Ru/Ce(OH)CO3NCs are attributed to high dispersion of Ru NPs on the Ce(OH)CO3nanorod.The formation of highly disperse Ru supported on Ce(OH) CO3nanorod may be due to a reaction between Ru (NH3)6Cl3and Ce(OH)CO3at 90℃[42].In addition,Ru/ Ce(OH)CO3-IMP and Ru/CeO2-IMP prepared with Ru (NH3)6Cl3precursor show a lower catalytic activity than those of Ru/Ce(OH)CO3-IMP and Ru/CeO2-IMP prepared with RuCl3precursor(Fig.S8).The Ru/Ce (OH)CO3NCs with a Ru loading of 6.0%(Fig.S9) displays a superior catalytic performance for the generation of hydrogen amongstall the Ru/Ce(OH)CO3catalysts with different Ru weight.Furthermore,the as-synthesized Ru/Ce(OH)CO3NCs show a robust durability for hydrolysis of AB even after eleven times of recycle(Fig.S10).The observed activity loss is probably due to the fact that the increase viscosity of the solution and/or deactivation effect of the increasing metaborate concentration during the hydrolysis of AB[7].
As shown in the Fig.S11,the Ru/Ce(OH)CO3NCs after heat treatment at 450℃for 2 h in argon atmosphere(i.e.Ru/CeO2)have been investigated for the hydrogen generation from AB(200 mmol·L-1,5 mL).A stoichiometric amountof hydrogen(72mL)is evolved in 10.2 min.Obviously,Ru/Ce(OH)CO3NCs after heated in the Aratmosphere(i.e.Ru/CeO2)exhibit a lower catalytic performance,in comparison to Ru/Ce (OH)CO3NCs,for the dehydrogenation of AB.The low activity of Ru/Ce(OH)CO3NCs after calcination could be attributed to the thermal decomposition of Ce(OH) CO3and the aggregated of Ru.
Three set of experiments about catalyst concentration,AB concentration,and temperature were carried out to investigate the kinetics of Ru/Ce(OH) CO3NCs toward hydrolysis of AB.Fig.5a shows the plot of the evolution of equivalent hydrogen permole of AB versus time for the hydrolysis of AB using Ru/ Ce(OH)CO3NCs as the catalyst in different Ru concentrations.When the catalyst concentration increases, the reaction time decreases obviously from 4.35 min
to 1.1 min for complete hydrogen release and the hydrogen generation rate rises gradually.The hydrogen generation rate was determined from the linear portion from 0 to 70mL of each plot.As shown in Fig.5b,the line slope of the plot of hydrogen evolution rate versus catalyst concentration in a log-log scale is 0.88,indicating that the hydrolysis of AB catalyzed by Ru/Ce (OH)CO3NCs are first order with respect to the catalyst concentration.Therefore,the hydrogen generation rate(r)increases by increasing catalyst concentration as expected(i.e.,ln r=ln cRu+3.59).Another set of experiments were carried out to study the effect of substrate concentration on the hydrogen generation rate.Fig.S12a shows the plot of the evolution of AB versus time for the hydrolysis of different AB concentrations using a constant Ru/Ce(OH)CO3NCs concentration at1.38mmol·L-1as the catalystat(25±0.5)℃. As shown in Fig.S12b,the line slope of the plot of hydrogen evolution rate versus initial concentration of AB,both in a log-log scale is 0.005,indicating a nearly zero-order regarding the AB concentration.The hydrogen generation rate keeps constant(ln r=4.2)for different AB concentrations from the catalytic hydrolysis of AB.
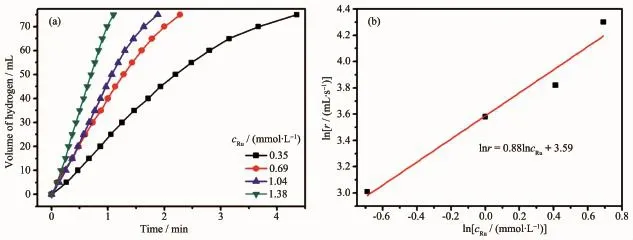
Fig.5(a)Plots of the volume of hydrogen generation from AB hydrolysis as a function of time at different catalyst concentrations catalyzed by Ru/Ce(OH)CO3NCs(cAB=200mmol·L-1);(b)ln cRuvs lnr plot
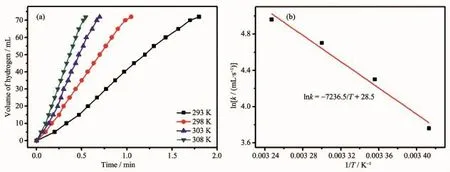
Fig.6(a)Plotof the volume of hydrogen generation from AB hydrolysis as a function of time at differenttemperatures catalyzed by Ru/Ce(OH)CO3NCs(nRu/nAB=0.006 9);(b)ln k vs 1/T p lot
In the final set of kinetic studies,the catalytic hydrolysis of AB was carried out at various temperatures in the range of 20~35℃.Obviously,as the reaction temperature increasing from 20 to 35℃,the hydrogen generation rates increase quickly(Fig.6a). The values of rate constant k at different temperatures are calculated from the slope of the linear partof each plot from Fig.6a.The Arrhenius plot of ln k vs 1/T for the catalyst is plotted in Fig.6b,from which the apparent activation energy is determined to be
approximately 60.16 kJ·mol-1.
3 Conclusions
In summary,the hydrogen production by hydrolysis of AB was studied by using Ru0NPs immobilized on Ce(OH)CO3.Ru NPs with average diameter about 4.8 nm were remarkably well-dispersed on the Ce(OH)CO3.The synthesized Ru/Ce(OH)CO3NCs exhibited a superior catalytic activity for the hydrolytic dehydrogenation of AB under ambient atmosphere at room temperature.The TOF for the hydrolysis of AB in the presence of Ru/Ce(OH)CO3NCs were measured to be 389.6 molH2·molRu-1·min-1.Moreover, Ru/Ce(OH)CO3nanocatalyst was reused for at least eleven cycles in complete hydrolysis of AB.This simple synthetic method can be extended to other Ce(OH)CO3-based metallic systems in more applications.
Supporting information isavailableathttp://www.wjhxxb.cn
[1]Zhang K,Han P,Hu Z,et al.Chem.Soc.Rev.,2015,44(3): 699-728
[2]Dem irci U B,Miele P.Energy Environ.Sci.,2011,4(9):3334-3341
[3]Han M,Zhao Q,Zhu Z,et al.Nanoscale,2015,7(43):18305-18311
[4]Lu Z H,Yao Q,Zhang Z,et al.J.Nanomater.,2014,2014: 729029
[5]Chandra M,Xu Q.J.Power Sources,2006,156(2):190-194
[6]Yao Q,Lu ZH,Wang Y,etal.J.Phys.Chem.C,2015,119 (25):14167-14174
[7]Yao Q,Lu ZH,HuangW,et al.J.Mater.Chem.A,2016,4 (22):8579-8583
[8]Chen Y Z,Xu Q,Yu SH,et al.Small,2015,11(1):71-76
[9]Yan JM,Wang Z L,Wang H L,etal.J.Mater.Chem.,2012, 22(22):10990-10993
[10]Lu Z H,Jiang H L,Yadav M,et al.J.Mater.Chem.,2012, 22(11):5065-5071
[11]Yan JM,Zhang X B,Akita T,et al.J.Am.Chem.Soc., 2010,132(15):5326-5327
[12]Aijaz A,Karkamkar A,Choi Y J,et al.J.Am.Chem.Soc., 2012,134(34):13926-13929
[13]Chen W,Ji J,Duan X,et al.Chem.Commun.,2014,50(17): 2142-2144
[14]Wang S,Zhang D,Ma Y,et al.ACSAppl.Mater.Interfaces, 2014,6(15):12429-12435
[15]Yao Q,Shi W,Feng G,et al.J.Power Sources,2014,257: 293-299
[16]Abo-Hamed E K,Pennycook T,Vaynzof Y,et al.Small, 2014,10(15):3145-3152
[17]Chen G,Desinan S,Nechache R,et al.Chem.Commun., 2011,47(22):6308-6310
[18]Yao Q,Lu ZH,Jia Y,et al.Int.J.Hydrogen Energy,2015, 40(5):2207-2215
[19]Zhang Z,Lu Z H,Tan H,et al.J.Mater.Chem.A,2015,3 (46):23520-23529
[20]Shen J,Yang L,Hu K,et al.Int.J.Hydrogen Energy,2015, 40(2):1062-1070
[21]Akbayrak S,Kaya M,Volkan M,et al.Appl.Catal.B,2014, 147:387-393
[22]LiD,Han J,Han L,etal.J.Environ.Sci.,2014,26(7):1497 -1504
[23]Chen J,Lu Z H,Wang Y,et al.Int.J.Hydrogen Energy, 2015,40(14):4777-4785
[24]Yao Q,Huang M,Lu Z H,et al.Dalton Trans.,2015,44(3): 1070-1076
[25]Yang Y,Lu ZH,Hu Y,et al.RSC Adv.,2014,4(27):13749-13752
[26]Kaya M,Zahmakiran M,Özkar S,et al.ACS Appl.Mater. Interfaces,2012,4(8):3866-3873
[27]Yao Q,Lu ZH,Zhang Z,et al.Sci.Rep.,2014,4:7597
[28]Yan JM,Zhang X B,Han S,et al.Angew.Chem.Int.Ed., 2008,47(12):2287-2289
[29]Lu Z H,Li J,Zhu A,et al.Int.J.Hydrogen Energy,2013, 38(13):5330-5337
[30]Glüer A,Förster M,Celinski V R,etal.ACSCatal.,2015,5 (12):7214-7217
[31]Wang H L,Yan JM,Wang Z L,et al.Int.J.Hydrogen Energy,2012,37(13):10229-10235
[32]Shan X,Du J,Cheng F,et al.Int.J.Hydrogen Energy, 2014,39(13):6987-6994
[33]Lu ZH,Li J,Feng G,etal.Int.J.Hydrogen Energy,2014, 39(25):13389-13395
[34]Metin O,Mazumder V,Ozkar S,et al.J.Am.Chem.Soc., 2010,132(5):1468-1469
[35]Wang H,Zhao Y,Cheng F,etal.Catal.Sci.Technol.,2016,6 (10):3443-3448
[36]Sahiner N,Yasar A O.Int.J.Hydrogen Energy,2014,39 (20):10476-10484
[37]YANG Yu-Wen(杨宇雯),FENGGang(冯刚),LU Zhang-Hui
(卢章辉),etal.Acta Phys.-Chim.Sin.(物理化学学报),2014, 30(6),1180-1186
[38]Yu P J,Lee M H,Hsu H M,et al.RSC Adv.,2015,5(18): 13985-13992
[39]Wang X,Liu D,Song S,et al.Chem.Commun.,2012,48 (82):10207-10209
[40]Gao K,Zhu Y Y,Tong D Q,et al.Chin.Chem.Lett.,2014, 25(2):383-386
[41]ZHANG Ze-Fang(张泽芳),YU Lei(俞磊),LIUWei-Li(刘卫丽),et al.Chinese J.Inorg.Chem.(无机化学学报),2011,27 (4):759-763
[42]Zhu F,Chen G,Sun S,et al.J.Mater.Chem.A,2013,1(2): 288-294
[43]Qao Q,Lu,ZH,Yang K,et al.Sci.Rep.,2015,5:15186
[44]Chen J,Qao Q,Zhu J,et al.Int.J.Hydrogen Energy,2016, 41(6):3946-3954
[45]Rachiero G P,Dem irci U B,Miele P.Catal.Today,2011, 170(1):85-92
[46]Qadir K,Joo SH,Mun B S,et al.Nano Lett.,2012,12(11): 5761-5768
[47]Dai H B,Kang X D,Wang P.Int.J.Hydrogen Energy, 2010,35(19):10317-10323
[48]Joo S H,Park JY,Renzas JR,et al.Nano Lett.,2010,10 (7):2709-2713
[49]Can H,MetinÖ.Appl.Catal.B,2012,125:304-310
[50]Chen G,Sun S,Zhao W,et al.J.Phys.Chem.C,2008,112 (51):20217-20221
[51]Wang L,Zhang L F,Zhong SL,et al.Appl.Surf.Sci.,2012, 263:769-776
[52]Li X,Zeng C,Fan G.Int.J.Hydrogen Energy,2015,40(10): 3883-3891
[53]Liang H,Chen G,Desinan S,et al.Int.J.Hydrogen Energy, 2012,37(23):17921-17927
[54]Akbayrak S,Özkar S.ACS Appl.Mater.Interfaces,2012,4 (11):6302-6310
[55]Rakap M.Appl.Catal.A,2014,478:15-20
[56]Durap F,Zahmakıran M,Özkar S.Appl.Catal.A,2009,369 (1):53-59
[57]Hu Y,Wang Y,Lu Z H,et al.Appl.Surf.Sci.,2015,341: 185-189
[58]Sahiner N,Demir S,Yildiz S.Colloids Surf.A,2014,449: 87-95
[59]Yan JM,Zhang X B,Shioyama H,et al.J.Power Sources, 2010,195(4):1091-1094
[60]Peng C Y,Kang L,Cao S,et al.Angew.Chem.Int.Ed., 2015,54(52):15725-15729
Hydrolysis of Ammonia Borane by Ru/Ce(OH)CO3Nanocom posites for Hydrogen Production
CHEN Jian-Min LU Zhang-Hui*XIONG Li-Hua
(Jiangxi Inorganic Membrane Materials Engineering Research Centre,College of Chemistry and
Chemical Engineering,Jiangxi Normal University,Nanchang 330022,China)
A facile method for fabrication of Ru/Ce(OH)CO3nanocomposites(NCs)undermild conditions was reported.The as-synthesized catalysts were characterized by TG,XRD,TEM,EDX,XPS,ICP,and used for H2production from hydrolysis of ammonia borane(AB).All the characterized results exhibit that Ru nanoparticles (NPs)around 4.8 nm are highly dispersed on Ce(OH)CO3nanorods.The as-synthesized Ru/Ce(OH)CO3NCs show a high catalytic activity for hydrogen generation from the aqueous of ammonia borane complex with a turnover frequency(TOF)of 389.6 molH·2molRu-1·min-1at room temperature,being higher than that ofmost reported metalbased catalysts.Especially,the catalyst can keep a superior activity for hydrolysis of AB even after eleven times of recycle.
energy storagematerials;ammonia borane;rare earth oxide;catalytic hydrolysis;hydrogen generation
O643.36
A
1001-4861(2016)10-1816-09
10.11862/CJIC.2016.228