外源H2O2对混合盐碱胁迫下燕麦幼苗叶片脯氨酸积累和代谢途径的影响
刘建新 王金成 刘秀丽 王风琴
(1.陇东学院生命科学与技术学院,庆阳 745000; 2.甘肃省高校陇东生物资源保护与利用省级重点实验室,庆阳 745000)
外源H2O2对混合盐碱胁迫下燕麦幼苗叶片脯氨酸积累和代谢途径的影响
刘建新 王金成 刘秀丽 王风琴
(1.陇东学院生命科学与技术学院,庆阳 745000;2.甘肃省高校陇东生物资源保护与利用省级重点实验室,庆阳 745000)
为探讨H2O2对盐碱胁迫下植物脯氨酸代谢的调控机理,以燕麦新品种‘定莜6号’幼苗为材料,采用水培法研究了外源H2O2对混合盐碱胁迫下燕麦脯氨酸积累和代谢途径的影响。结果表明,75 mmol·L-1混合盐碱(NaCl∶Na2SO4∶NaHCO3∶Na2CO3=12∶8∶9∶1)胁迫可促进燕麦幼苗叶片脯氨酸的积累,提高脯氨酸合成的鸟氨酸途径关键酶鸟氨酸δ-氨基转移酶(δ-OAT)活性,抑制脯氨酸合成的谷氨酸途径关键酶Δ1-吡咯啉-5-羧酸合成酶(P5CS)及脯氨酸降解限速酶脯氨酸脱氢酶(ProDH)活性。在75 mmol·L-1混合盐碱胁迫下添加0.01~1 000 μmol·L-1H2O2可显著提高燕麦幼苗叶片的脯氨酸含量,其中10 μmol·L-1H2O2的作用最明显;10 μmol·L-1H2O2上调了75 mmol·L-1混合盐碱胁迫下燕麦幼苗叶片的P5CS和δ-OAT活性,降低了ProDH活性。此外,10 μmol·L-1H2O2使75 mmol·L-1混合盐碱胁迫下燕麦幼苗叶片内源性H2O2含量急剧升高后迅速降低。表明外源H2O2能够提高混合盐碱胁迫下燕麦幼苗内源H2O2的含量,并通过活化脯氨酸合成的谷氨酸途径和鸟氨酸途径,抑制脯氨酸的降解,促进混合盐碱胁迫下燕麦幼苗脯氨酸的积累。
燕麦;混合盐碱胁迫;过氧化氢;脯氨酸代谢
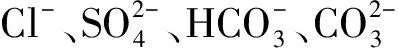
过氧化氢(hydrogen peroxide,H2O2)是植物细胞代谢过程中产生的一种活性氧(reactive oxygen species,ROS),高浓度时可损伤生物大分子,而低浓度的H2O2是一种信号分子,参与逆境胁迫应答[7],提高植物的耐盐性[8]。有研究表明,外源H2O2预处理可提高胁迫蛋白的表达减轻盐胁迫对小麦幼苗的氧化伤害[9],诱导抗氧化防御机制产生提高水稻的耐盐性[10]。盐碱胁迫导致植物水分亏缺和碳氮代谢失调,碳氮代谢转向渗透溶质积累以适应环境胁迫[11]。脯氨酸(proline,Pro)是其中一种重要的渗透调节物质,并具有清除ROS,减轻环境胁迫对植物伤害等作用[12]。植物体内Pro的合成有两条途径:一是谷氨酸(glutanate,Glu)途径,Δ1-吡咯啉-5-羧酸合成酶(Δ1-pyrroline-5-carboxylate synthetase,P5CS)是关键酶;二是鸟氨酸(ornithine,Orn)途径,Orn-δ-氨基转移酶(ornithine-δ-aminotransferase,δ-OAT)是限速酶;Pro降解的关键酶是脯氨酸脱氢酶(proline dehydrogenase,ProDH)。其中P5CS定位在细胞质中,而δ-OAT和ProDH存在于线粒体中[13]。混合盐碱胁迫可诱导青山杨(Populuspseudo-cathayana×P.deltoides)Pro的积累[1];外源H2O2能够通过激活白刺(NitrariatangutorumBobr)Glu激酶活性和降低ProDH活性促进Pro积累[14];并通过提高可溶性糖和谷胱甘肽含量增强小麦(Triticumaestivum)幼苗对NaCl胁迫的抗性[15]。然而,外源H2O2对混合盐碱胁迫下植物Pro积累及其代谢途径影响的研究尚未见报道。
燕麦(Avenanuda)是一种喜阴凉、耐盐碱的粮饲兼用型作物,其籽粒有独特的营养和保健功效[16],被称为盐碱地改良的先锋作物,在我国内蒙古、河北、山西、甘肃等省区广泛种植,年种植面积达55万hm2[17]。‘定莜6号’燕麦是甘肃省定西市旱作农业科研推广中心选育的燕麦新品种,具有抗旱、丰产和品质优等特点。‘定莜6号’幼苗对盐胁迫的响应及H2O2对响应的调节已有研究报道[18],为进一步探讨H2O2对其混合盐碱胁迫下Pro代谢的调节机制,根据甘肃省燕麦栽培地土壤盐分的组成,将两种中性盐NaCl、Na2SO4和两种碱性盐NaHCO3、Na2CO3按不同摩尔质量比例混合,研究外源H2O2对混合盐碱胁迫下‘定莜6号’幼苗Pro积累和代谢途径的影响,以期为进一步阐明燕麦耐盐碱机理提供理论依据。
1 材料与方法
1.1 材料来源和混合盐碱溶液配比

1.2 材料培养和实验设计
燕麦种子用1% CuSO4溶液表面消毒20 min,浸种6 h后播种在垫有3层湿润吸水纸的瓷盘中,置培养箱中25℃催芽3 d,挑选发芽一致的萌发种子播种在塑料盆(上口径20 cm,高14 cm)的珍珠岩中,浇水后置日光温室培养,昼/夜温度(22~33)℃/(15~19)℃,相对湿度65%~75%,光照强度485~690 μmol·m-2·s-1。幼苗长至2叶1心时选生长一致的壮苗,经自来水和蒸馏水冲洗后,移栽至装有5 L Hoagland完全营养液的水培池中培养,每池定植240株左右,营养液每2 d更换1次。培养7 d后进行如下处理:(1)Hoagland营养液,对照(CK);(2)含75 mmol·L-1混合盐碱的Hoagland溶液,CSAS(complex saline-alkali stress);(3)含75 mmol·L-1混合盐碱和10 μmol·L-1H2O2的Hoagland溶液,CSAS+ H2O2;(4)含10 μmol·L-1H2O2的Hoagland营养液,H2O2。对2叶1心期的燕麦幼苗用不同浓度的混合盐碱处理的预备实验中发现,75 mmol·L-1浓度处理下植株生物量显著下降,且叶色暗绿,株高明显降低,但植株能够承受胁迫而不会导致死亡。因此,选用75 mmol·L-1作为混合盐碱胁迫的浓度。实验处理过程中每天更换1次溶液。
1.3 测定指标和方法
1.3.1H2O2含量
H2O2含量测定采用Sergiev[19]的方法略有改动:称取0.50 g叶片,用预冷的5 mL 0.001 g·L-1三氯乙酸研磨,以每分钟15 000转离心20 min,取0.7 mL上清液加0.7 mL 10 mmol·L-1磷酸缓冲液(pH7)和1.4 mL 1 mol·L-1的KI,测定390 nm波长的吸光值,由标准曲线计算出单位鲜重材料所含H2O2的含量。
1.3.2 Pro含量
采用李合生[20]的磺基水杨酸-酸性茚三酮显色法测定波长520 nm吸光值,以Pro梯度溶液做标准曲线计算Pro含量,结果以单位鲜重材料所含Pro的μg数表示。
1.3.3 P5CS、δ-OAT和ProDH活性
P5CS、δ-OAT和ProDH活性均按赵贵林等[21]的方法测定。以每小时ΔOD535变化0.01表示一个P5CS活性单位(U);以每小时生成1 mmol P5C的量为一个δ-OAT活性单位(U);以分钟ΔOD600减少0.001为一个ProDH活性单位(U)。所有酶活性以每g鲜重材料所含的活性单位数表示。
1.4 统计分析
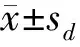
2 结果与分析
2.1H2O2实验浓度的筛选
为确定混合盐碱胁迫下对Pro积累影响的适宜H2O2处理浓度,在75 mmol·L-1混合盐碱胁迫下分别添加0、0.01、0.1、1、10、100和1 000 μmol·L-1的H2O2溶液处理燕麦幼苗6 d,并对幼苗叶片Pro含量进行分析。结果如图1所示,随着H2O2浓度的递增,混合盐碱胁迫下燕麦幼苗叶片内的Pro含量显著提升。当H2O2浓度为10 μmol·L-1时Pro含量出现最大值,是未经H2O2处理的7.1倍(P<0.05)。尔后,随H2O2浓度增加,Pro含量不断降低,但仍高于未经H2O2处理的幼苗。此外,不同浓度H2O2对未经混合盐碱胁迫的燕麦幼苗叶片中Pro含量的影响与混合盐碱胁迫下的变化趋势类似,只是Pro含量明显偏低。因此,选用10 μmol·L-1H2O2作为混合盐碱胁迫下对Pro代谢影响的实验浓度。

图1 不同浓度H2O2对75 mmol·L-1混合盐碱胁迫下燕麦幼苗叶片脯氨酸含量的影响Fig.1 Effect of different concentration of H2O2 on proline contents in leaves of oat seedlings under complex saline-alkali stress
2.2外源H2O2对混合盐碱胁迫下燕麦叶片Pro和H2O2含量的影响
未经混合盐碱胁迫的燕麦幼苗叶片Pro含量在处理期间无明显变化(P>0.05);75 mmol·L-1混合盐碱胁迫显著提高了燕麦叶片的Pro含量,并随处理时间延长呈逐渐上升趋势,胁迫6 d时的Pro含量比CK提高了5.0倍;添加10 μmol·L-1H2O2不仅能提高正常生长燕麦幼苗叶片的Pro含量,更能显著增加混合盐碱胁迫下燕麦叶片的Pro含量(图2)。表明外源H2O2可促进混合盐碱胁迫下燕麦幼苗Pro的积累。
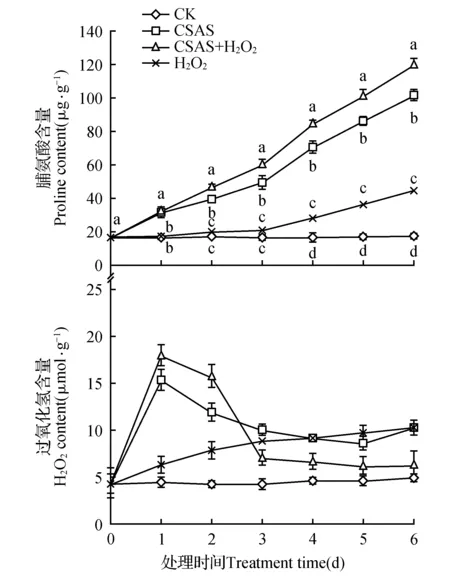
图2 10 μmol·L-1 H2O2对75 mmol·L-1混合盐碱胁迫下燕麦幼苗叶片脯氨酸和内源H2O2含量的影响CK.对照;CSAS.混合盐碱胁迫;CSAS+H2O2.混合盐碱胁迫+过氧化氢;H2O2.过氧化氢 图中不同字母表示同一时间不同处理在5%水平差异显著,下同。Fig.2 Effect of 10 μmol·L-1 H2O2 on contents of proline and endogenous H2O2 in leaves of oat seedlings under 75 mmol·L-1 complex saline-alkali stress CK.Control; CSAS.Complex saline-alkali stress; CSAS+H2O2.Complex saline-alkali stress+Hydrogen peroxide; H2O2.Hydrogen peroxide Bars superscripted with different letters are significantly different at 0.05 levels for the same time,the same as below.
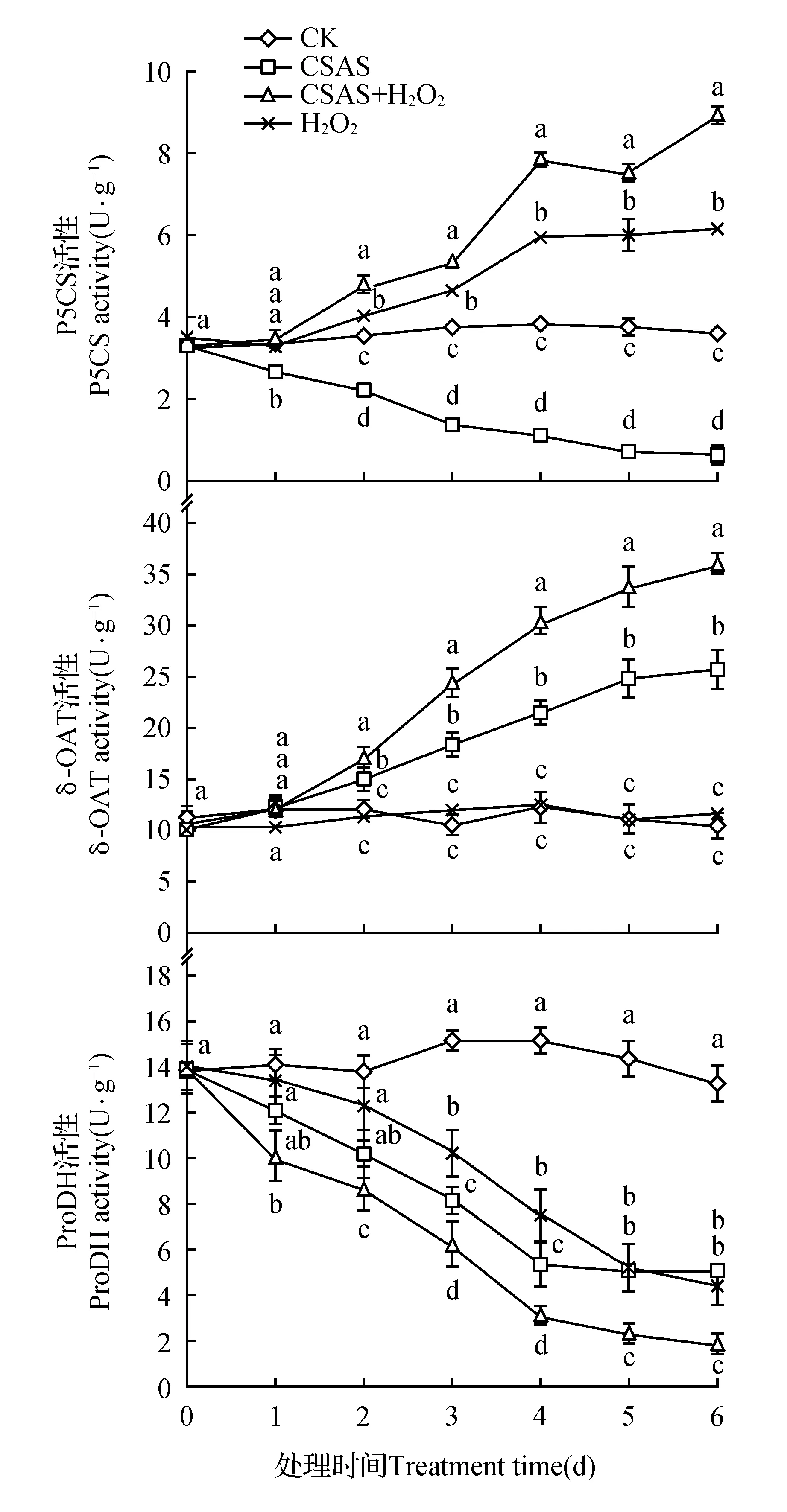
图3 10 μmol·L-1 H2O2对75 mmol·L-1混合盐碱胁迫下燕麦幼苗叶片脯氨酸代谢关键酶活性的影响Fig.3 Effect of exogenous H2O2 on activities of key enzymes of proline biosynthesis in leaves of oat seedlings under complex saline-alkali stress
CK燕麦幼苗叶片H2O2含量在处理期间变化不大(P>0.05);单独H2O2处理使燕麦叶片H2O2含量随处理时间延长不断提高(P<0.05);混合盐碱胁迫和混合盐碱胁迫下添加H2O2处理均使燕麦叶片H2O2含量随处理时间延长呈快速升高之后急剧下降变化,处理1 d时H2O2均出现峰值,且处理第1和2 d的H2O2含量表现为混合盐碱胁迫下添加H2O2处理显著大于单独混合盐碱胁迫处理,但在处理的第3~6 d时H2O2含量变化却相反(图2)。
2.3外源H2O2对混合盐碱胁迫下燕麦幼苗叶片Pro代谢关键酶活性的影响
P5CS和δ-OAT分别是Pro生物合成的Glu途径和Orn途径关键酶;而ProDH是Pro降解途径的限速酶[13]。如图3所示,与CK相比,混合盐碱胁迫显著抑制了燕麦幼苗叶片P5CS和ProDH活性,却显著增强了δ-OAT活性,受抑和增强的程度随胁迫时间延长而提高;外源H2O2虽未引起正常生长燕麦幼苗叶片在处理期间δ-OAT活性的明显改变(P>0.05),但显著提高了正常生长燕麦幼苗叶片中P5CS的活性及混合盐碱胁迫下燕麦叶片的P5CS和δ-OAT活性,增幅随处理时间延长而提高(P<0.05),胁迫6 d时增幅分别为72.6%、151.1%和39.8%。外施H2O2使正常生长和混合盐碱胁迫燕麦幼苗叶片的ProDH活性随处理时间延长不断下降,胁迫6 d时降幅分别达67.2%%和61.7%。说明外源H2O2促进混合盐碱胁迫下燕麦幼苗Pro的合成,抑制Pro的降解。
3 讨论
Wahid等[2]研究表明,盐胁迫可诱导H2O2产生并积累,进而调节小麦的抗盐反应。本研究显示,75 mmol·L-1混合盐碱胁迫1 d即可引起燕麦幼苗体内H2O2爆发式骤增,然后随胁迫时间延长逐渐下降,说明H2O2可能作为信使分子参与燕麦对混合盐碱胁迫的响应。添加10 μmol·L-1外源H2O2处理进一步提高了混合盐碱胁迫前期(1~3 d)燕麦幼苗叶片的H2O2含量,但胁迫后期(4~6 d)H2O2含量明显下降(图2),这可能是外源H2O2处理提高的内源H2O2作为信号分子刺激了抗氧化系统的应答,增强了抗氧化酶活性和相关基因表达[9],从而降低了混合盐碱胁迫后期燕麦幼苗H2O2的积累。
渗透调节是植物适应盐碱胁迫的重要生理机制,而Pro被认为是最重要的渗透调节物质之一[22]。闫永庆等[1]研究表明,混合盐碱胁迫诱导青山杨叶片Pro积累。外源H2O2处理能够提高小麦幼苗的耐盐性[15]。本研究表明,0.01~1 000 μmol·L-1的外源H2O2能够提高75 mmol·L-1混合盐碱胁迫6 d燕麦幼苗叶片的Pro含量,其中10 μmol·L-1H2O2促进Pro积累的作用最明显(图1),且10 μmol·L-1H2O2处理下燕麦叶片的Pro含量随胁迫时间延长不断增加(图2),结合外源H2O2能够提高混合盐碱胁迫前期(1~3 d)燕麦幼苗叶片H2O2含量的结果,表明外源H2O2处理可能通过提高内源H2O2含量参与盐碱胁迫下燕麦Pro积累的调控。然而,外源H2O2调控盐碱胁迫植物Pro积累的机制目前尚不清楚。本研究表明,混合盐碱胁迫下,燕麦幼苗叶片Pro合成的Glu途径关键酶P5CS和Pro降解限速酶ProDH活性显著下降,而Orn途径关键酶δ-OAT活性明显增强,降幅和增幅均随胁迫时间延长持续提高(图3)。说明混合盐碱胁迫燕麦Pro的积累是以鸟氨酸途径为主的合成增加和降解减少共同作用的结果。这与夏方山等[23]以碱地风毛菊(Saussurearuncinata)在碱性盐胁迫下的结果一致,而与董秋丽等[24]在芨芨草(Achnatherumsplendens)上的研究结果不同。混合盐碱胁迫下燕麦幼苗P5CS活性下降可能是Pro积累后反馈抑制的结果,而δ-OAT活性却不受此影响[13]。进一步研究表明,10 μmol·L-1H2O2可显著提高混合盐碱胁迫下燕麦幼苗P5CS和δ-OAT活性,抑制ProDH活性,并且这种作用程度随胁迫时间延长而增强(图3)。说明外源H2O2通过活化Pro合成的Glu和Orn途径及抑制Pro的降解过程上调了混合盐碱胁迫下燕麦幼苗Pro的积累。H2O2作为信号分子在感知胁迫信号和调控防御系统响应,提高植物抗逆反应中发挥着重要作用[25]。然而,Pro积累是涉及许多因素调控的复杂信号转导过程[26]。H2O2信号调控植物Pro代谢途径的分子机理及与其它信号的交叉对话机制还需进一步深入探究。
4 结论
混合盐碱胁迫下,外源H2O2可诱导燕麦幼苗叶片内H2O2含量急剧增加后快速下降,Pro不断积累;外源H2O2提高混合盐碱胁迫下燕麦幼苗Pro代谢关键酶P5CS、δ-OAT活性和抑制分解代谢限速酶ProDH活性与其促进Pro的积累密切相关。
1.闫永庆,王文杰,朱虹,等.混合盐碱胁迫对青山杨渗透调节物质及活性氧代谢的影响[J].应用生态学报,2009,20(9):2085-2091.
Yan Yongqing,Wang Wenjie,Zhu Hong,et al.Effects of salt-alkali stress on osmoregulation substance and active oxygen metabolism of Qingshan poplar(Populuspseudo-cathayana×P.deltoides)[J].Chinese Journal of Applied Ecology,2009,20(9):2085-2091.
2.Wahid A,Perveen M,Gelani S,et al.Pretreatment of seed with H2O2improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins[J].Journal of Plant Physiology,2007,164(3):283-294.
3.Tanou G,Molassiotis A,Diamantidis G.Hydrogen peroxide and nitric oxide-induced systemic antioxidant primelike activity under NaCl-stress and stress-free conditions incitrus plants[J].Journal of Plant Physiology,2009,166(17):1904 -1913.
4.刘建新,王金成,王鑫,等.外源NO对NaHCO3胁迫下黑麦草幼苗光合生理响应的调节[J].生态学报,2012,32(11):3460-3466.
Liu Jianxin,Wang Jincheng,Wang Xin,et al.Regulation of exogenous nitric oxide on photosynthetic physiological response ofLoliumperenneseedlings under NaHCO3Stress[J].Acta Ecologica Sinica,2012,32(11):3460-3466.
5.颜宏,赵伟,盛艳敏,等.碱胁迫对羊草和向日葵的影响[J].应用生态学报,2005,16(8):1497-1501.
Yan Hong,Zhao Wei,Sheng Yanmin,et al.Effects of alkali-stress onAneurolepidiumchinenseandHelianthusannuus[J].Chinese Journal of Applied Ecology,2005,16(8):1497-1501.
6.Shi D C,Sheng Y M.Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors[J].Environmental and Experimental Botany,2005,54(1):8-21.
7.冯汉青,白晶月,管冬冬,等.胞外H2O2及NADPH氧化酶参与了铜胁迫对植物细胞死亡的诱导[J].植物研究,2015,35(5):710-715.
Feng Hanqing,Bai Jingyue,Guan Dongdong,et al.Extracellular H2O2and NADPH Oxidase are Involved in the Copper-Induced Cell Death[J].Bulletin of Botanical Research,2015,35(5):710-715.
8.Jand-Venes Rolim Medeiros,Enéas Gomes-Filho.Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants[J].Journal of Plant Physiology,2005,162(10):1114-1122.
9.Abdul Wahid,Mubaraka Perveena,Sadia Gelania,et al.Pretreatment of seed with H2O2improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins[J].Journal of Plant Physiology,2005,164(3):283-294.
10.Uchida A,Jagendorf A T,Hibino T,et al.Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice[J].Plant Science,2002,163(3):515-523.
11.Shi D C,Wang D L.Effects of various salt-alkaline mixed stresses onAncurolepidiumchinense(Trin.) Kitag[J].Plant and Soil,2005,271(1-2):15-26.
12.Dinakar N,Nagajyothi P C,Suresh S,et al.Cadmium induced changes on proline,antioxidant enzymes,nitrate and nitrite reductases inArachishypogaeaL.[J].Journal of Environmental Biology,2009,30(2):289-294.
13.Kavi Kishor P B,Sangam S.Regulation of proline biosynthesis,degradation,uptake and transport in higher plants:Its implications in plant growth and abiotic stress tolerance[J].Current Science,2005,88(3):424-438.
14.张园园,吕秀军,张辉,等.外源H2O2处理对唐古特白刺愈伤组织脯氨酸代谢的影响[J].植物研究,2011,31(2):213-217.
Zhang Yuanyuan,LÜ Xiujun,Zhang Hui,et al.Effect of exogenous hydrogen peroxide on the proline metabolism inNitrariatangutorumBobr.Callus[J].Bulletin of Botanical Research,2011,31(2):213-217.
15.张波,张怀刚.外源H2O2对小麦幼苗耐盐性的调节作用[J].西北植物学报,2007,27(12):2491-2495.
Zhang Bo,Zhang Huaigang.Regulation of exogenous hydrogen peroxide on wheat seedling salinity tolerance[J].Acta Botanica Boreali-Occidentalia Sinica,2007,27(12):2491-2495.
16.Drzikova B,Dongowski G,Gebhardt E.Dietary fibre-rich oat-based products affect serum lipids,microbiota,formation of short-chain fatty acids and steroids in rats[J].British Journal of Nutrition,2005,94(6):1012-1025.
17.林叶春,曾昭海,任长忠,等.局部根区灌溉对裸燕麦光合特征曲线及叶绿素荧光特性的影响[J].作物学报,2012,38(6):1062-1070.
Lin Yechun,Zeng Zhaohai,Ren Changzhong,et al.Effects of partial root zone irrigation on leaf photosynthetic curves and chlorophyll fluorescence parameters in naked oat[J].Acta Agronomica Sinica,2012,38(6):1062-1070.
18.刘建新,王金成,王瑞娟,等.燕麦幼苗对盐胁迫的响应及过氧化氢对响应的调节[J].生态学杂志,2014,33(1):89-97.
Liu Jianxin,Wang Jincheng,Wang Ruijuan,et al.Response ofAvenanudaL. seedlings to salt stress and the modulation of hydrogen peroxide[J].Chinese Journal of Ecology,2014,33(1):89-97.
19.Sergiev I,lexieva V,karanov E.Effect of spermine,atrazine andcorabination between them on someendogenous rotective systems and stress markers in plants[J].Comptes rendus de l’Académie bulgare des Sciences,1997,51(2):122-124.
20.李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000.
Li Hesheng.Principles and techniques of plant physiological biochemical experiment [M].Beijing:Higher Education Press,2000.
21.赵贵林,陈强,胡国霞,等.水稻脯氨酸代谢关键酶对水分胁迫的响应[J].干旱地区农业研究,2011,29(3):80-83.
Zhao Guilin,Chen Qiang,Hu Guoxia,et al.Responses of the key enzymes involved in proline metabolism in rice seedling under water stress[J].Agricultural Research in the Arid Areas,2011,29(3):80-83.
22.Szabados L,Savoure A.Proline:a multifunctional amino acid[J].Trends in Plant Science,2010,15(2):89-97.
23.夏方山,董秋丽,董宽虎.碱性盐胁迫对碱地风毛菊苗期脯氨酸代谢途径的影响[J].中国草地学报,2011,33(1):48-53.
Xia Fangshan,Dong Qiuli,Dong Kuanhu.Effect of alkaline salts on proline metabolism ofSaussurearuncinataat seedling stage[J].Chinese Journal of Grassland,2011,33(1):48-53.
24.董秋丽,夏方山,董宽虎.NaCl胁迫对芨芨草苗期脯氨酸代谢的影响[J].草业学报,2010,19(5):71-76.
Dong Qiuli,Xia Fangshan,Dong Kuanhu.Effects of NaCl stress on proline metabolism ofAchnatherumsplendensseedling[J].Acta Prataculturae Sinica,2010,19(5):71-76.
25.Orozco-Cardenas M,Ryan C A.Hydrogen peroxide is generated systemically in plant leaves by wounding and system in via the octadecanoid pathway[J].Proceedings of the National Academy of Sciences of the United States of America,1999,96(11):6553-6557.
26.邓凤飞,杨双龙,龚明.外源ABA对低温胁迫下小桐子幼苗脯氨酸积累及其代谢途径的影响[J].植物生理学报,2015,51(2):221-226.
Deng Fengfei,Yang Shuanglong,Gong Ming.Effect of exogenous abscisic acid on proline accumulation and metabolic pathways inJatrophacurcasseedlings under cold stress[J].Plant Physiology Journal,2015,51(2):221-226.
The project was financially supported by the science and technology plan project of Qingyang city in Gansu province(KZ2014-19)
introduction:LIU Jian-Xin(1964—),male,professor,mainly engaged in Plant stress physiology.
date:2015-12-08
EffectofExogenousH2O2onProlineAccumulationandMetabolicPathwayinLeavesofOatSeedlingsunderComplexSaline-AlkaliStress
LIU Jian-Xin WANG Jin-Cheng LIU Xiu-Li WANG Feng-Qin
(1.College of Life Science and Technology,Longdong University,Qingyang 745000;2.University Provincial Key Laboratory for Protection and Utilization of Longdong Bio-resources in Gansu Province,Qingyang 745000)
Saline-alkali stress interferes with cell metabolism, and inhibits plant growth and development. Proline metabolism is closely related with plant salt-alkali resistance. As signaling molecules, hydrogen peroxide(H2O2) plays vital roles in the regulation of plant cell metabolism, as well as in adaptation to the environmental stress. In order to understand the regulatory mechanism of exogenous H2O2on proline metabolism in oat(Avena nuda) under salinity-alkalinity stresses, the ‘Dingyou No.6’(a new oat cultivar) seedlings with two leaves were used to investigate the effects of exogenous H2O2on proline accumulation in leaves of the seedlings under complex saline-alkali stress by the method of solution culture. The results showed that 75 mmol·L-1complex saline-alkali stress(molar ratio of NaCl∶Na2SO4∶NaHCO3∶Na2CO3=12∶8∶9∶1) led to a significant accumulation of proline in leaves, and induced a rapid increase of activities of the key enzymes ornithine-δ-aminotransferase(δ-OAT) of proline biosynthesis, and a decrease of activities of the key enzymes Δ1-pyrroline-5-carboxylate synthetase(P5CS) of proline biosynthesis, as well as the key enzyme proline dehydrogenase(ProDH) of proline degradation. Moreover, treatments with 0.01-1 000 μmol·L-1, especially, 10 μmol·L-1H2O2could enhance the accumulation of proline in oat seedling leaves under complex saline-alkali stress. H2O2of 10 μmol·L-1also increased the activities of P5CS and δ-OAT, and decreased the activity of ProDH in leaves under complex saline-alkali stress. In addition, 10 μmol·L-1H2O2treatments could rapidly increase the endogenous H2O2levels in oat seedling leaves under complex saline-alkali stress. The exogenous H2O2treatment resulted in the increase of endogenous H2O2content in oat seedlings under complex saline-alkali stress. H2O2induced proline accumulation might be a combined result of the activation of glutamate and ornithine pathways of proline biosynthesis and inhibition of proline degradation pathway.
oat;complex saline-alkali stress;hydrogen peroxide;proline metabolism
甘肃省庆阳市科技计划项目(KZ2014-19)资助
刘建新(1964—),男,教授,主要从事植物逆境生理研究。
2015-12-08
Q945.78
A
10.7525/j.issn.1673-5102.2016.03.017

