沉默DNA依赖蛋白激酶亚基对肝癌耐药细胞Bel-7402/5-Fu DNA损伤修复功能的影响*
李大玉,梁大敏,束波,杨加伟,生欣,李长福,余春波,范芳
(遵义医学院生化教研室,贵州遵义563099)
沉默DNA依赖蛋白激酶亚基对肝癌耐药细胞Bel-7402/5-Fu DNA损伤修复功能的影响*
李大玉,梁大敏,束波,杨加伟,生欣,李长福,余春波,范芳
(遵义医学院生化教研室,贵州遵义563099)
目的探讨DNA依赖蛋白激酶亚基(DNA-PKcs)基因沉默后对肝癌耐药细胞Bel-7402/5-Fu DNA损伤修复功能的影响。方法采用阳离子脂质体法将shDNA-PKcs干扰质粒转染Bel-7402/5-Fu细胞,根据荧光细胞数计算转染率,实时荧光定量聚合酶链反应(qR T-PCR)及Western blot检测DNA-PKcs的沉默效率;Western blot检测TopoⅡ、γ-H2AX蛋白表达;5-乙炔基-2'脱氧尿嘧啶核苷法检测细胞DNA合成。结果质粒的转染效率为67%;qR T-PCR及Western blot检测结果显示,DNA-PKcs mR NA及蛋白水平的沉默效率分别为82.6%和71.8%;Western blot结果显示,实验组TopoⅡ蛋白表达水平比对照组低,差异有统计学意义(P<0.05),γ-H2AX蛋白表达水平比对照组高,差异有统计学意义(P<0.01)。5-乙炔基-2'脱氧尿嘧啶核苷法结果显示,实验组DNA合成率比对照组低,差异有统计学意义(P<0.05)。结论shDNA-PKcs干扰质粒能下调Bel-7402/5-Fu细胞中DNA-PKcs、TopoⅡ蛋白的表达,抑制Bel-7402/5-Fu细胞DNA合成。
DNA依赖性蛋白激酶亚基;肝癌耐药细胞/Bel-7402/5-Fu细胞;DNA损伤修复
DNA双链断裂(double-stranded breaks,DSBs),是细胞最严重的一种损伤,可导致细胞失去分裂增殖而死亡,是多数抗癌药物杀死肿瘤细胞的重要机制。DNA依赖蛋白激酶亚基(DNA-dependent ki nase catalytic subunit,DNA-PKcs)是非同源重组修复(nonhomologous end-joining,NHEJ)途径的核心成分,在许多DNA损伤修复途径中扮演着重要角色,如NHEJ、VDJ重组。同时可以磷酸化许多修复基因和转录因子[1-2]。肿瘤耐药与DNA损伤修复能力增强有关[3],本文将沉默DNA-PKcs的表达,将干扰质粒shDNA-PKcs转染Bel-7402/5-Fu肝癌耐药细胞,检测DNA-PKcs沉默后,对细胞DNA损伤修复功能的影响,以探讨DNA-PKcs在肝癌耐药的发生发展中的作用机制。
1 材料与方法
1.1材料
Bel-7402/5-Fu细胞株购于南京凯基生物科技发展有限公司,PRNAi-U6.1-Neo shRNA质粒购于百奥迈科生物科技有限公司,LipofectamineTM2000 Reagent购于美国Invitrogen公司,DNA-PKcs抗体购于美国Assay biotech公司,TopoⅡ抗体购于英国Abcam公司,γ-H2AX抗体购于美国ABGENT公司,其他试剂为国产分析纯。
1.2方法
1.2.1细胞培养将Bel-7402/5-Fu细胞放在含有10%的胎牛血清的1640培养液中,置于37℃、5%的二氧化碳培养箱中培养,每天观察细胞生长状态,取对数期细胞用于实验。
1.2.2分组与转染分为空白对照组、脂质体组、质粒对照组、shDNA-PKcs实验组。细胞接种于6孔板,每孔种5×105个细胞,融合度达到60%~80%时进行转染。配制质粒-脂质体复合物,并加入对应孔中,每孔200μl,4~6h后换液。48h后荧光显微镜下计数,计算转染率(每个视野下的荧光细胞个数/相同视野下的总细胞个数×100%)。
1.2.3实时荧光定量聚合酶链反应检测DNA-PKcs mR NA的表达水平总RNA的提取按TIANGEN公司总RNA提取试剂盒(离心柱型)说明书进行,实时荧光定量聚合酶链反应(real-time quantitative polymerase chain reaction,qRT-PCR)引物:DNA-PKcs正向引物5'-GTGACAAGGCAACTGTATGAGC-3',反向引物3'-TTCTCTAAAAACACCAGCCACA-5',扩增片段长度163 bp;β-actin正向引物5'-TGACGTGG ACATCCGCAAAG-3',反向引物3'-GGAGCGACAGG TGGAAGGTC-5',扩增片段长度206 bp,每个样品DNA-PKcs mRNA表达量经qRT-PCR检测,PCR产物作熔解曲线分析,根据公式2-△△CT相对定量分析计算DNA-PKcs mRNA相对表达量。
1.2.4Western blot检测DNA-PKcs、TopoII、γ-H2AX蛋白表达提取各组细胞总蛋白,聚氰基丙烯酸正丁酯法定量样品蛋白含量。Western blot法检测DNA-PKcs、TopoII、γ-H2AX蛋白在各组中的表达。
1.2.5EdU法检测细胞DNA合成率细胞处理48 h后,按照广州锐博5-乙炔基-2'脱氧尿嘧啶核苷(5-Ethynyl-2'-deoxyuridine,EdU)检测试剂盒操作说明书进行操作,荧光显微镜下观察细胞,拍照。由绿光激发蓝色荧光,由紫光激发红色荧光。每组取5个视野,计数相同视野下EdU标记细胞及总细胞数,计算DNA合成率。
1.3统计学方法
采用SPSS 18.0统计软件对数据进行分析,计量数据以均数±标准差(±s)表示,用单因素方差分析和LSD-t检验,P<0.05为差异有统计学意义。
2 结果
2.1shDNA-PKcs质粒的转染效率
shDNA-PKcs质粒转染效率约为67%。见图1。
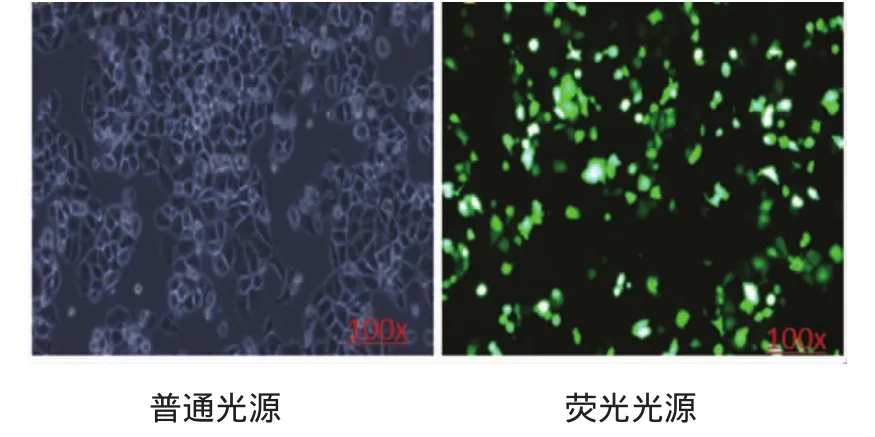
图1 shDNA-PKcs质粒转染效率
2.2DNA-PKcs mRNA的表达水平
各组DNA-PKcs mRNA水平比较,经单因素方差分析,差异有统计学意义(F=74.404,P=0.000)。各组DNA-PKcs相对倍数变化比较,经单因素方差分析,差异有统计学意义(F=160.518,P=0.000)。shDNAPKcs实验组与空白对照组DNA-PKcs mRNA水平比较,经LSD-t检验,差异有统计学意义(t=2.919,P= 0.004),shDNA-PKcs实验组mRNA水平高于空白对照组;shDNA-PKcs实验组与空白对照组DNA-PKcs相对倍数变化比较,经LSD-t检验,差异有统计学意义(t= 17.237,P=0.001),即shDNA-PKcs实验组DNA-PKcs mRNA相对表达量低于空白对照组。DNA-PKcs mRNA水平的沉默效率为82.6%。见附表和图2。
附表各组DNA-PKcs mRNA相对表达量比较(n=3±s)
注:†与对照组比较,P<0.01
DNA-PKcs相对倍数空白对照组23.133±0.16417.207±0.3371.000±0.000脂质体组22.493±0.13516.630±0.4781.045±0.101质粒对照组22.680±0.27417.047±0.6901.226±0.052 shDNA-PKcs实验组26.013±0.562†17.537±0.4680.174±0.083†F值74.404160.518 P值0.0000.000组别DNA-PKcs mRNA β-actin mRNA
2.3DNA-PKcs、TopoII、γ-H2AX的蛋白表达水平
各组DNA-PKcs、TopoⅡ、γ-H2AX蛋白相对表达量比较,经单因素方差分析,差异有统计学意义(DNA-PKcs:F=23.478,P=0.000;TopoⅡ:P=15.564,P=0.001;γ-H2AX:F=157.096,P=0.000)。shDNAPKcs实验组DNA-PKcs、TopoⅡ、γ-H2AX蛋白相对表达量与对照组比较,经LSD-t检验,差异有统计学意义(DNA-PKcs:t=8.407,P=0.006;TopoⅡ:t= 5.225,P=0.017;t=12.348,P=0.003),表明shDNAPKcs实验组DNA-PKcs、TopoⅡ蛋白相对表达量低于空白对照组,γ-H2AX蛋白相对表达量高于空白对照组。DNA-PKcs蛋白水平的沉默效率为71.8%。见图3。
2.4DNA合成率
各组DNA合成率,经单因素方差分析,差异有统计学意义(F=49.296,P=0.000),而shDNA-PKcs实验组DNA合成率与空白对照组比较,经LSD-t检验,差异具有统计学意义(t=12.290,P=0.003),即shDNA-PKcs实验组DNA合成率低于空白对照组。可见DNA-PKcs的沉默可降低肝癌耐药细胞Bel-7402/5-Fu DNA的合成。见图4。

图4 各组DNA合成率比较
3 讨论
肝癌是临床常见的恶性肿瘤,目前临床常用手术结合放疗和化疗。但长期放疗和化疗后,容易出现耐药,这可能与DNA损伤信号紊乱和DNA损伤修复有关[4-5]。很多因素均会引起DNA损伤。其中,由电离辐射、抗癌药物(如阿霉素)等引起的DNA双链断裂是最严重的损伤。在哺乳动物细胞中,以DNA依赖蛋白酶(DNA-dependent protein kinase,DNA-PK)为核心组件的NHEJ途径,在DNA双链断裂的修复中有着非常重要的作用[6-7]。
DNA-PKcs是DNA-PK的催化亚单位,位于染色体8ql1,由4 128个氨基酸组成的大分子量蛋白质,相对分子量为470 kD,主要包括DNA断裂结合区、催化功能区、Ku结合区3个功能区,属于胞内磷脂酰肌醇激酶(phosphatidylinositol 3-kinases,PI3K)家族。DNA双链断裂后,DNA-PKcs结合到DNA断裂末端,通过自身磷酸化,以及磷酸化下游蛋白,如Artemis、DNA ligase4、XRCC4和Cernunnos等完成DNA损伤修复[8-9]。DNA-PKcs能维持基因组的稳定性,具有肿瘤抑制功能[10]。目前有研究[11]表明,下调DNA-PKcs表达能增加肿瘤细胞对药物的敏感性。有研究显示,在耐药的神经胶质瘤、口腔癌的放疗患者中,发现DNA-PKcs表达量增加[12],表明放疗和化疗后,机体产生了耐药性。另外,在一些耐药细胞中发现DNA-PKcs表达和活性增加[13-14]。本实验已通过沉默DNA-PKcs发现耐药蛋白P-gp表达降低,因此推测耐药细胞Bel-7402/5-Fu耐药性降低可能与DNA-PKcs沉默所致的P-gp下调有关。可能原因是DNA-PKcs介入到耐药细胞Bel-7402/5-Fu DNA损伤修复系统所致。DNA-PKcs在某些耐药细胞中表达增加和活性增强,提高了耐药细胞DNA损伤修复的能力,从而对抗化疗药物所致的DNA损伤而引起耐药。本实验采用RNAi技术沉默DNA-PKcs,检测肝癌耐药细胞中γ-H2AX的表达情况。结果发现,沉默后肝癌耐药细胞中γ-H2AX明显增加,说明DNA-PKcs沉默后能够增加DNA的损伤。为了进一步说明沉默DNA-PKcs后能增加DNA的损伤情况,本实验还检测沉默后DNA的合成情况和TopoⅡ蛋白的表达情况,结果发现沉默后DNA合成降低,TopoⅡ表达也降低。以此可以推测沉默DNAPKcs后能增加DNA损伤,降低DNA损伤修复功能。可能是DNA-PK为核心的NHEJ途径在肝癌耐药细胞DNA损伤修复中有着重要的作用,DNA-PKcs是否还通过其他机制调节肝癌耐药细胞Bel-7402/5-Fu DNA损伤修复功能,还有待进一步实验研究。
[1]LEE K J,LIN Y F,CHOU H Y,et al.Involvement of DNA-dependent protein kinase in normalcellcycleprogression through mitosis[J].J Biol Chem,2011,286(14):12796-12802.
[2]HSU F M,ZHANG S,CHEN B P.Role of DNA-dependent protein kinasecatalytic subunit in cancerdevelopment and treatment[J]. Transl Cancer Res,2012,1(1):22-34.
[3]CHENG L,LUO S,JIN C,et al.FUT family mediates the multidrug resistance ofhumanhepatocellularcarcinoma via the PI3K/Akt signaling pathway[J].Cell Death and Disease,2013,4: 1-12.
[4]张阳德,王宁,廖明媚,等.DNA损伤和修复在肝癌中的研究进展[J].中国现代医学杂志,2012,22(13):57-61.
[5]BOUWMAN P,JONKERS J.The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance[J].Nat Rev Cancer,2012,12(9):587-598.
[6]CURTIN N J.DNA repair dysregulation from cancer driver to therapeutic target[J].Nat Rev Cancer,2012,12(12):801-817.
[7]ROTHKAMM K,KRÜGER I,THOMPSON L H,et al.Pathways of DNA double-strand break repair during the mammalian cell cycle[J].Mol Cell Biol,2003,23:5706-5715.
[8]WOODS D S,SEARS C R,TURCHI J J.Recognition of DNA Termini by the C-Terminal region of the Ku80 and the DNADependent protein kinase catalytic subunit[J].PLoS One,2015, 10(5):1-19.
[9]WILLIAMS G J,HAMMEL M,RADHAKRISHNAN S K,et al. Structural insights into NHEJ:building up an integrated picture of the dynamic DSB repair super complex,one component and interaction at a time[J].DNA Repair(Amst),2014,17:110-120.
[10]江娜,沈瑛,孔宪明,等.DNA-PK蛋白功能及其调控机制[J].医学分子生物学杂志,2011,8(3):278-282.
[11]SEO S B,HUR J G,KIM M J,et al.TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases[J].Mol Cancer,2010,9:199.
[12]SÖDERLUND LEIFLER K,QUESETH S,FORNANDER T,et al.Low expression of Ku70/80,but high expression of DNAPKcs,predict good response to radiotherapy in early breast cancer[J].Int J Oncol,2010,37(6):1547-1554.
[13]RYU J S,UM J H,KANG C D,et al.Fractionated irradiation leads to restoration of drug sensitivity in MDR cells that correlates with down-regulation of P-gp and DNA-dependent protein kinase activity[J].Radiat Res,2004,162(5):527-535.
[14]NISHIDA Y,MIZUTANI N,INOUE M,et al.Phosphorylated Sp1 is the regulator of DNA-PKcs and DNA ligase IV tran scription of daunorubicin-resistant leukemia cell lines[J].Biochim Biophys Acta,2014,1839(4):265-274.
(申海菊编辑)
Effects of DNA-PKcs gene silence on repair of damaged DNA in multidrug-resistant hepatocellular carcinoma cell line Bel7402/5-Fu*
Da-yu Li,Da-min Liang,Bo Shu,Jia-wei Yang,Xin Sheng,Chang-fu Li,Chun-bo Yu,Fang Fan
(Department of Biochemistry,Zunyi Medical College,Zunyi,Guizhou 563099,China)
Objective To investigate the effect of shDNA-PKcs on DNA damage repair function of the multidrug-resistant hepatocellular carcinoma cell line Bel7402/5-Fu.Methods shDNA-PKcs plasmids were transiently transfected into Bel7402/5-Fu cells via cathodolyte liposome transfection method.Transfection efficiency was evaluated by calculating the fluorescent cells and silence efficiency was determined using qRTPCR and Western blot.qRT-PCR and Western blot were used to detect mRNA and protein expressions of TopoⅡand γ-H2AX.The cellular DNA synthesis was detected by 5-ethynyl-2'-deoxyuridine(EdU).Results Transfection efficiency was 67%.qRT-PCR and Western blot results showed the efficiency of RNA and protein interference for DNA-PKcs was 82.6%and 71.8%.Western blot analysis showed that the protein expression of TopoⅡin the shDNA-PKcs group was significantly lower than that in the control group(P<0.05),while γ-H2AX expression was higher than that of the control group(P<0.01).EdU detection results showed the DNA synthesis of the shDNA-PKcs group decreased compared with that of the control group(P<0.05). Conclusions shDNA PKcs interference plasmid can down-regulate the expressions of DNA-PKcs and TopoⅡand inhibit the DNA synthesis of Bel7402/5-Fu cells.
DNA-PKcs;Bel7402/5-Fu cell;DNA damage repair
R 735.7
A
10.3969/j.issn.1005-8982.2016.17.004
1005-8982(2016)17-0019-04
2015-10-30
贵州省社发攻关项目(No:[2009]3066);贵州省社发攻关项目[黔科合SY(2013)3008]
范芳,E-mail:fanf1970@126.com

