早期输注20%脂肪乳剂对大鼠急性胰腺炎的影响*
赵艳梅,菅志远
[湖北省十堰市太和医院(湖北医药学院附属医院)肝胆胰外科诊疗中心,湖北 十堰 442000]
早期输注20%脂肪乳剂对大鼠急性胰腺炎的影响*
赵艳梅,菅志远
[湖北省十堰市太和医院(湖北医药学院附属医院)肝胆胰外科诊疗中心,湖北 十堰 442000]
目的探讨早期经外周静脉注射20%脂肪乳剂对大鼠急性胰腺炎(AP)严重程度的影响及其作用机制。方法以SD大鼠为研究对象,将其随机分为A组(模型对照组)、B组(脂肪乳剂干预组)和C组(AP生理盐水组),每组15只,建立大鼠AP模型。B组在建模手术后1、3和9 h经外周静脉输注20%脂肪乳剂,C组在建模手术后1、3和9h经外周静脉注射生理盐水。检测大鼠6、12和24h血清三酰甘油、胆固醇及微循环相关因子的变化,并观察大鼠AP严重程度的病理变化。结果成功建立大鼠AP模型。B、C组6h的病理学评分比较,差异无统计学意义;12和24 h的病理学评分比较,差异有统计学意义。B组6h的血清三酰甘油、胆固醇、C-反应蛋白(CR P)、血管紧张素-Ⅱ(AngⅡ)及血栓素B2(TX B2)水平高于C组。B组12 h的血清三酰甘油、胆固醇、CR P及TX B2水平高于C组。B组24h的血清三酰甘油、胆固醇、CR P、AngⅡ及TX B2水平高于C组。结论大鼠AP早期经外周静脉注射脂肪乳剂可使大鼠的血脂升高,其可能通过影响微循环使大鼠AP程度加重。
急性胰腺炎;血脂;微循环
急性胰腺炎(acute pancreatitis,AP)患者早期存在胰岛素抵抗,因此应用葡萄糖作为营养供能受到限制。而高脂血症是AP的重要诱因和加重因素之一。目前多数学者认为AP发生后,在肠外营养治疗中,不建议使用脂肪乳剂,以防进一步加重病情[1-2]。然而使用脂肪乳剂后是否真正加重AP病情,以及相关机制,尚不明确。本研究通过建立大鼠AP动物模型,自外周静脉输注20%脂肪乳剂后,观察大鼠AP病理改变严重程度和血脂水平的变化。
1 材料与方法
1.1实验材料与试剂
无特定病原体级SD大鼠45只,10~12周龄,体重200~300g,雌雄不限,由湖北医药学院动物实验中心提供[实验动物使用许可证编号:SYXK(鄂)2011-0031]。将大鼠随机分为A组(模型对照组)、B组(脂肪乳剂干预组)和C组(AP生理盐水组),每组15只。牛磺胆酸钠购自美国Sigma公司,血管紧张素-Ⅱ(human angiotensionⅡ,AngⅡ)和血栓素B2(Thromboxane B2,TXB2)酶联免疫吸附实验(enzyme linked immunosorbent assay,ELISA)试剂盒购自北京尚柏生物医学技术有限公司。
1.2AP动物模型制备
参照Aho法制备大鼠AP动物模型。实验动物术前禁食12h,自由饮水。称重后给予2%戊巴比妥腹腔麻醉。仰卧位固定,腹部正中切口进腹,经下腔静脉穿刺抽血1ml备检。找到胰管,在显微镜下用3.5号细针头穿刺胰管后插入胰管,近端采用血管夹夹闭。B、C组采用静脉输液泵向远端胰管内缓慢注射5%牛磺胆酸钠溶液(0.1 ml/100 g),保留针头5 min,松开血管夹,抽出针头,关腹。A组向胰管内缓慢注射同等剂量的生理盐水。
1.3实验动物的药物处理
A组术后1和3h分别经尾静脉注射生理盐水5 ml/(kg·次),B组术后第1、3和9 h分别经尾静脉注射20%力能脂肪乳(江苏华瑞公司)5ml/(kg·次),C组术后第1、3和9h分别经尾静脉注射生理盐水5ml/(kg·次)。
各组动物麻醉清醒后均可自由饮水。A组于术后6h全部处死,B、C组于术后6、12和24 h分批处死,每批5只,经腹主动脉采血进行相关的血清指标分析。动物处死后首先观察腹水的量和颜色,并取胰腺组织用福尔马林固定,苏木精-伊红染色法染色。按Kusske法进行胰腺组织的病理学评分,即按照水肿、出血、坏死和炎细胞浸润程度4个方面进行盲法评分,每项记分根据无、轻度及重度分别记为0、1和2分,每只大鼠的AP病理学评分为0~8分。每例标本经2名病理科医师阅片后进行评分,取其平均值作为每只大鼠的最后病理学评分。
所有的血液标本采集后立即注入抗凝管,1 000 r/min离心5 min,分离血清置入-20℃冰箱冷冻保存待检。血清淀粉酶、三酰甘油、血清胆固醇、C反应蛋白(C-reactive protein,CRP)的测定由十堰市太和医院检验科采用血液生化仪测定,AngⅡ和TXB2采用ELISA法测定。ELISA法步骤:将稀释的血清标本0.1 ml加入已经准备的反应孔中,以空白为对照,37℃孵育1 h,洗涤后加入稀释的酶标抗体0.1ml,37℃孵育45min,洗涤后加入3,3',5,5'-四甲基联苯胺底物溶液进行显色,然后37℃孵育30 min后,加入2mol/L硫酸0.05ml终止反应,检测各组的光密度(optical density,OD)值,计算血清中AngⅡ和TXB2浓度。
1.4统计学方法
采用SPSS 19.0统计软件进行数据分析,计量资料以均数±标准差(±s)表示,用t检验,P<0.05为差异有统计学意义。
2 结果
2.1AP模型
6 h处死的大鼠中,A组大鼠只有少量腹水,B、C组可见明显的腹水。病理学检查:A组胰腺组织未见明显的水肿和充血;B、C组可见胰腺间质、被膜下水肿,血管充血,炎症细胞浸润等AP病理改变。B、C组大鼠AP模型建立成功。见图1。
2.2B、C组各时间胰腺炎病理改变及评分
B、C组术后6h主要表现为胰腺间质、被膜下水肿,血管充血,炎症细胞浸润。随者时间延长,病理改变程度加重,24h时胰腺坏死的范围、水肿程度均较12h严重,可见胰腺呈片状凝固性坏死、实质局灶性坏死及间质血管坏死出血表现。而在相同时间点,B组大鼠的AP程度较C组严重。两组胰腺炎严重程度的组织病理学切片比较,经2名病理科医师阅片后进行评分。6h时处死大鼠,B、C组的病理学评分比较,差异无统计学意义(t=2.121,P=0.067);12 h时,B组的病理学评分高于C组(t=2.456,P=0.040);24h时,B组的病理学评分仍高于C组(t=3.130,P= 0.014)。见表1和图2。
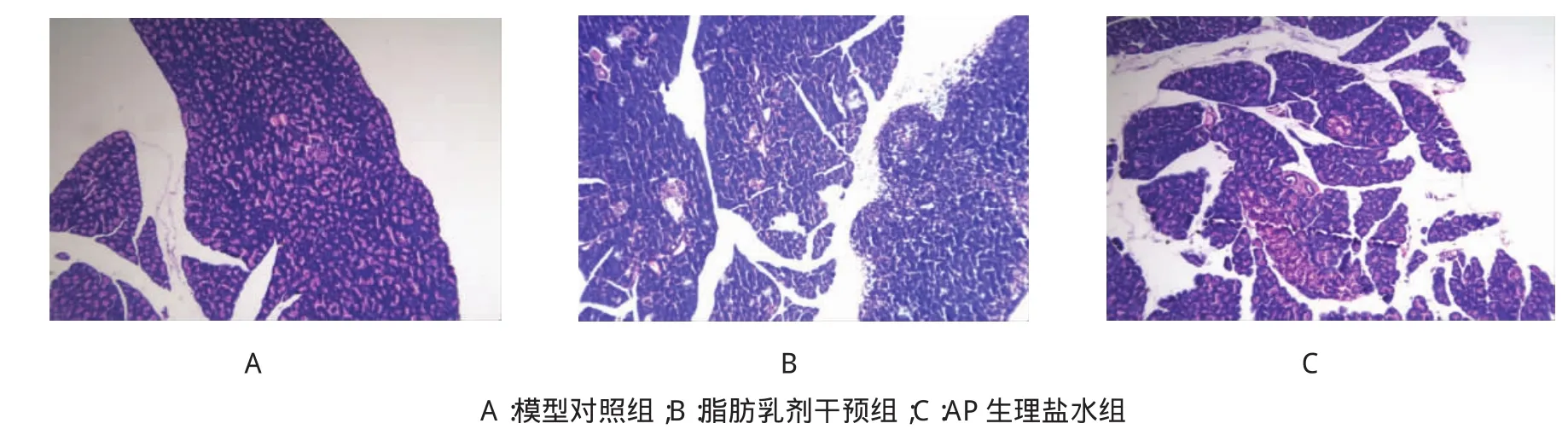
图1 造模后3组大鼠胰腺组织的病理切片(×100)
表1 B、C组大鼠不同时间的病理学评分比较(±s)
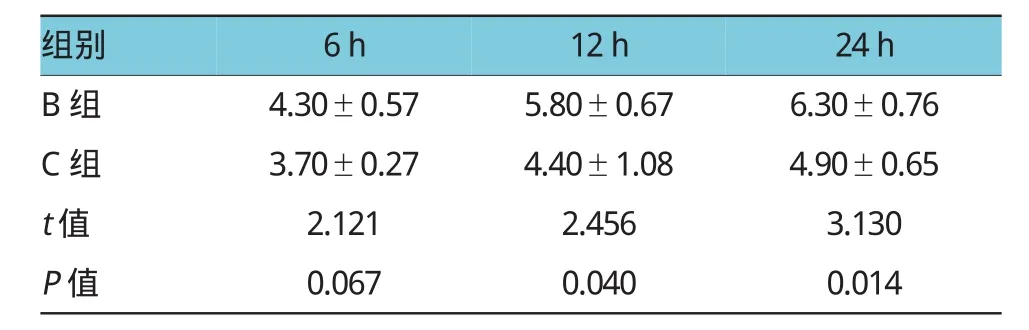
表1 B、C组大鼠不同时间的病理学评分比较(±s)
组别6h12h24h B组4.30±0.575.80±0.676.30±0.76 C组3.70±0.274.40±1.084.90±0.65 t值2.1212.4563.130 P值0.0670.0400.014

图2 B、C组12和24 h大鼠胰腺组织的病理学切片(×200)
2.3B、C组各时间大鼠血清血脂比较
分别对6、12和24h处死的大鼠抽取血液,进行血清三酰甘油、胆固醇及CRP检验,结果显示,B组大鼠的血清三酰甘油和胆固醇含量高于C组(P<0.05)。见表2。
表2 B、C组大鼠不同时间的指标比较(±s)

表2 B、C组大鼠不同时间的指标比较(±s)
组别血淀粉酶/(IU/L)6h12h 24h24h三酰甘油/(mmol/L)6h12h胆固醇/(mmol/L)6h12h24h B组1448.80±458.90 1514.80±375.60 2167.80±196.50 3.60±0.855.97±0.707.00±1.272.81±0.262.98±0.302.88±0.45 C组1126.80±450.40 1008.20±98.801685.80±661.40 1.00±0.151.19±0.151.60±0.791.48±0.332.06±0.432.28±0.26 t值1.1202.9171.5626.70514.9928.0897.0013.9352.595 P值0.2950.0290.1570.0000.0000.0000.0000.0040.032组别6h CRP/(mg/L)12h6h 24h AngⅡ/(ng/L)12h6h 24h TXB2/(ng/L)12h24h B组1028.80±316.601368.40±286.301898.20±319.60914.60±45.10 986.00±105.701038.80±46.40 498.80±65.90 554.80±47.60 578.20±51.10 C组582.60±98.80931.20±283.70 1208.80±164.60828.80±38.10 903.00±14.50 903.40±19.10 357.60±40.10 412.60±24.50 449.40±34.30 t值3.0092.4264.2883.2491.7406.0344.0945.9424.679 P值0.0170.0410.0030.0120.1200.0000.0030.0000.002
2.4B、C组各时间大鼠血清AngⅡ、TXB2比较
B组大鼠的AngⅡ水平在6、12和24 h逐渐升高,C组大鼠的AngⅡ水平在术后6~12 h明显升高,而12~24 h几乎无变化。B组大鼠的AngⅡ水平在6和24h高于C组,差异有统计学意义(P<0.05)。而B、C组的TXB2在6、12和24 h逐渐升高,B组各时间的血清TXB2水平均高于C组(P<0.05)。见表2。
3 讨论
国内外对血脂水平与AP的关系已经进行大量研究。目前认为,高脂血症是AP发病的重要危险因素之一,而AP发生后,往往存在脂质代谢异常,部分AP患者伴有三酰甘油的升高[3-4]。因此多数学者认为,高脂血症既是AP的病因,又是AP代谢紊乱的表现,两者形成恶性循环,因此常常将脂肪乳剂作为AP患者应用的禁忌证[4-5]。另有学者认为,在应激状态下,机体的葡萄糖代谢受到抑制,脂肪成为体内主要的能源,应用脂肪乳剂为主的肠外营养治疗可减少葡萄糖的利用,有利于AP患者血糖的控制,若应用适当,对AP的治疗利大于弊[6-7]。目前,对于AP早期是否应用脂肪乳剂,尚存在较大争论。本研究中,笔者成功建立AP大鼠模型。B组在造模后1、3和9h分别经尾静脉注入大剂量20%脂肪乳剂,结果显示,在6、12和24h,血清三酰甘油和胆固醇的含量明显高于C组,而两组大鼠的胰腺炎病理学评分中,B组大鼠12和24 h胰腺炎严重程度的评分均较C组高。本研究结果显示,B组大鼠的CRP较C组明显升高,而CRP作为AP严重程度和炎症反应综合征的一个重要指标[8-9]。因此,笔者认为,大鼠AP早期应用大剂量的脂肪乳剂,可使血脂升高,加重AP程度。
关于输注脂肪乳剂加重AP的相关机制,笔者推测主要原因可能为脂肪乳剂中的脂肪酸引起血脂升高。有研究认为,游离脂肪酸可通过诱发酸中毒、激活胰蛋白酶、对腺泡细胞膜的直接毒性作用,以及影响微循环等多个途径使AP加重[10-11]。除此之外,脂肪酸和胆固醇可通过直接影响胰腺微循环,激活血小板产生具有强烈缩血管作用的血栓素,造成胰腺腺泡的坏死[12-13]。笔者从病理切片发现,12和24 h胰腺炎病理学评分增高的主要原因是胰腺坏死范围增加,其是否与上述因素相关尚需进一步明确。
胰腺缺血在导致水肿性胰腺炎向坏死性胰腺炎发展的过程中起重要作用。研究认为,AP发生时,肾素-血管紧张素系统的激活可影响胰腺的血供,而AngⅡ是该系统的重要因子[14-16]。前列腺素类的平衡失调是影响胰腺微循环障碍的另一重要原因。AP时磷脂酶A2的释放加速花生四烯酸产生,后者可生成大量血栓素A2,血栓素A2是强烈的血小板凝聚剂和血管收缩剂,可导致胰腺微循环障碍[17]。由于血栓素A2代谢较快,常通过检测其中稳定的代谢产物TXB2来反映其水平。笔者前期实验发现,通过抑制AngⅡ和TXB2可改善大鼠重症急性胰腺炎的严重程度[18]。本研究中,检测大鼠血清中的AngⅡ和TXB2水平,结果显示,B组大鼠输注脂肪乳剂后,血清中AngⅡ和TXB2水平较C组升高,提示在AP早期,大剂量输注脂肪乳剂,客观上使大鼠胰腺的微循环进一步恶化,加速胰腺炎由轻型向重型转化。
综上所述,在大鼠AP早期,通过外周静脉大剂量输注脂肪乳剂可使大鼠的血脂水平升高,可能通过相关的因子导致胰腺的微循环障碍,加重大鼠AP程度,但需进行深入的实验研究。
[1]JANISCH N H,GARDNER T B.Advances in management of acute pancreatitis[J].Gastroenterol Clin North Am,2016,45(1):1-8.
[2]PHILLIP V,STEINER J M,ALGÜL H.Early phase of acute pancreatitis:assessment and management[J].World J Gastrointest Pathophysiol,2014,5(3):158-168.
[3]CHARLESWORTH A,STEGER A,CROOK M A.Acute pancreatitis sociated with severe hypertriglyceridaemia;a retrospective cohort study[J].Int J Surg,2015,23(Pt A):23-27.
[4]PATEL K,TRIVEDI R N,DURGAMPUDI C,et al.Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation[J].Am J Pathol,2015,185(3):808-819.
[5]EWALD N,HARDT P D,KLOER H U.Severe hypertriglyceridemia and pancreatitis:presentation and management[J].Curr Opin Lipidol,2009,20(6):497-504.
[6]PATEL K S,NOEL P,SINGH V P.Potential influence of intravenous lipids on the outcomes of acute pancreatitis[J].Nutr Clin Pract,2014,29(3):291-294.
[7]MIRTALLO J M,DASTA J F,KLEINSCHMIDT K C,et al.State of the art review:intravenous fat emulsions:current applications, safety profile,and clinicalimplications[J].Ann Pharmacother, 2010,44(4):688-700.
[8]YIN G,HU G,CANG X,et al.C-reactive protein:rethinking its role in evaluating the severity of hyperlipidemic acute pancreatitis[J]. Pancreas,2014,43(8):1323-1328.
[9]LEE K J,KIM H M,CHOI J S,et al.Comparison of predictive systems in severe acute pancreatitis according to the revised atlanta classification[J].Pancreas,2016,45(1):46-50.
[10]WANG Y,STERNFELD L,YANG F,et al.Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells[J].Gut,2009,58(3):422-430.
[11]KULIAVIENE I,GULBINAS A,CREMERS J,et al.Fatty acids of erythrocyte membrane in acute pancreatitis patients[J].World J Gastroenterol,2013,19(34):5678-5684.
[12]YANG F,WANG Y,STERNFELD L,et al.The role of free fatty acids,pancreatic lipase and Ca+signalling in injury of isolated acinar cells and pancreatitis model in lipoprotein lipase-deficient mice[J].Acta Physiol(Oxf),2009,195(1):13-28.
[13]MATEU A,RAMUDO L,MANSO M A,et al.Acinar inflammatory response to lipid derivatives generated in necrotic fat during acute pancreatitis[J].Biochim Biophys Acta,2014,1842(9): 1879-1886.
[14]CHAN Y C,LEUNG P S.The renin-angiotensin system and reactive oxygen species:implications in pancreatitis[J].Antioxid Redox Signal,2011,15(10):2743-2755.
[15]LIU R,QI H,WANG J,et al.Angiotensin-converting enzyme (ACEand ACE2)imbalance correlates with the severity of cerulein-induced acute pancreatitis in mice[J].Exp Physiol, 2014,99(4):651-663.
[16]LIU R,QI H,WANG J,et al.Ulinastatin activates the reninangiotensin system to ameliorate the pathophysiology of severe acute pancreatitis[J].J Gastroenterol Hepatol,2014,29(6):1328-1337.
[17]HACKERT T,SPERBER R,HARTWIG W.et al.P-selectin inhibition reduces severity of acute experimental pancreatitis[J]. Pancreatology,2009,9(4):369-374.
[18]周平,张玉环,营志远,等.川芎嗪和缬沙坦对大鼠胰腺炎治疗作用的实验研究[J].肝胆外科杂志,2009,17(1):61-63.
(童颖丹编辑)
Influence of intravenous injection of 20%fat emulsion at early stage on acute pancreatitis of rat model*
Yan-mei Zhao,Zhi-yuan Jian
(Hepatobiliary and Pancreatic Surgery Center,Taihe Hospital,Hubei Medical University,Shiyan,Hubei 442000,China)
Objective To observe the effect of intravenous injection of 20%fat emulsion at early stage on the severity of the rat acute pancreatitis(AP).Methods Experimental pancreatitis model of SD rats were induced by an retro-pancreatic duct injection of Sodium Taurocholate,and the experimental rats were divided into model group(group A),study group(group B)and control group(group C)with 15 rats in each group. At the 1st,3rd and 9th hour after the modeling surgery of acute pancreatitis,the rats in the group B were injected with the 20%fat emulsion through the caudal vein,while those in the group C were injected with normal saline.At the 6th,12th and 24th hour after the modeling operation,serum triglyceride,cholesterol and the factors related to the microcirculation were examined by ELISA,and the score of the pathological grade of the AP severity was calculated.Results The rat AP model was successfully induced.There were significant differences in the pathological grade score between the group B and the group C at the 12th and 24th hour but not at the 6th hour after the modeling operation.At the 6th hour after the modeling operation,the serum levels of triglyceride,cholesterol,C-reaction protein(CPR),angiotensinⅡ(AngⅡ)and thromboxane B2(TXB2)in the group B were significantly higher than those in the group C.At the 12th hour,the serum levels of triglyceride,cholesterol,CPR and TXB2 in the group B were obviously higher than those in the group C.And at the 24th hour after the operation,the serum levels of triglyceride,cholesterol,CPR,AngⅡand TXB2 in the group B were significantly higher than those of the group C.Conclusions Peripheral intravenous injection of 20%fat emulsion at early stage could induce rat hyperlipidemia,which could increase the severity of rat acute pancreatitis possibly by affecting pancreatic microcirculation.
acute pancreatitis;blood lipid;pancreatic microcirculation
R 657.51
A
10.3969/j.issn.1005-8982.2016.19.005
1005-8982(2016)19-0023-05
2016-02-14
湖北省十堰市科技项目(No:ZD2011008)
菅志远,E-mail:jianzhiyuan2001@163.com;Tel:0719-8801497

