基于半刚性的4-羧基苯乙酸和富氮共配体组装的Zn(II)/Cd(II)配位聚合物的合成、结构和性质
鞠丰阳 李云平 李桂连 刘广臻*, 辛凌云 李晓玲
(1洛阳师范学院食品与药品学院,洛阳471934)
(2洛阳师范学院化学化工学院,洛阳471934)
基于半刚性的4-羧基苯乙酸和富氮共配体组装的Zn(II)/Cd(II)配位聚合物的合成、结构和性质
鞠丰阳1李云平2李桂连2刘广臻*,2辛凌云2李晓玲2
(1洛阳师范学院食品与药品学院,洛阳471934)
(2洛阳师范学院化学化工学院,洛阳471934)
采用溶剂热法合成了一系列Zn(II)/Cd(II)配位聚合物:{[Zn(cbaa)(bpmp)0.5(H2O)]·2H2O}n(1)、[Zn(cbaa)(bip)]n(2)、[Cd(cbaa) (Hizb)]n(3)和[Cd2(cbaa)2(itmb)(H2O)]n(4)(H2cbaa=4-羧基苯乙酸;bpmp=1,4-二(4-吡啶甲基)哌嗪;bip=3,5-双(1-咪唑基)吡啶;Hizb= 2-(4-咪唑-1-基苯基)-1H-苯并咪唑;itmb=1-(咪唑-1-基)-4-(1,2,4-三唑-1-基甲基)苯)。X射线单晶衍射结果表明,半刚性的4-羧基苯乙酸和富氮辅助配体构筑形成了4个多样化拓扑结构的配位聚合物。化合物1和2是Zn(II)配位聚合物:1是由2个Zn-羧酸盐链之间通过富氮配体桥连形成的一维梯形结构,而2是由Zn-羧酸盐链之间通过富氮配体拓展形成的二维单层结构;化合物3和4是Cd(II)配位聚合物:3是由Cd-O无机链之间通过羧酸配体的桥连拓展形成的二维单层结构,富氮配体作为伸出层平面的悬臂仅仅起到结构修饰作用,而4则形成了Cd-羧酸盐空旷双层结构,富氮配体填充在层内空腔中,从而导致了致密双层结构的产生。另外,考察了4个化合物的热稳定性和光致发光性能。
溶剂热合成;锌;镉;配位聚合物;荧光
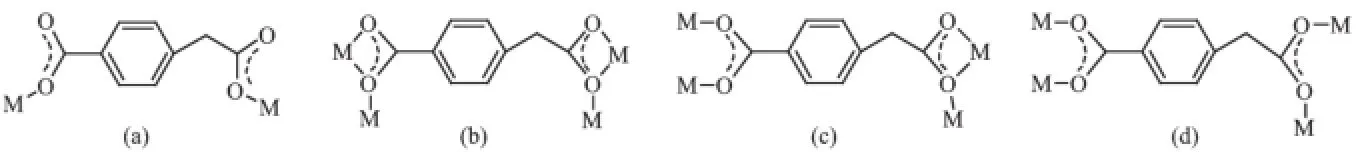
Scheme 1 Coordinationmodes of cbaa2-observed in comp lexes 1~4
Carboxylate ligands are desirable candidates for the assembly processes of metal-organic coordination polymers(MOCPs)due to their excellent coordination capability and versatile bindingmodes under different reaction conditions[1-3].Additionally,the deprotonation of carboxyl groups can compensate for the metal charge,which is necessary to avoid inclusion of unligated anionic species[4-5].As is well known,the carboxylate groups are able to adoptnumerous binding modes such asmonodentate,bidentate,tridentate and tetradentate coordination[6-7].The bidentate coordination modes are themost common ones and can be detailed as chelating,bridging syn-anti inμ2-1,1 mode,synsyn,syn-anti,and anti-anti inμ2-1,3mode[8-11].And the tridentate modes can be illustrated as anti-syn-syn, anti-syn-anti inμ3-1,1,3 mode,and so on[12-13].To satisfy the coordination requirement,the tetradentate coordination mode called as syn-anti-syn-anti inμ4-1,1,3,3 mode and the pentadentate coordination mode may be employed[14].The rigid aromatic carboxylate ligands have been investigated universally due to the fact that their inherent large molecule stiffness can afford the MOCPs with high thermal stability[15-17].And several researches based on flexible aromatic carboxylate ligandshavebeen documented recently[18-21]. On the one hand,using flexible carboxylate ligands can result in“losing control”over the design and assembly of relevant MOCPs because flexible ligands are more sensitive to reaction conditions.Moreover, the rotation about the C-C bonds can lead to changeable conformation and low symmetry of carboxylate ligands.On the other hand,the flexibility of ligandsmay provide rare opportunities to construct some interesting crystalline architectures with particular attributes deriving from so-called breathing ability.Semirigid aromatic dicarboxylate ligands combined with rigid-COOH and flexible-CH2COOH groups possess the merits of both rigid and flexible carboxylate groups,and have caught our attention successfully although they are underdeveloped in the chemistry of MOCPs[22-23].
Several complicated factors such as the nature of metal ions and carboxylate ligands,the concentration of starting species,solvent,temperature,pH value and N-donor co-ligands can influence the coordination modes of the carboxylate groups,in turn deciding the structures,properties and potential applications of resulted MOCPs[24-25].Using N-donor co-ligands to meet or mediate the coordination modes of the carboxylate groups has been probed widely and always been an efficient approach for reconstructing the motifs of MOCPs[26-27].Bis-pyridyl-type ligands are the most common ones among numerous N-donor coligands.And various nitrogen-rich co-ligands including a number of imidazolyl,triazolyl,tetrazolyl,pyrazolyl, benzimidazolyl and so on,which can provide more coordination sides and more chance to produce H-bonds interaction and aromatic interaction,have been involved and played important role in the assembly of MOCPs[28-30].
Herein,a series of four zinc and cadmium coordination polymers originating from Zn(II)/Cd(II)acetate,semirigid 4-carboxybenzeneaceticacid(H2cbaa) and various nitrogen-rich co-ligands have been exemplified.Various coordination modes of cbaa2-observed in complexes 1~4 are shown in Scheme 1a~d.
And the preparations,X-ray structures,fluorescent properties and thermal stabilities of all these compounds are described below.
1 Experimental
1.1 Materials and m ethods
All reagents were commercially purchased and used directly without further purification.Elemental analyses for C,H and N were performed on a Flash EA 2000 elemental analyzer.Infrared spectra(IR) were obtained by a Nicolet 6700 FT-IR spectrophotometer over a range of 4 000~600 cm-1.The powder X-ray diffraction(PXRD)patterns of the products were recorded with a Bruker AXS D8 Advance diffractometer using monochromated Cu Kαadiation(λ= 0.154 18 nm;generator current:40 mA;generator voltage:40 kV;scanning scope:2θ=5°~50o).The thermogravimetric analyses(TGA)were carried out on a SⅡEXStar6000 TG/DTA6300 analyzer with a heating rate of 10℃·min-1under N2atmosphere.Luminescence spectra of the solid samples were obtained at the room temperature on a Hitachi F-4500 spectrophotometerwith xenon arc lamp as the light source.
1.2 Preparation of complexes
The compounds were synthesized by the solvothermal methods using a mixed solvent of absolute methanol(3.50 mL),deionized water(3.50 mL)and NaOH(0.002 0 g,0.05 mmol).Starting materials were placed in a sealed 23 mL PTFE-lined stainless steel autoclave and kept under autogenous pressure at 120℃for 4 days,whereupon it was cooled slowly to the room temperature.The crystals were isolated after being washed with distilled water and acetone,and allowed to dry in air.The PXRD patterns of the bulk products are in well agreement with the simulated patterns based on the structure solutions(Fig.S1~S4).
Synthesis of{[Zn(cbaa)(bpmp)0.5(H2O)]·2H2O}n(1): A mixture of Zn(OAc)2·2H2O(0.044 g,0.20 mmol), H2cbaa(0.018 g,0.10 mmol)and bpmp(0.027 g,0.10 mmol)were dissolved in the mixed solvent,giving colourless blocked crystals afte solvothermal reaction (Yield:83%based on Zn).Anal.Calcd.for C17H21N2O7Zn(%):C,47.40;H,4.91;N,6.50.Found(%):C, 47.33;H,4.56;N,6.47.IR(KBr,cm-1):3 619(w,br), 2 933(w,br),2 815(m),1 618(s),1 578(s),1 525(s), 1 424(s),1 350(s),1 299(m),1 158(m),1 012(m),929 (w),841(m),780(m),727(m),687(w),615(w).
Synthesis of[Zn(cbaa)(bip)]n(2):A mixture of Zn(OAc)2·2H2O(0.022 g,0.10mmol),H2cbaa(0.009 0 g,0.050 mmol)and bip(0.010 g,0.050 mmol)were dissolved in the mixed solvent,giving colourless blocked crystals after solvothermal reaction(Yield: 41%based on Zn).Anal.Calcd.for C20H15N5O4Zn(%): C,52.82;H,3.32;N,15.40.Found(%):C,52.79;H, 3.34;N,15.38.IR(KBr,cm-1):3 124(m),3 023(w), 2 893(w),1 607(s),1 561(s),1 509(s),1 359(s),1 317 (m),1 266(m),1 120(m),1 064(m),1 007(m),946(m), 882(m),823(m),732(m),646(s).
Synthesis of[Cd(cbaa)(Hizb)]n(3):A mixture of Cd(OAc)2·2H2O(0.053 g,0.20mmol),H2cbaa(0.018 g,0.10 mmol)and Hizb(0.026 g,0.10 mmol)were dissolved in the mixed solvent,giving colourless blocked crystals after solvothermal reaction(Yield: 37%based on Cd).Anal.Calcd for C25H18CdN4O4(%): C,54.51;H,3.29;N,10.17.Found(%):C,54.60;H, 3.22;N,10.13.IR(KBr,cm-1):3 415(w,br),3 191(w, br),1 599(m),1 563(m),1 508(s),1 391(s),1 313(m), 1 277(w),1 064(w),843(w),747(m),679(w).
Synthesisof[Cd2(cbaa)2(itmb)(H2O)]n(4):Amixture of Cd(OAc)2·2H2O(0.053 g,0.20 mmol),H2cbaa (0.018 g,0.10 mmol)and itmb(0.045 g,0.20 mmol) were dissolved in themixed solvent,giving colourless blocked crystals after solvothermal reaction(Yield: 64%based on Cd).Anal.Calcd for C30H25Cd2N5O9(%): C,43.71;H,3.06;N,8.50.Found(%):C,43.67;H, 3.11;N,8.48.IR(KBr,cm-1):3 292(w,br),3 135(m), 1 607(s),1 549(s),1 523(s),1 384(s),1 276(m),1 140 (m),1 059(w),1 018(w),931(w),835(w),783(m),739 (s),659(s).
1.3 X-ray crystallography
Single-crystal X-ray diffraction data for complexes 1~4 were recorded at room temperature on a Bruker SMARTAPEXⅡCCD diffractometerequipped with graphite-monochromated Mo Kαradiation(λ= 0.071 073 nm).All the structures were solved by the
direct method followed by successive difference Fourier syntheses and refined by full-matrix leastsquares techniques using the SHELX-97 program package with anisotropic thermal parameters for all non-hydrogen atoms[31-32].Hydrogen atoms of the organic molecules were placed in calculated positions and refined isotropically with a riding model.The hydrogen atoms associated with the H2O molecule were initially located in a difference Fouriermap and included in the final refinement by use of geometrical restraints with the O-H distances being fixed at 0.085 nm and Uiso(H)equivalent to 1.5 times of Ueq(O).The details of the structure solution and final refinements are listed in Table 1.
CCDC:906895,1;1443786,2;906897,3;906898, 4.
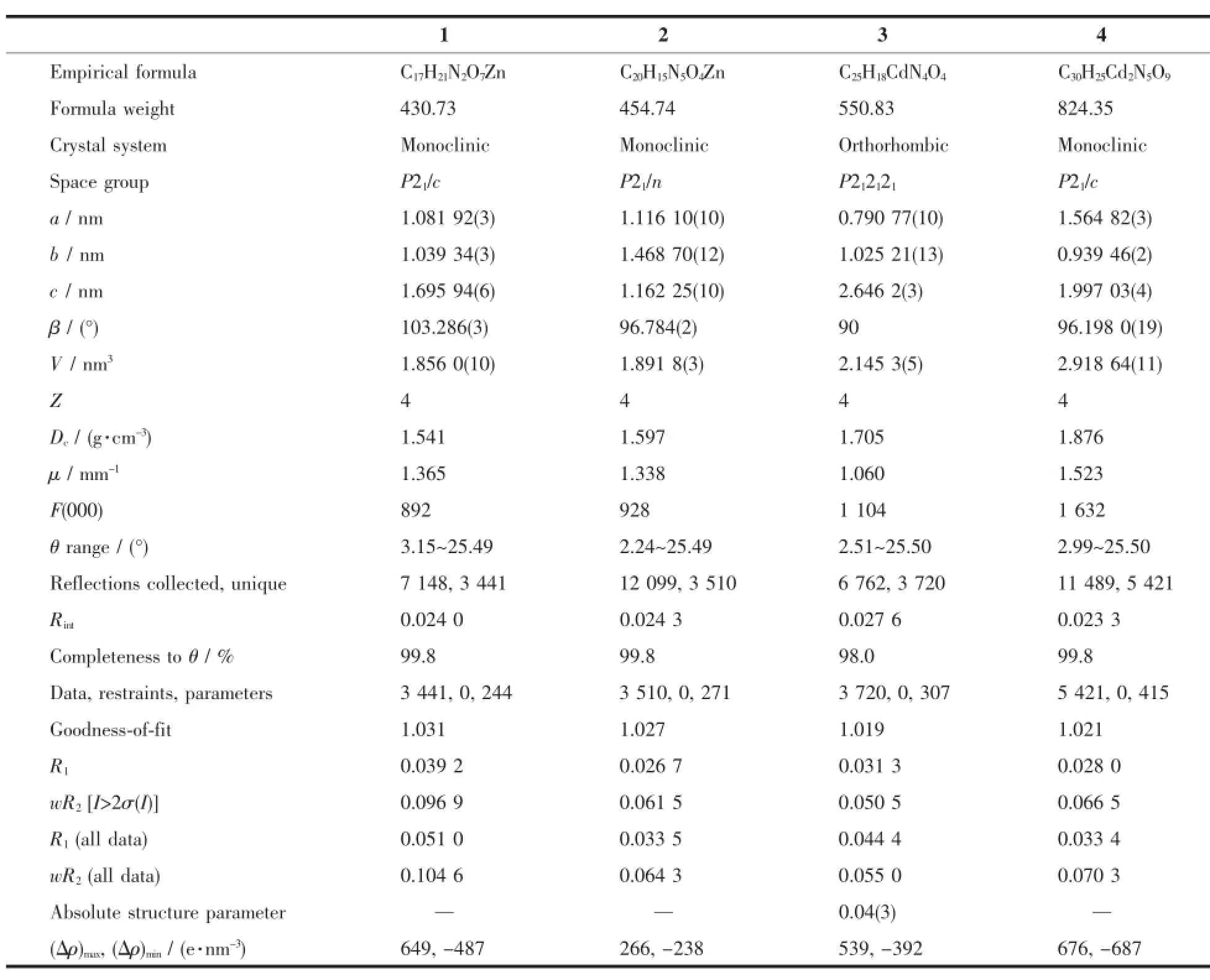
Table 1 Crystal and structure refinement data for 1~4
2 Results and discussion
2.1 Structural description of{[Zn(cbaa)(bpmp)0.5(H2O)]·2H2O}n(1)
The asymmetric unit of complex 1 comprises one crystallographically distinct Zn(II)cation,one cbaa2-, half bpmp molecule,one coordinating H2O molecule and two free H2O molecules(Fig.1a).The Zn1 atom forms a slightly distorted tetrahedron[ZnNO2(H2O)]by one-COO-oxygen atom and one-CH2COO-oxygen atom from two symmetry-related cbaa2-,one pyridyl nitrogen atom of bpmp ligand and one O atom of coordinated water.The Zn-O bond lengths range from 0.196 4(2)to 0.204 7(2)nm,and the Zn-N bond length is 0.204 1(2)nm.The isolated[ZnNO2(H2O)]tetrahedras are spaced by cbaa2-ligand to produce Zncarboxylate chains,and the neighbor chains are linked by bpmp co-ligands to generate a ladderlike
coordination polymer propagating along the b direction (Fig.1b).Each stile of the ladder is comprised of [ZnNO2(H2O)]tetrahedra connected byμ2-cbaa2-ligand with a bismonodentate mode for both carboxyalte groups.Theμ2-bpmp ligand acts as the rung of the ladder joining to Zn(II)centers of the stiles through terminal pyridyl nitrogen atoms.
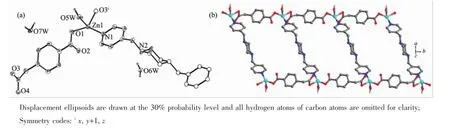
Fig.1(a)View of coordination environment of Zn(II)in 1;(b)Ladderlike coordination polymer along the b direction
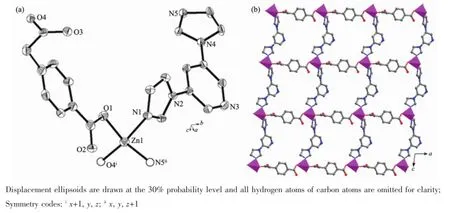
Fig.2(a)Coordinated environmentof Zn(II)in 2;(b)Monolayer structure featuring[ZnN2O2]units spaced by carboxylate and bip molecules
The parall ladders are closed packing such that each ladder is surrounded by six neighbours and cohered together by H-bonding interactions between the coordinated water and lattice water,leading to no solvent-accessible volume for the supramolecular structure(Fig.S5).
2.2 Structural description of[Zn(cbaa)(bip)]n(2)
The asymmetric unit of complex 2 consists of one Zn(II)cation,one cbaa2-and one bip molecule,as shown in Fig.2a.The unique Zn atom forms a slightly distorted tetrahedron[ZnN2O2]by one-COO-oxygen atom and one-CH2COO-oxygen atom from two symmetry-related cbaa2-,and two N atoms from two symmetry-related bip ligands.The Cd-O distances are 0.194 03(16)and 0.195 57(16)nm while the Cd-N distances are 0.201 18(16)and 0.203 78(16)nm.The [ZnN2O2]units are connected byμ2-cbaa2-ligandswith both carboxylate groups in monodentatemode to form a metal-carboxylate chain along the a direction and futher linked by the bipmolecules along the c diretion to generate a square(4,4)grid monolayer(Fig.2b). The neighboring layers are cohered to produce a layer-pair by strongπ-πstacking interactions derived from the pyridine rings of bip molecules with the centroid-centroid distances of 0.407 10(2)nm.And the layer-pairs are further stacked to form the ultimate 3D framework by relatively weak van der Waals interactions(Fig.S6).
2.3 Structural description of[Cd(cbaa)(Hizb)]n(3)
The asymmetric unit of complex 3 consists of one Cd(II)cation,one cbaa2-and one Hizb molecule,as shown in Fig.3a.The unique Cd atom displays a [CdNO6]heptacoordinated geometry with the coordination sphere defined by three-COO-oxygen atoms, three-CH2COO-oxygen atoms and one imidazolyl N atom.The Cd-O distances range from 0.223 6(3)to 0.271 47(31)nm,and the Cd-N distance is 0.225 1(3) nm.
The Cd1 cations are doubly bridged with each other by twoμ2-O bridges from carboxylate groups to produce 1D motifs constructed by edge-shared [CdNO6]polyhedra(Fig.3b).And the 1D motifs are interlinked byμ2-cbaa2-ligands to produce a monolayer structure in the ab plane with the Hizb ligands as pendentarms(Fig.3c and 3d),wherein both carboxylates of each cbaa2-adopt a tridentate coordination mode.There are only intralayer H-bonds between benzimidazolyl N atoms and carboxylate O atoms,and the final 3D supramolecular structure is only stabilized by weak van derWaals forces between the layers.
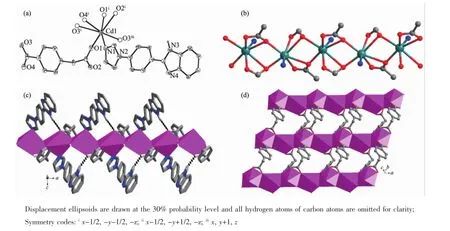
Fig.3(a)Coordinated environmentof Cd(II)in 3;(b)Cd-carboxylate chain featuring edge-shared[CdNO6]polyhedra; (c)Monolayer structure with Hizb pendent arms;the intralayer H-bonding interactions are labeled as dark dotted lines;(d)Cd-carboxylate sheetmotif showing coordinated details of cbaa2-ligand
2.4 Structural description of[Cd2(cbaa)2(itmb) (H2O)]n(4)
The asymmetric unit of complex 4 consists of two Cd(II)cations,two cbaa2-,one itmb molecule and one guest water molecule,as shown in Fig.4a.Both Cd atoms display octahedrally coordinated geometries. The coordination sphere of Cd1 is formed by two -COO-oxygen atoms,two-CH2COO-oxygen atoms, one water O atom and one terminal N atom of one itmb while the coordination sphere of Cd2 is defined by two-COO-oxygen atom,three-CH2COO-oxygen atoms and one termial N atom of one itmb ligand.The Cd-O distances range from 0.221 9(2)to 0.251 5(2)nm, and the Cd-N distances are 0.232 8(3)and 0.234 7(3) nm,respectively.
Two Cd octahedra are triply bridged by the combination of one-CH2COO-oxygen atom and two -COO-carboxylate groups in bridging bidentatemode to form one[Cd2O(OCO)2]dinuclear unit with the Cd…Cd seperation of 0.367 18(3)nm.The adjacent dinuclear units are further connected by one bridging bidentate-CH2COO-carboxylate group to generate a single-stranded Cd-carboxylate chain along the b direction(Fig.4b).These Cd-carboxylate chains are
extended byμ2-cbaa2-ligands with two carboxylate groups in bridging bidentate and bridging tridentate coordination mode to produce one Cd-carboxylate sheetmotif(Fig.4c).And the neighbor sheetmotifs are connected through anotherμ2-cbaa2-ligands with both carboxylate groups in bridging bidentate mode to generate one open Cd-carboxylate bilayer with linear channels paralleling to the chain motifs(Fig.S7). Furthermore,each channel is filled with two stacking of itmb ligands acting simultaneously as the linkers between the chain motifs,thus leading to final dense bilayer(Fig.4d).Meanwile,there are interlayer H-bonds between coordinated H2Omolecules and carboxylate O atoms to stabilize its entire 3D supramolecular structure(Fig.S8).
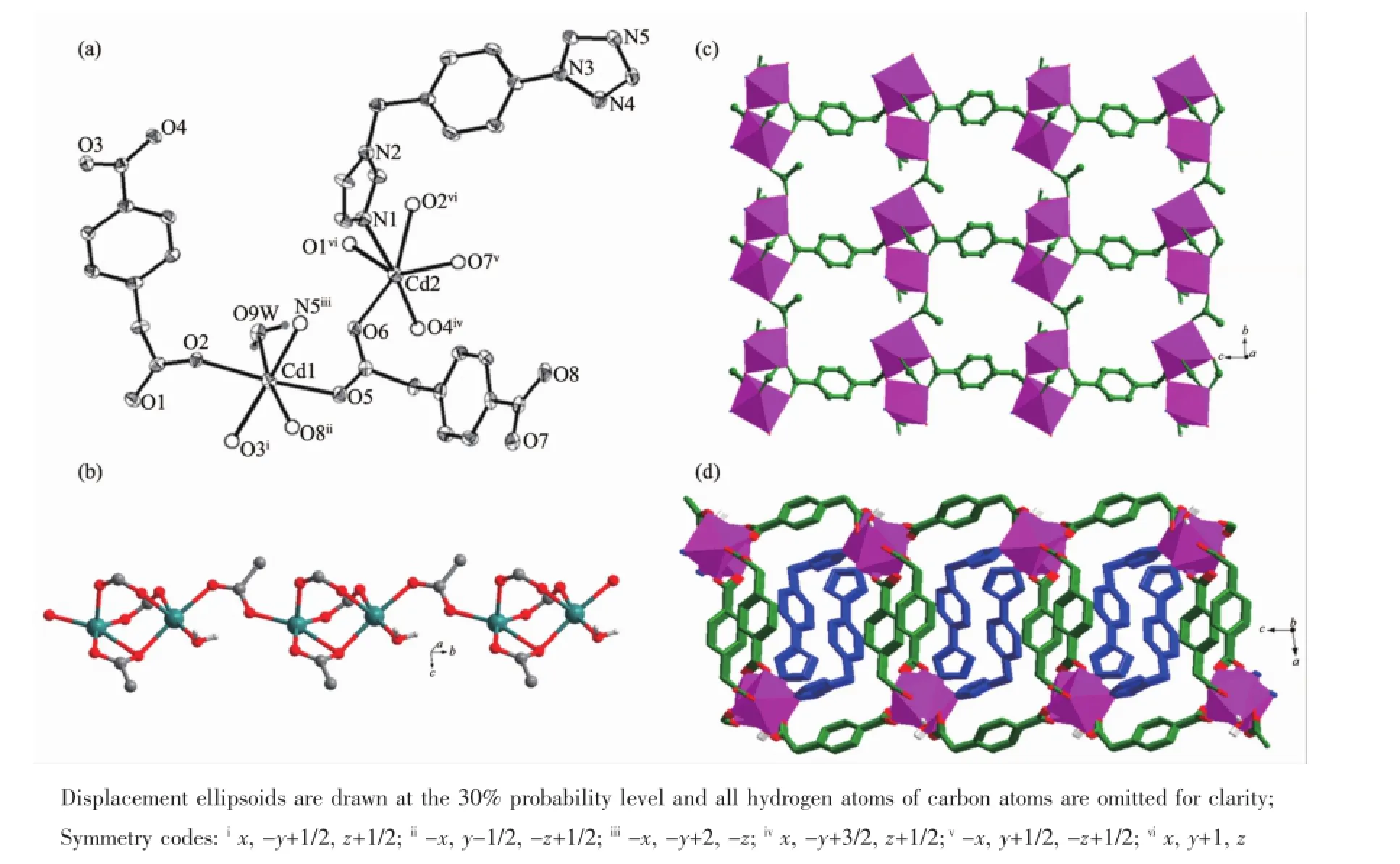
Fig.4(a)Coordinated environment of Cd(II)in 4;(b)Single-stranded chain featuring{Cd2O(OCO)2}dinuclear unit spaced by bridging bidentate-CH2COO-carboxylate groups;(c)Cd-carboxylate sheetmotif showing coordinated details of cbaa2-ligands;(d)Dense bilayer structure featuring open Cd-carboxylate bilayerwith each channel filled with two stacking of itmb ligands acting simultaneously as the linkers between the chainmotifs
2.5 TGA and fluorescent properties
Thermogravimetric analysis(TGA)for complexes 1~4 were carried outon crystalline samples from room temperature to 900℃to investigate their thermal stabilities,as shown in Fig.5.For complex 1,the release of two guest watermolecules was observed in the range from 61 to 182℃with two obviousprocesses (Calcd.8.36%,Found 7.84%).And the succeeding weight loss owing to the removal of one coordinated water and decomposition of organic ligands takes place between 280 and 860℃.The final residue holding a weight of 18.67%of the total sample is attributed to ZnO phase(Calcd.18.89%).Complex 2 can survive below 310℃and then a series of consecutive weight losses are observed and do not stop until the heating end.Similar to complex 2,complex 3 can survive below 350℃and then decomposes gradually until the heating end.The final residue is attributed to CdO component(Calcd.23.31%,Found 24.02%).Complex 4 undergoes the firstweight loss of 2.03%in the temperature range of 110~155℃, which could be assigned to the release of one coordinated watermolecule(Calcd.2.18%).And the full decomposition of organic ligands appears at the temperature of 155~535℃.The remnant holding a
weight of 30.17%of the total sample is CdO phase (Calcd.31.15%).
The fluorescent properties of complexes 1~4 as well as the related ligands were investigated in the solid state at ambient temperature,as depicted in Fig. 6.The free H2cbaa ligand has the emission with a maximum at 445 nm(λex=353 nm).And the free nitrogen-rich co-ligands display featureless emissions with themaximum at 418 nm(λex=290 nm)for bip, 466 nm(λex=384 nm)for Hizb and 416 nm(λex=350 nm)for itmp while the bpmp molecule shows weak fluorescent emission in the high-energy region and makes almost no contribution to the emission of the complex 1[33].Furthermore,there are multifarious emission behaviorswith themaximum at 468 nm(λex= 300 nm)for 1,425 nm(λex=349 nm)for 2,417 and 452nm(λex=340 nm)for 3,and 393 nm(λex=300 nm) for 4,respectively.They can probably be assigned to the ligand localized emission but neither metal-toligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT)in nature for the Zn2+and Cd2+with d10configuration are difficult to oxidize or reduce[34-35].Crystalsof1,2 and 4 show emission bands with spectrum features similar to that of the powdered H2cbaa ligand.In comparison with the free H2cbaa precursor,red shifts of emission bands for 1 and blue shifts of emission bands for 2 and 4 have been observed,which may be attributed to the deprotonated effect of H2cbaa and the coordination interactions of the cbaa2-ligands around the centralmetal ions[36-37]. In addition,complex 3 displays fluorescence emission with bimodal bands which may be related to the cooperative actions of cbaa2-ligands and Hizb ligands.
3 Conclusions
In summary,the reactions of Zn(II)/Cd(II)acetate with semirigid 4-carboxybenzeneacetate and different nitrogen-rich co-ligands bymild solvothermalmethods generate four new coordination polymers.Complex 1 and 2 possess 1D ladder and 2D monolayer structure featuring Zn-carboxylate chains linked by different nitrogen-rich co-ligands,respectively.Complex 3 exhibit 2D monolayer containing Cd-O inorganic chains cross-linked further by cbaa carboxylates with Hizb co-ligands acting as pendentarms,while complex 4 is 2D dense bilayer containing Cd-carboxylate open bilayer with linear channels filled with itmb coligands.Moreover,the solid state luminescences of all compounds are attributed to the ligand localized emission.
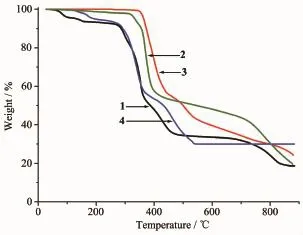
Fig.5 TGA curves of complexes 1~4
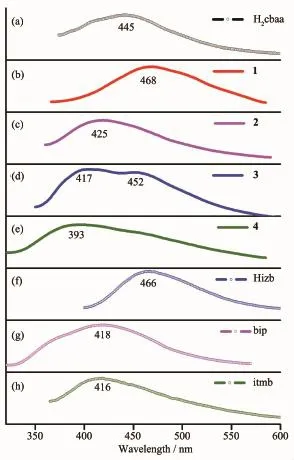
Fig.6 Solid-state emission spectra of complexes 1~4 and the related ligands at ambient temperature
Supporting information isavailable athttp://www.wjhxxb.cn
[1]Yang L B,Wang H C,Fang X D,et al.CrystEngComm, 2016,18:130-142
[2]Zeng M H,Wang Q X,Tan Y X,et al.J.Am.Chem.Soc., 2010,132:2561-2563
[3]Li G L,Liu G Z,Ma L F,et al.Chem.Commun.,2014,50: 2615-2617
[4]Du M,Jiang X J,Zhao X J.Inorg.Chem.,2007,46:3984-3995
[5]Liu G Z,Li SH,Wang L Y.CrystEngComm,2012,14:880-889
[6]Ghosh A K,Shatruk M,Bertolasi V,et al.Inorg.Chem., 2013,52:13894-13903
[7]Zhang JP,Huang X C,Chen X M.Chem.Soc.Rev.,2009, 38:2385-2396
[8]Lama P,Aijaz A,Neogi S,et al.Cryst.Growth Des.,2010, 10:3410-3417
[9]Li X L,Liu G Z,Xin L Y,et al.Synth.React.Inorg.Met.-Org.Chem.,2015,45:914-920
[10]Li G L,Yin W D,Li X L,et al.Synth.React.Inorg.Met.-Org.Chem.,2015,45:869-874
[11]Ghosh S K,Ribas J,Bharadwaj P K.Cryst.Growth Des., 2005,5:623-629
[12]Su Z,Fan J,Chen M,et al.Cryst.Growth Des.,2011,11: 1159-1169
[13]Dietzel P D C,Johnsen R E,Blom R,et al.Chem.Eur.J., 2008,14:2389-2397
[14]Xie F T,Bie H Y,Duan L M,et al.J.Solid State Chem., 2005,178:2858-2866
[15]Liu G Z,Li S H,Li X L,et al.CrystEngComm,2013,15: 4571-4580
[16]Xin L Y,Li X L,Liu G Z.Synth.React.Inorg.Met.-Org. Chem.,2013,43:1013-1018
[17]YINWei-Dong(尹卫东),LIGui-Lian(李桂连),LIU Guang-Zhen(刘广臻),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(7):1439-1446
[18]Liu TF,LüJ,Cao R.CrystEngComm,2010,12:660-670
[19]Pigge FC.CrystEngComm,2011,13:1733-1748
[20]Zhou Y F,Wang R H,Wu B L,et al.J.Mol.Struct.,2004, 697:73-79
[21]Xin L Y,Liu G Z,Ma L F,et al.Aust.J.Chem.,2015,68: 758-765
[22]Liu G Z,Li X D,Li X L,et al.CrystEngComm,2013,15: 2428-2437
[23]Tian Z F,Su Y,Lin JG,et al.Polyhedron,2007,26:2829-2836
[24]Long L S.CrystEngComm,2010,12:1354-1365
[25]Ma L F,Liu B,Wang L Y,et al.Dalton Trans.,2010,39: 2301-2308
[26]Li C P,Chen J,Yu Q,et al.Cryst.Growth Des.,2010,10: 1623-1632
[27]Jia W W,Luo JH,Zhu M L.Cryst.Growth Des.,2011,11: 2386-2397
[28]Xue L P,Chang X H,Li SH,et al.Dalton Trans.,2014,43: 7219-7226
[29]Xu JK,Sun X C,Yang L R,et al.Z.Anorg.Allg.Chem., 2014,640:236-242
[30]YINWei-Dong(尹卫东),LIGui-Lian(李桂连),LIXiao-Ling (李晓玲),et al.Chinese J.Inorg.Chem.(无机化学学报), 2016,32(4):662-668
[31]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[32]Sheldrick G M.SHELXL-97,Program for Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[33]Sahu J,Ahmad M,Bharadwaj P K.Cryst.Growth Des., 2013,13:2618-2627
[34]Zhang L P,Ma JF,Yang J,et al.Inorg.Chem.,2010,49: 1535-1550
[35]QIAO Yu(乔宇),MABo-Nan(马博男),LIXiu-Ying(李秀颖), et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(6): 1245-1251
[36]Chang X H,Zhao Y,Feng X,et al.Polyhedron,2014,83: 159-166
[37]Li F F,Ma J F,Song S Y,et al.Inorg.Chem.,2005,44: 9374-9383
Zinc(II)and Cadm ium(II)Coordination Polymersw ith Various Polynuclears Spaced by Sem irigid 4-Carboxybenzeneacetate and Nitrogen-Rich Co-ligands:Syntheses,Structures and Properties
JU Feng-Yang1LIYun-Ping2LIGui-Lian2LIUGuang-Zhen*,2XIN Ling-Yun2LIXiao-Ling2
(1School of Food and Drug,Luoyang Normal University,Luoyang,Henan 471934,China)
(2College of Chemistry and Chemical Engineering,Luoyang Normal University,Luoyang,Henan 471934,China)
A series of four Zn(II)/Cd(II))coordination polymers,{[Zn(cbaa)(bpmp)0.5(H2O)]·2H2O}n(1),[Zn(cbaa) (bip)]n(2),[Cd(cbaa)(Hizb)]n(3)and[Cd2(cbaa)2(itmb)(H2O)]n(4)have been obtained by the solvothermal reaction of Zn(II)/Cd(II)acetate with semirigid 4-carboxybenzeneacetic acid(H2cbaa)and co-ligands,1,4-bis(4-pyridylmethy)piperazine(bpmp),3,5-bis(1-imidazoly)pyridine(bip),2-(4-imidazol-l-yl-phenyl)-1H-benzimidazole(Hizb) and 1-(imidazo-1-ly)-4-(1,2,4-trazol-1-ylmethyl)benzene(itmb).The single-crystal X-ray diffraction analyses show that four compounds feature various polynuclears spaced by 4-carboxybenzeneacetate and N-rich co-ligands and display various topology structures.Two zinc complexes are 1D ladder featuring Zn-carboxylate chains linked further by bpmp co-ligands for 1 and 2D monolayer containing Zn-carboxylate chains cross-linked further by bip co-ligands for 2,respectively.And two cadmium complexes exhibit2Dmonolayer featuring Cd-O inorganic chains cross-linked further by cbaa carboxylateswith Hizb co-ligands acting as pendent arms for 3 and 2D dense bilayer
国家自然科学基金(No.21571093)、河南省高校科技创新人才项目(No.14HASTIT017)、河南省高校科技创新团队项目(No.14IRTSTHN008)和河南省科技攻关计划(No.162102210304)资助。
*通信联系人。E-mail:gzliuly@126.com
4-carboxybenzeneacetic acid;zinc;cadmium;coordination polymers;fluorescent properties
2016-06-30。收修改稿日期:2016-09-14。
O0614.24+1;O614.24+2
A
1001-4861(2016)10-1876-09
10.11862/CJIC.2016.234
containing Cd-carboxylate open bilayer with itmb co-ligands encapsulated within the cavities for 4,respectively. The fluorescent properties and thermal stabilities of all these compounds have been investigated.CCDC:906895, 1;1443786,2;906897,3;906898,4.