Cytokines imbalance in patients with neurocysticercosis:a review and meta-analysis
CHEN Xiao-hua,WANG Fei,ZOU Yang,TIAN Xiao-jun,GU Jun-chao
(Beijing Friendship Hospital, Capital Medical University / Beijing Tropical Medicine Research Institute/Beijing Key Laboratory for Research on Prevention and Treatment of Tropical Diseases, Beijing 100050, China)
Cytokines imbalance in patients with neurocysticercosis:a review and meta-analysis
CHEN Xiao-hua,WANG Fei,ZOU Yang,TIAN Xiao-jun,GU Jun-chao
(Beijing Friendship Hospital, Capital Medical University / Beijing Tropical Medicine Research Institute/BeijingKeyLaboratoryforResearchonPreventionandTreatmentofTropicalDiseases,Beijing100050,China)
Abstract:Neurocysticercosis is associated with central and peripheral immune system dysfunction; however, the cytokine expressions in individual studies are conflicting. The present paper aims to systematically review evidence of central and peripheral cytokine alterations in neurocysticercosis integrating findings from various affective states. A meta-analysis was conducted in comparison of central and peripheral cytokine concentrations in patients with neurocysticercosis with control subjects. Fourteen articles with a total of 518 neurocysticercosis patients and 386 controls were included. Overall, concentrations of interferon-γ (IFN-γ) (P=0.000 5) and IL-2 (P<0.000 01) were significantly lower, but tumor necrosis factor-a (TNF-a) (P<0.000 01), IL-4 (P=0.000 01), IL-10(P<0.000 01) and soluble ICAM-1(P<0.000 01), sIL-2R (P<0.000 01) were significantly higher in neurocysticercosis patients compared with controls. There were no significant differences of IL-6 and IL-8 levels between neurocysticercosis patients and control subjects although sIL-2R and IL-6 were low in CSF of neurocysticercosis patients, which was supported by only one study. These results indicate that a Th1/Th2 imbalance exists in neurocysticercosis, which may be involved in the pathogenesis of the disease.
Keywords:cytokines; neurocysticercosis; meta-analysis; inflammation; biomarker
Neurocysticercosis, caused by the larvae of the pork tapewormTaeniasolium(TsM), is the most common helminth infection of the CNS in humans worldwide[1]. Neurocysticercosis (NCC) is also the leading cause of epilepsy in developing world.
Immune mechanisms are involved in the pathogenesis of neurocysticercosis. Cysticercal lesions develop in brain depending upon a combination of host immune-inflammatory response, mainly mediated by cytokines produced by cysticercal antigens[2]. The invasion of the central nervous system by the TsM induces a blood brain barrier (BBB) disruption. As the parasite dies, the bioactive molecules ofTaeniasoliummetacestode are released, and a strong inflammatory response are induced, that may contribute to the development of clinical symptoms and signs[3].
Thus, disorder of immune system may involve in pathogenesis of NCC. We conducted a meta-analysis of CSF, sera and non TsM antigen stimulated peripheral blood mononuclear cell (PBMC) cytokines, cytokine receptors and soluble intercellular adhesion molecule (sICAM) levels in neurocysticercosis so as to investigate the influence of TsM infection to host immune responses in the neurocysticercosis patient in comparison with the control subject. Additionally, we discussed the effect of different body fluid components, different assay methods and the influence of anti-parasite treatment on cytokine- and cytokine receptor levels.
Methods and materials
A protocol for the review was prepared before the data collection.
Eligibility criteria
Inclusion and exclusion criteria were determined before the search was conducted. We included human studies comparing patients with neurocysticercosis with the controls, in which one or more of the following inflammatory markers was measured in the CSF, sera and/or no TsM antigen stimulated PBMC: IFN-γ, IL-2, IL-2 receptor (sIL-2R) , IL-4 ,TNF-a, IL-6, IL-8, IL-10, IL-23, IL-17 and sICAM-1 . The exclusion criteria were: (i) case reports or very small case series; (ii) same patient data (such as duplicate publication); (iii) review articles, editorial, letters, author reply, comments, erratum, conference proceedings; (iv) insufficient data to reassess sensitivity or specificity from individual studies; (v) articles not within the field of our study.
Search strategy
The systematic search was performed by Chen XH and Wang F in PubMed, CNKI, Wan Fang and VIP Web of Science from 1990 to December 2014. The search strategy employed the following keywords: inflammation, cytokine, interleukin, inflammatory markers, tumor necrosis factor, immune and neurocysticercosis. Reference lists of retrieved articles were also searched by hand.
Study selection and data extraction
Data were collected using a standard data extraction form (Table 1). Information from articles in a language of English or Chinese was extracted.

Tab.1 Characteristics of the included studies

Tab.1(Continued)
Two reviewers (Zou Y. and Tian XJ) independently screened all titles, abstracts and full texts of selected articles and conducted the data extraction and the quality assessment. Disagreements were resolved by consensus.
Cytokine, cytokine receptor and soluble intercellular adhesion molecule levels (mean and standard deviation) were extracted for each group of study participants along with relevant clinical data (age, sex, anti-parasite treatment, epilepsy or not, ) and information on assay method and body fluid fractions was analyzed. For two studies evaluating anti-parasite treatment on TNF-α level of neurocysticercosis patients, the levels of pre-treatment were chosen for comparison with post-treatment.
Review Manager v.5.2 (Cochrane Collaboration, Oxford, United Kingdom) was used for the analysis. We chose to calculate a standardized mean difference (SMD) for the principal summary measure for every cytokine included because of the variable assay types used (i.e. Flow Cytometry and ELISA) in evaluating cytokines, cytokine receptors and soluble intercellular adhesion molecule concentrations, and because different body fluid components were analyzed in the included studies, yielding very varying concentrations between studies. The standardized mean difference was calculated using a random effects model, which is more appropriate than a fixed-effect model when the true effect likely varies between studies due to heterogeneity not readily explained.
Cytokine parameters evaluated in only one study were not included in the quantitative analysis. Heterogeneity was investigated for combined measures by the chi-square test and inconsistency, indicating the impact of heterogeneity on the meta analysis, was calculated using the I-square value. Possible sources of significant heterogeneity were assessed and sensitivity analysis and subgroup analysis were performed where data permitted this.
Results of the data analysis were presented overall for neurocysticercosis patients versus control subjects, with subgroup results according to different body fluid component (Serum, PBMC and CSF), different measure method (ELISA or Flow Cytometry) or whether received anti parasite treatment (before treatment and after treatment) where applicable.
Results
Study selection
The search yielded 87 articles, of which 15 were included in the meta-analysis[4-18]. Figure 1 depicts the study selection process.
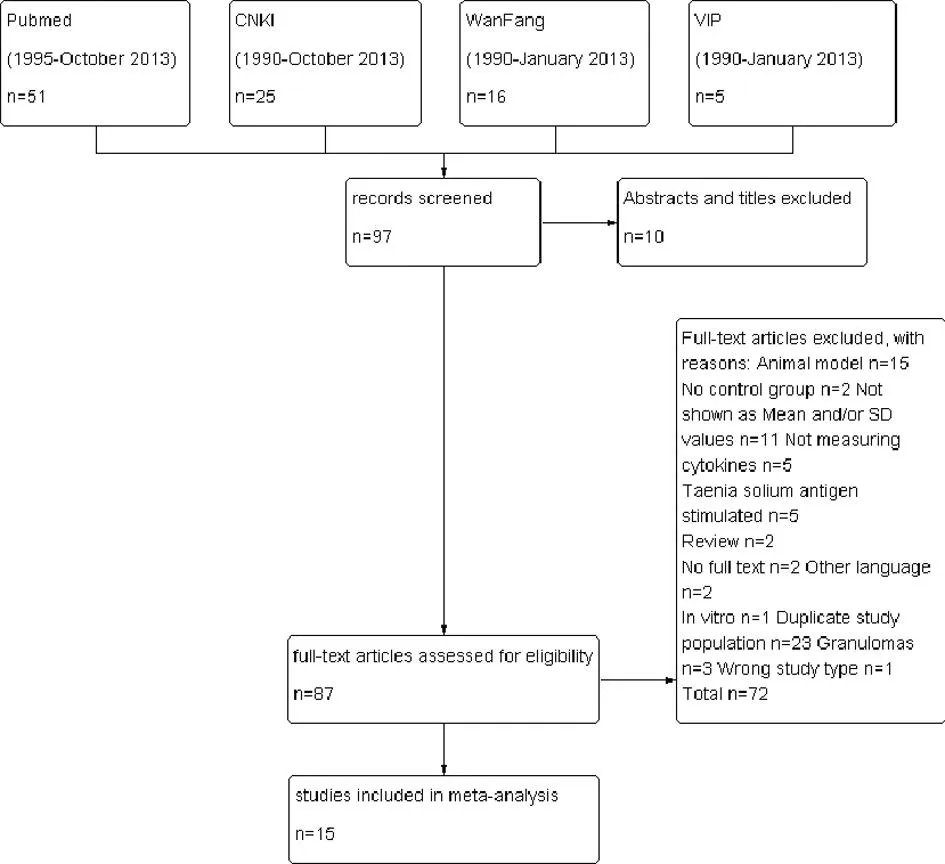
Fig.1Flowchart of literature search, study screening and selection process
In total 904 individuals (518 patients with neurocysticercosis) were included. Patients with neurocysticercosis and controls were comparable as to age and gender (Table 2). The neurocysticercosis was diagnosed by the physician according to clinical symptoms, CSF/serum diagnosis and image examination. The sample of control comes from really healthy population or other diseases of differential diagnosis from neurocysticercosis.

Tab.2 Cytokine expression in patients with neurocysticercosis compared with those of the control group
IFN-γ
IFN-γ was investigated in six studies comprising 261 neurocysticercosis patients and 225 controls (WangYing 2002, XuHongxiu2001, YanXiaobo 2004, Yehong2005, Zhang Weizhe 1997, Avantika Verma 2011). Concentrations of IFN-γ were significantly lower in neurocysticercosis patients compared with controls (SMD-19.13 [-29.84, -8.41],P=0.000 5) (Figure 2). Removing any component subgroup (CSF, Serum or PBMC) did not change the overall result.

CI: confidence interval.
IL-2
Five studies evaluated IL-2, involving 189 neurocysticercosis patients and 140 controls (WangYing2002, WenTingyuan2002, Yehong2005, Zhang Weizhe1997, and Zhoufen2002). neurocysticercosis patients had significantly lower concentrations of IL-2 compared with controls (SMD -4.60, CI: [-5.93, -3.26],P<0.000 01) (Figure 3). Removing the different body fluid subgroup (Serum or PBMC) did not change the overall result.

CI: confidence interval.
TNF-α
Levels of TNF-α were examined in eleven studies (YanXiaobo 2004, WangYing 2002, Yehong2005, Zhang Weizhe1997, Wangfeng2003, Sunxiaohong2006, liujunnian2000, Wangminghui2001, Chenchuan2000, Avantika Verma 2011, Wangxiaojuan2004) involving a total of 425 neurocysticercosis patients and 389 controls. Overall, concentrations of TNF-α were higher among neurocysticercosis patients compared with controls (SMD 0.49, CI: [0.22, 0.76],P=0.000 04) (Figure 4). Removing different body fluid component subgroup (CSF, Serum or PBMC) did not alter the overall result.

CI: confidence interval.
IL-4
Concentrations of IL-4 were evaluated among 118 neurocysticercosis patients and 125 controls in three studies (XuHongxiu2001, Yehong2005, and Avantika Verma 2011). In all, levels of IL-4 were significantly higher in neurocysticercosis patients compared with controls (SMD20.46, CI: [12.11, 28.81],P<0.000 01) (Figure 5). Removing the different body fluid subgroup (serum or PBMC) did not alter the overall result.

CI: confidence interval.
IL-10
Concentrations of IL-10 were evaluated among 118 neurocysticercosis patients and 125 controls in three studies (Avantika Verma 2011, XuHongxiu2001, and Yehong 2005). Over all, levels of IL-10 were significantly higher in neurocysticercosis patients compared with controls (SMD69.78, CI: [40.20, 99.36],P<0.000 01) (Figure 6). Removing any body fluid subgroup (serum or PBMC) did not alter the overall result.

CI: confidence interval.
sICAM-1
Levels of sICAM-1 were examined in two studies (Avantika Verma 2011, WangYing 2002) involving a total of 98 neurocysticercosis patients and 104 controls. The concentrations of sICAM-1 were higher among neurocysticercosis patients compared with controls (SMD 0.62, CI: 0.36-0.88,P<0.001) (Figure 7). As all data of sICAM-1 come from serum of subjects, no different body fluid subgroup could be compared in this group.
sIL-2R
sIL-2R was investigated in three studies comprising 196 neurocysticercosis patients and 146 controls (Liuruichun2006, WangYing 2002, and Zhoufen2002). Concentrations of sIL-2R were significantly higher in neurocysticercosis patients compared with controls (SMD 72.95, CI: [51.84, 94.07],P<0.000 01). Whereas, removal of the serum subgroup resulted in significantly lower sIL-2R (SMD -2.37, CI: [-2.78, -1.96],P<0.000 01), which was supported by only one study (Liuruichun2006) (Figure 8).

CI: confidence interval.

CI: confidence interval.
IL-6 and IL-8
There were no significant differences overall, for replicated evaluations of IL-6 and IL-8 (Table 2, Figure 9 & Figure 10). In the subgroup analysis, no overall result was changed when removing any body fluid component subgroup but for IL-6, where removal of the serum subgroup resulted in an overall significantly higher standardized mean difference, indicating higher levels among neurocysticercosis patients compared with control subjects in CSF. This, however, was based on one remaining study (Liuruichun2006).

CI: confidence interval.
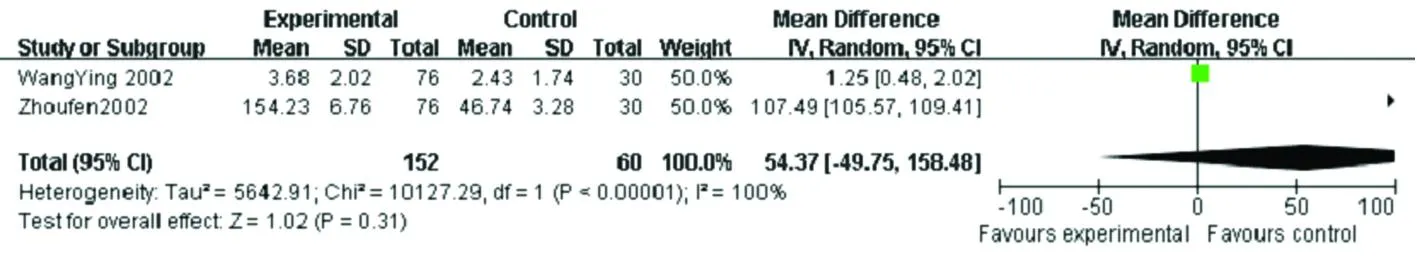
CI: confidence interval.
IL-5, IL-1β, IL-23 and IL-17
Concentrations of IL-5, IL-1β, IL-23 and IL-17 were only evaluated in one study respectively, thus not yielding replicated findings.
Correlation with clinical features
The influence of anti parasite treatment used in individual studies was evaluated where study numbers allowed this. Correlation between prevention of anti parasite treatment and cytokine parameter levels were reported in two studies for TNF-a, with no replication of positive findings. (WangYing 2002, Zhoufen 2002). There is no significant findings in TNF-α level in before-treatment and after treatment (SMD: -3.25, CI: [-8.72, 2.21],P=0.24) (Figure 11). As no enough data, correlation between clinical features (BBB disorder vs. BBB normal, epilepsy vs. non-epilepsy, symptomatic vs. asymptomatic and different clinic stages) and cytokine parameter levels could not be compared by meta analysis.

CI: confidence interval.
Fig.11Forest graph shows the meta-analysis of the TNF-α expression with the neurocysticercosis patients before treatment and after treatment
Heterogeneity and sensitivity analysis
Heterogeneity in the standardized mean difference was assessed for each cytokine for all replicated comparisons between neurocysticercosis patients and control subjects, showing signs of significant heterogeneity for all comparisons (Table 2).
In the sensitivity analysis, the influence of body fluid components used in individual studies (CSF, Serum or PBMC) was evaluated where study numbers allowed this. For the overall significant finding of IFN-γ, IL-2, TNF-α, IL-4, IL-10 and sICAM-1, removal of studies analyzing CSF, serum or PBMC samples had no impact on results. Of the studies with overall no significant findings in IL-8, results were not altered when removing serum studies analyzing. but for sIL-2R and IL-6, where the overall concentration of sIL-2R and IL-6 became significantly higher among neurocysticercosis patients compared with control subjects when removing the rest studies analyzing on serum.
In addition, sensitivity analysis was performed, where possible, for the impact of assay type used for cytokine analysis. For the comparisons with overall significant standardized mean differences between neurocysticercosis patients and control subjects, the result of IFN-γ level remained significant after removal of studies employing flow cytometric assays (Figure 12).

CI: confidence.
Discussion
This meta-analysis included 15 studies, totally 518 neurocysticercosis patients in various stage of infection and 386 control subjects. Of the 13 different cytokines, cytokine receptor and soluble inter-cellular adhesion molecule investigated, 9 were examined in multiple studies with overall significant findings for six cytokine parameters. Specifically, the meta-analysis demonstrated significantly elevated levels of TNF-α, IL-4, IL-10 and sICAM-1 in neurocysticercosis patients compared with controls. Overall depressed levels of IFN-γ and IL-2 in neurocysticercosis patients compared with control subjects were significant. There were no significant differences in levels of sIL-2R, IL-6 and IL-8 between neurocysticercosis patients and control subjects, while there were no replicated comparisons for IL-5, IL-1β, IL-23 and IL-17. The sensitivity analysis revealed no clear effect of assay type used for the analysis and no significant findings in TNF-α level in before-treatment and after treatment, but sIL-2R and IL-6 may have different expression characters in CSF and serum (Liuruichun 2006).
Primarily, Th1 and Th2 immune response were involved in the brain granulomas nearby TsM lesions. The brain granulomas in neurocysticercosis patients[19], naturally infected swine[20]and murine[21]were associated with a Th1 (IFN-γ and IL-18 )and Th2 profile(IL-4, IL-13, and IL-10). In CSF, Th2 cytokines IL-5, IL-6, IL-10 and proinflammatory cytokine TNF-α were elevated of inflammation and active nuerocysticercosis patients compared with non-inflammation or inactive of those[22-24]. As for reasons such as not expressed by mean SD, no control group nor whole text available, those articles were not include in meta analysis. Whereas, those results were partly consistent with our meta analysis result which indicates that pro-inflammation and Th2 cytokines were predominant in CSF of NCC immune, especially in symptomatic and inflammatory patients. But for expression of Th1 cytokines, such as IFN-γ in CSF, serum and PBMC (TsM antigen stimulated), the result was paradoxical. It is supposed that IFN-γ level was no significant difference in CSF of inflammation and non-inflammation neurocysticercosis patients and a little lower in CSF of active NCC and inactive NCC patients than those of controls[24-25]. But inTaeniasoliumcyst fluid antigens stimulated PBMC, IFN-γ level was significantly higher in symptomatic compared with asymptomatic neurocysticercosis patients[26]. In references included in our meta analysis,only one result support IFN-γ in elevated in serum of NCC patients(Avantika Verma 2011), but most studies showed that which was lower in central and peripheral(serum and no TsM antigen stimulated PBMC) of NCC group than those of controls(YanXiaobo 2004, XuHongxiu2001,WangYing 2002, Yehong2005, Zhang Weizhe1997). The above results imply that NCC has different immune characters in central and peripheral immune system. Th2 cytokines were predominant in NCC immune response, whereas the trend of Th1 cytokines still needs more researches.
While the interplay between the peripheral immune system and the central immune system is not clearly understood, there is some evidences shown that neurocysticercosis is a systemic condition. There is no influence of removing the PBMC subgroup on overall significant finding regarding IFN-γ, IL-2, TNF, IL-4 and IL-10, which implies that certainly cytokines’ character is identical in CNS and peripheral system of neurocysticercosis patients. However, the overall no significant finding regarding sIL-2R and IL-6 became significant after removing the PBMC subgroup. Although which was based on two comparisons in a single study (Liuruichun2006), the result still implies that certainly cytokines’ character is different in CNS and peripheral system of neurocysticercosis patients.
Several limitations in the present study need attention. First, the collection criteria of control were different which could influence cytokine parameter levels. Second, the collection criteria of neurocysticercosis patients was obscure, the inclusion of multi-stages patients could pose significant challenges. Third, the inclusion of studies employing various assay procedures and analyzing different body fluid components, could potentially introduce heterogeneity and bias results.
Our sensitivity analysis, demonstrated no significant impact of assay type on overall significant results. The present results should be interpreted with caution because of significant heterogeneity between studies and low study numbers for some cytokine measures. There are some measures to minimize heterogeneity within studies: strictly criterion of enrolled controls, maximize excluded out the disease impacted cytokine levels; matched pair samples in central and peripheral system; chosen sensitive and consistent laboratory methods, and assessing intra-individual alteration of immune responses.
Conclusion
In conclusion, this meta-analysis demonstrated decreased level of IFN-γ and IL-2, but elevated levels of TNF-α, IL-4, IL-10 and sICAM-1 in neurocysticercosis patients compared with controls, which implies disorder of Th1/Th2 and predominant of pro-inflammation cytokines were involved in the pathogenesis of the disease. Further researches on the role of cytokine played in central and peripheral immune system in neurocysticercosis are warranted.
References
[1]Singh AK, Prasad KN, Prasad A, et al. Immune responses to viable and degenerative metacestodes ofTaeniasoliumin naturally infected swine[J]. Int J Parasitol, 2013, 43(14): 1101-1107. DOI: 10.1016/j.ijpara.2013.07.009
[2]Kashyap B, Das S, Jain S, et al. Correlation between the clinico radiological heterogeneity and the immune-inflammatory profiles in pediatric patients withneurocysticercosis from a tertiary referral centre[J]. J Trop Pediatr, 2012, 58(4): 320-323. DOI: 10.1093/tropej/fmr093
[3]Alvarez JI, Krishnamurthy J, Teale JM. Doxycycline treatment decreases morbidity and mortality of murine neurocysticercosis: evidence for reduction of apoptosis and matrix metalloproteinase activity[J]. Am J Pathol, 2009, 175(2): 685-695. DOI: 10.2353/ajpath.2009.081073
[4]Yan XB, Yu CJ, Wang WZ. Study of the correlation between cytokines and BBB in serum and CSF of patients with cerebral cysticercosis[J]. Chin J Neuroimmunol Neurol, 2004, 11(5): 297-299. (in Chinese)
[5]Xu HX, Liu BY, Sun GP, et al. Investigation on five cytokines in serum of patients with neurocysticercosis[J]. Endem Dis Bull, 2001, 16(2): 14-15. (in Chinese)
[6]Wang Y, Zhang WZ, Li GQ, et al. Detections of adhesion molecule and cytokines in neurocystivercosis patients[J]. Acta Parasitol Med Entomol Sin, 2002, 9(4): 204-209. (in Chinese)
[7]Wen TY, Hu MY. Roles of unbalanced secretion of cytokine of Th cell in etiopathogenes is of cerebral cysticercosis[J]. Chin General Pract, 2002, 5(11): 877-878. (in Chinese)
[8]Ye H, Wang L. Detection Th1/Th2 cytokines in patients with neurocysticercosis[J]. Chin J Zoonoses, 2005, 21(4): 361-363.
[9]Zhang WZ, Xu ZJ, Zhao YY. Studies on the activity of IL-2 and IFN- γ induced in vitro from PBMC of patients with cerebral cysticercosis[J]. Acta Parasitol Med Entomol Sin, 1997, 4(4): 207-210.
[10]Wang F, Yang XP, Ding Y, et al. Study on cerebrospinal fluid nitric oxide, interleukin-1β and tumor necrosis factor α in patients with neurocysticercosis[J]. Henan J Pract Nervous Dis, 2003, 6(4): 24, 50. (in Chinese)
[11]Sun XH, Wang XL, Jiang J, et al. Concentration of NO and TNF-α in cerebrospinal fluid of patients with neurocysticercosis[J]. Chin J Zoonoses, 2006, 22(1): 83-84. (in Chinese)
[12] Wang XJ, Wu LK, Li CY. Expression of NO and TNF-α in cerebrospinal fluid of patients with neurocysticercosis[J]. Chin J Nerv Ment Dis, 2004, 30(4): 255-257. (in Chinese)
[13]Liu JN, Kong FY, Pan YY, et al. Expression of NO and TNF-α in CSF of patients with cerebral cysticercosis[J]. J Ningxia Med Coll, 2000, 22(1): 3-4. (in Chinese)
[14]Liu RC, Yan JL, Guo L. The detection of sIL-2R and IL-6 serum and CSF in patients with cerebral cysticercosis and the relations with epilepsy seizures[J]. J Brain Nervous Dis, 2006, 14(1): 13-15.
[15]Wang MH, Li SH, Qiao ZX. Clinical significance of detection of serum tumor necrosis factor-alpha in children with cerebral cysticercosis[J]. Chin J Parasitol Parasit Dis, 2001, 19(2): 124. (in Chinese)
[16]Chen C, Wei QD, Zhu JY. Level of nitric oxide and TNF-α in sera of patient with cerebral cysticercosis[J]. J Pract Parasit Dis, 2000, 8(1): 15-16. (in Chinese)
[17]Zhou F, Zi JW, Liu WB. Study on cellular immunity function in child patient with cerebral cysticercosis[J]. Chin J Parasitol Parasit Dis, 2002, 20(1): 61. (in Chinese)
[18]Verma A, Prasad KN, Cheekatla SS, et al. Immune response in symptomatic and asymptomatic neurocysticercosis[J]. Med Microbiol Immunol, 2011, 200(4): 255-261. DOI: 10.1007/s00430-011-0198-x
[19]Restrepo BI, Alvarez JI, Castano JA, et, al. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile[J]. Infect Immun, 2001, 69 (7): 4554-4560.
[20]Singh AK, Prasad KN, Prasad A, et, al. Immune responses to viable and degenerative metacestodes ofTaeniasoliumin naturally infected swine[J]. Int J Parasitol, 2013, 43(14): 1101-1107. DOI: 10.1016/j.ijpara.2013.07.009
[21]Robinson P, Atmar RL, Lewis DE, et al. Granuloma cytokines in murine cysticercosis[J]. Infect Immun, 1997, 65(7): 2925-2931.
[22]ChavarrIa A, Fleury A, GarcIa E, et al. Relationship between the clinical heterogeneity of neurocysticercosis and the immune-inflammatory profiles[J]. Clin Immunol, 2005, 116(3): 271-278.
[23]Aguilar-Rebolledo F, Cedillo-Rivera R, Llaguno-Violante P, et al. Interleukin levels in cerebrospinal fluid from children with neurocysticercosis[J]. Am J Trop Med Hyg, 2001, 64(1/2): 35-40.
[24]Rodrigues V Jr, de-Mello FA, Magalhaes EP, et al. Interleukin-5 and interleukin-10 are major cytokines in cerebrospinal fluid from patients with active neurocysticercosis[J]. Braz J Med Biol Res, 2000, 33(9): 1059-1063.
[25]Saenz B, Fleury A, Chavarria A, et al. Neurocysticercosis: local and systemic immune-inflammatory features related to severity[J]. Med Microbiol Immunol, 2012, 201(1): 73-80. DOI: 10.1007/s00430-011-0207-0
[26]Prasad A, Prasad KN, Gupta RK, et al. Increased expression of ICAM-1 among symptomatic neurocysticercosis[J]. J Neuroimmunol, 2009, 206(1/2): 118-120. DOI: 10.1016/j.jneuroim.2008.09.015
Received:2015-10-19;Revision accepted:2015-11-27
国家自然科学基金青年科学基金项目(No.81101269);首都医科大学省部级重点实验室开放研究课题基金(No.2013RDBF04)联合资助
DOI:10.3969/j.issn.1002-2694.2016.02.008
Corresponding author:Gu Jun-chao, Email: gujunchao668@163.com
中图分类号:R532.33
文献标识码:A
文章编号:1002-2694(2016)02-0137-11
通讯作者:谷俊朝, Email: gujunchao668@163.com
作者单位:首都医科大学附属北京友谊医院,北京热带医学研究所,热带病防治研究北京市重点实验室,北京100050
脑囊虫病患者细胞因子表达不平衡
——系统性回顾及Meta分析
陈小华,王非,邹洋,田小军,谷俊朝
摘要:脑囊虫病可能导致患者中枢和外周免疫系统的功能障碍,然而,在个别研究中细胞因子表达特征的结果是相互矛盾的。本研究目的是系统地分析囊尾蚴感染状态下脑囊虫病患者细胞因子表达特征研究结果,整合中枢和外周细胞因子变化的证据。我们进行了一项meta分析,针对脑囊虫病患者中枢及外周细胞因子浓度与对照组比较的研究。该研究纳入14个研究,共518例脑囊虫病和386个对照包括在内。总体而言,干扰素- γ (IFN-γ)的浓度(P值=0.000 5)和IL- 2(P<0.000 01)显著较低,但肿瘤坏死因子-α (TNF-α) (P<0.000 01),IL- 4(P=0.000 01),IL- 10(P<0.000 01)和sIL-2R(P<0.000 01)在脑囊尾蚴病的患者显著高于对照组。对IL- 6和IL- 8表达水平在囊虫患者和对照组之间没有显著差异。sIL -2R和IL - 6在脑囊虫病患者脑脊液水平显著降低,该结果仅由唯一的一项研究支持。这项分析提示TH1/TH2细胞因子表达失衡可能参与脑囊虫病患者发病机理。
关键词:细胞因子;脑囊虫病;Meta分析;炎症;生物标志物
Supported by the National Natural Science Foundation of China (No. 81101269), and the Capital Medical University Provincial Key Laboratory Opening Foundation (No. 2013RDBF04)

