Preparation and characterization of hexagonal SrMnO3 nanofibers by electrospinning
Zhu Hua, Zhong Xin
(School of Chemistry and Chemical Engineering, Sichuan University of Arts and Science, Dazhou 635000)
Preparation and characterization of hexagonal SrMnO3nanofibers by electrospinning
Zhu Hua, Zhong Xin
(School of Chemistry and Chemical Engineering, Sichuan University of Arts and Science, Dazhou 635000)
SrMnO3/polyvinylpyrrolidone(PVP)compositenanofibershavebeenpreparedsuccessfullybyelectrospinningprocessfromstrontium(Ⅱ)nitrateandmanganese(Ⅱ)acetatetetrahydrate.ThehexagonalSrMnO3nanofiberswasobtainedaftercalcinationat800 ℃for3h.TheeffectofPVPconcentrationonthefiberstructurewasinvestigated.Thestructureandpropertiesofnanofiberswerecharacterized.TheresultsshowedthatSrMnO3/PVPcompositenanofibersbecameuniformandthebeadedstructuredisappearedwhenthemassfractionofPVPreached8%;PVPcompletelydecomposedandallthefeedstockstransformedintoSrMnO3duringthecalcinationat700 ℃,thusagoodpurityhexagonalSrMnO3fiberof150-200nminthediameterwereobtained.
strontiummanganate;polyvinylpyrrolidone;electrospinning;calcination;nanofibers
Electrospinningtechniquehasbeenactivelyexploitedasasimpleandversatilemethodforgeneratingcontinuousnanofiberswhichhashighporousstructure,lowdensityandhighspecificsurfacearea[1-3].DuetothespecialskeletalstructurewhichwasconsistedofathreedimensionalnetworkofMnO6octahedra,strontiummanganate(SrMnO3)hassomepropertiessuchaselectronicproperties,thermochromism,thermalconductivity,magneticproperty,etc[4].Recently,themixtureofinorganicandorganicsaltsdissolvinginpolyvinylpyrrolidone(PVP)asstartingmaterials,inorganic-polymericfibershavepreparedbyelectrospinning.Theinorganic-polymericfiberswouldtransformintonanofibersbysubsequentcalcinationsathightemperature.Asfarasweknow,thepreparationofSrMnO3nanofibershasneverbeenreported.HerewereportthepreparedofSrMnO3nanofibersviaelectrospinningcombinedwithsol-gelprocess,andthepropertieswerecharacterizedforitsfutureapplications.
1 Experimental
Strontium(Ⅱ)nitrateandmanganese(Ⅱ)acetatetetrahydratewereusedastherawmaterialsforthepreparationofSrMnO3nanofibers.PVPwiththerelativemolecularmassof1.3×106wasusedasaviscosity-controllingagent.Asolutionwaspreparedbydissolving4mmolstrontium(Ⅱ)nitrateand4mmolmanganese(Ⅱ)acetatetetrahydratein3mLdemonizedwater,whichwasaddedwithacertainamountofPVPdissolvingin11mLethanolaqueous,stirringfor12hatroomtemperature.Thenthehomogeneoushybridsolwasobtained.Theaboveprecursorsolwasloadedina20mLplasticsyringeof25gaugeswithstainlesssteelneedle.Thedistancebetweenthespinneretandcollectorwasfixedas10cmandthehigh-voltagesupplywasmaintainedat15kV.Thespinningratewascontrolledat1.5mL/h.SrMnO3/PVPcompositenanofiberswascollectedonthecollector.Thesefiberswerecalcinatedatarateof2 ℃/minandremainedinairatmospherefor3hat800 ℃.ThushexagonalSrMnO3nanofiberswereobtained.
TheX-raydiffraction(XRD)patternsweremeasuredonaRigakuGeigerfluxinstrument.ThesizeandmorphologyofSrMnO3fiberswereobservedwithJEOLJSM-6390scanningelectronmicroscope(SEM).EnergydispersiveX-ray(EDX)spectrographwasrecordedonINCA200EDSattachedwithSEMtostudythephasepurityofSrMnO3nanofibersthroughtheelementalanalysis.Thermogravimetric(TG)analysiswascarriedoutonaTAQ50thermogravimetricanalyzerinnitrogenatmosphere,andthetemperature-risingratewas10 ℃/min.Fouriertransformationinfrared(FTIR)spectroscopywasrecordedonaPerkinElmerspectrum100.
2 Results and discussion
2.1SEMandEDXanalysis
Fig.1showsthattheconcentrationofPVPhasasignificanteffectontheformation,uniformityandhomogeneityofnanofibers.Thesizeandmorphologyhasmarkeddifferenceunderdifferentconcentrations.Non-uniformfiberswithbeadedstructureareformedwhenthemassfractionofPVPwasbelow6%.WiththeincreaseofPVPconcentration,thesurfacetensionandviscosityarecorrespondinglyincreased,theresistanceairflowtensileforceandstaticelectricityarealsoincreasedintheelectrospinningprocess,andthestructureoftheobtainedfibersbecomeuniform.ThebeadedstructuredisappearsandthefibersbecomeuniformatthePVPmassfractionof8%.Therandomlyorientedhybridfiberswithsmoothsurfaceandaveragediameterof300-400nmcanbeobservedasthemassfractionofPVPwas10%.TheobtainedfiberismoreuniformthanthatoflowPVPconcentration.Theresultsarewellconsistentwiththepublishedliterature[5].
AsFig.2ashown,thefibrousstructureisretainedonthewholeexceptthatasmallamountoffibersarebrokenafterannealingat800 ℃.Andtheaveragediameterisreducedto150-200nmduetotheevaporationanddecompositionofPVPandvolatilecomponents.ThefiberssurfacebecomesroughbecauseofthedecompositionofPVPandtheformationofcrystallites.SrMnO3nucleialsogrowtoformlargernanoparticles.AsFig.2bshown,allpeaksarecorrespondingtoSr,MnandO,exceptAupeak(Auissprayedontothenanofibersbeforescanninginordertogethighqualitymorphologyimages).TheresultshowsthattheSrMnO3nanofibersholdgoodpurityafterremovalofPVP.
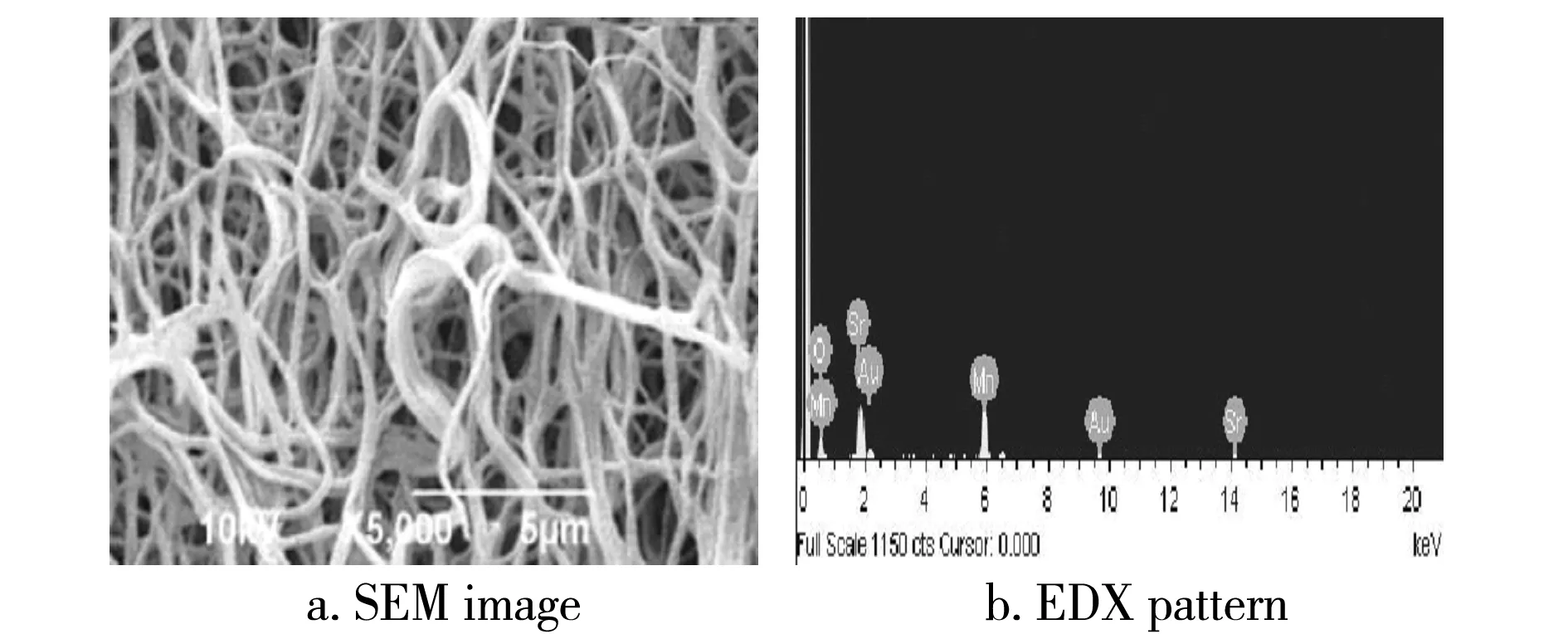
Fig.2 SEM images and EDX pattern of SrMnO3 nanofibers
2.2XRDanalysis
AsindicatedinFig.3,strongpeaksareobservedat2θof32.84°associatedwith(110)planes.Othermaindiffractionpeaksat2θof27.22°, 35.19°, 43.19°, 48.90°, 58.62°, 60.17°and68.87°areassignedtothediffractionof(102), (103), (202), (203), (300), (213)and(220)crystalfacets,respectively.AlldiffractionpeaksshowgoodconsistencywithJCPDSCardNo.24—1213ofperovskitephaseofSrMnO3[6].Asinglehexagonalperovskitesystemwasdetermined.NotypicalpeakofpolymerisobservedinFig.3,indicatingthatPVPwasdecomposedandremovedafterannealingat800 ℃.
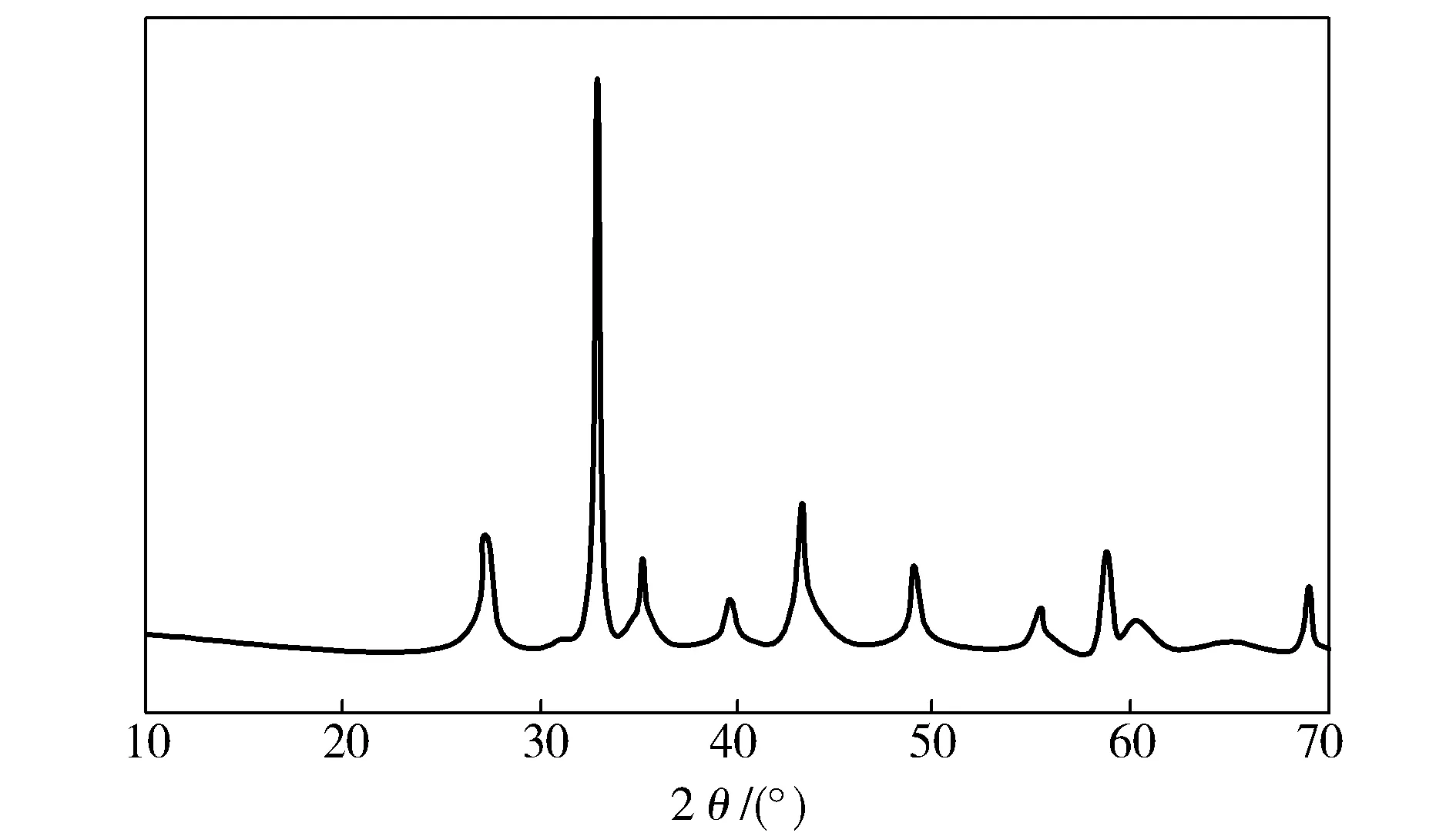
Fig.3 XRD patterns of SrMnO3 nanofibers
2.3Thermogravimetricanalysis
AsshowninFig.4,theweightlossprocesshas3stages.Theweightlossis11.4 %atthefirststagebelow120 ℃,whichwascausedbythelossofsurfaceadsorbatesandresidualmoisture[7];theweightlossof35.5%over250-400 ℃isduetothedecompositionoforganiccontents(PVP)[5];similarly,theweightlossof33.7%over400-700 ℃isduetothedecompositionoftheorganometallicprecursor(manganeseacetate)andstrontiumnitrate;theweightlossiscompletedbelow700 ℃andthetotalweightlossisabout80.4%;noweightlossisobservedabove700 ℃andtheTGcurvebecomeshorizontal.TheTGcurveindicatesthatSrMnO3fibersamplescanbefabricatedatatemperatureof700 ℃andabove.

Fig.4 TG curve of SrMnO3/PVP composite nanofiber
2.4FTIRspectrometry
AsFig.5shown,forPVP,thebroadbandaround3 200-3 600cm-1correspondstoO—Hstretchingvibration;thetripletpeakspresentat2 954cm-1correspondingtotheasymmetricandsymmetricC—Hstretchingvibrationsofmethylgroups(fromacetate)[8];andtheotherthreedominantpeaksatabout1 654, 1 442and1 292cm-1correspondtothestretchingvibrationofCO,C—HandC—Nbonds,respectively[9].ForSrMnO3/PVPcompositenanofiber,theCOstretchingvibrationcharacteristicpeakred-shiftsto1 652cm-1,theC—Hstretchingvibrationpeaksblue-shiftsto2 956and1 444cm-1,respectively;andthestretchingofC—Nat1 292cm-1isweakened.ForSrMnO3nanofiber,allthePVPpeaksvanish,indicatingthatthePVPwasfulldecomposed,threenewpeaksappearat765, 663, 542cm-1,whichareascribedtothestretchingvibrationofmetal-oxide(M—O)bondsinSrMnO3,matchingwellwiththepublishedliterature[6].TheFTIRresultsareingoodagreementwiththeSEM,XRDandTGresults.
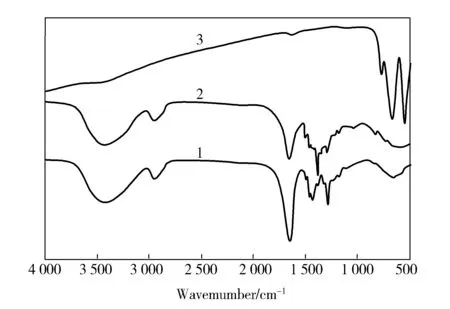
Fig.5 FTIR spectra of PVP and SrMnO3/PVP composite nanofiber and SrMnO3 nanofiber1—PVP;2—SrMnO3/PVP composite nanofiber;3—SrMnO3 nanofiber
3 Conclusions
a.SrMnO3/PVPcompositenanofiberswithadiameterof300-400nmweresuccessfullyfabricatedbyelectrospinningtechniqueandpurehexagonalSrMnO3fiberswithadiameterof150-200nmwerepreparedbytheheattreatmentofSrMnO3/PVPcompositenanofibersat800 ℃ .
b.PVPconcentrationplaysanimportantroleintheformation,uniformityandhomogeneityofnanofibers.ThefiberbecameuniformwhenthePVPmassfractionreached8%.
c.PVPwasdecomposedandremovedfromSrMnO3/PVPcompositefiberaftertheheattreatmentat700 ℃ .
References
[1]WangJinxian,ZhengXiaoqiu,DongXiangting,etal.SynthesisofLaMnO3nanofibersviaelectrospinning[J].ApplPhysRes, 2009, 1(2): 30-36.
[2]BhardwajN,KunduSC.Electrospinning:Afascinatingfiberfabricationtechnique[J].BiotechnolAdv,2010,28(3):325-347.
[3]LiDan,XiaYounan.Directfabricationofcompositeandceramichollownanofibersbyelectrospinning[J].NanoLett, 2004, 4(5):933-938.
[4]HeirasJ,PichardoE,MahmoodA,etal.Thermochromismin(Ba,Sr)-Mnoxides[J].JPhysChemSolid, 2002, 63(4):591-595.
[5]ChandradassJ,KimH,MomadeFWY.SynthesisofultrafineMgFe2O4,nanofibersviaelectrospiningusingsol-gelprecursor[J].JSol-GelSciTechnol, 2013, 65(2):189-194.
[6]KhazaeiM,MalekzadehA,AminiF,etal.Effectofcitricacidconcentrationasemulsifieronperovskitephaseformationofnano-sizedSrMnO3andSrCoO3samples[J].CrystResTechnol, 2010, 45(10):1064-1068.
[7]ImranZ,BatoolSS,IsrarMQ,etal.Fabricationofcadmiumtitanatenanofibersviaelectrospinningtechnique[J].CeramInt, 2012, 38(4):3361-3365.
[8]TianHuyong,LuoWeigen,PuXinghua,etal.SynthesisandanalysesofthermaldecompositionandmicrostructureofSr-dopedbariumtitanatealkoxidederivedprecipitatesandthinfilms[J].ThermochimActa, 2000, 360(1):57-62.
[9]CuiQizheng,DongXiangting,WangJinxian,etal.Directfabricationofceriumoxidehollownanofibersbyelectrospinning[J].JRareEarth, 2008, 26(5):664-669.
静电纺丝技术合成SrMnO3纳米纤维及结构表征朱华,钟欣(四川文理学院化学化工学院,四川 达州 635000)摘要:以四水乙酸锰和硝酸锶为原料,通过静电纺丝法制备了锰酸锶(SrMnO3)/聚乙烯吡咯烷酮(PVP)复合纳米纤维,在800 ℃下处理3h,得到六方晶形结构的SrMnO3纳米纤维,考察了PVP浓度对纤维结构的影响,并对纤维的结构与性能进行了表征。结果表明:PVP质量分数为8%时,SrMnO3/PVP复合纤维表面光滑,均匀性好;热处理温度达700 ℃时,SrMnO3/PVP复合纤维中PVP完全分解,原料全部转化为SrMnO3,所得SrMnO3纳米纤维直径为150~200nm的六方晶形结构,且具有良好的纯度。关键词:锰酸锶聚乙烯吡咯烷酮静电纺丝法烧结纳米纤维
date:10- 06- 2016;reviseddate: 25- 06- 2016.
AppliedBasicResearchProgramsofScienceandTechnologyDepartmentofSichuan(2015JY0254).
TQ343.2Documentcode:AArticleID: 1001- 0041(2016)04- 0058- 03
Biography:HuaZhu(1981-),male,lecturer,beengagedinthestudyoffinechemicals.E-mail:Zhuhua2006@163.com.

