西那卡塞对终末期肾病患者继发性甲状旁腺功能亢进影响的Meta分析
王喆,魏芳,陈海燕,姜埃利
西那卡塞对终末期肾病患者继发性甲状旁腺功能亢进影响的Meta分析
王喆,魏芳,陈海燕,姜埃利
目的评价西那卡塞治疗终末期肾病患者继发性甲状旁腺功能亢进的有效性和安全性。方法纳入拟钙剂治疗终末期肾病患者继发性甲状旁腺功能亢进的随机对照研究。计算机检索MEDLINE(1966.1—2014.9)、OVID(1963.1—2014.9)、中文万方数据库(1996.1—2014.9)、CNKI(1979.1—2014.9)、Cochrane图书馆临床对照试验资料库。手工检索已发表或未发表的相关文献,包括会议摘要等。由2名评价员独立对纳入的文献进行质量评价和数据提取,用RevMan5.2软件进行Meta分析。结果共纳入19项随机对照试验,共7 702例患者。Meta分析结果显示,西那卡塞与传统治疗方法相比,可以显著降低甲状旁腺素(WMD=-301.54µg/L,95%CI:-344.38~-258.7µg/L,P<0.05),降低血钙(WMD=-8.3 mg/L,95%CI:-9.1~-7.4 mg/L,P<0.05),降低血磷(WMD=-3.4 mg/L,95%CI:-4.6~-2.3 mg/L,P<0.05)。两组总体不良反应发生率相近(RR=1.03,95%CI:0.98~1.09,P>0.05)。西那卡塞组主要不良反应包括恶心(RR=2.05,95%CI:1.53~2.75,P<0.05),呕吐(RR=2.00,95%CI:1.78~2.23,P<0.05),腹泻(RR=1.15,95%CI:1.03~1.30,P<0.05),以及无症状的低钙血症(RR=7.60,95%CI:5.61~10.30,P<0.05),但均为短暂且不严重的不良反应。2组病死率相近(RR =0.97,95%CI:0.89~1.05,P>0.05)。结论西那卡塞抑制透析患者继发性甲状旁腺功能亢进,降低血钙和血磷,不增加病死率,但增加恶心、呕吐、腹泻和低钙血症的风险。
受体,钙敏感;肾透析;甲状旁腺功能亢进症,继发性;Meta分析;西那卡塞;拟钙剂;终末期肾病
继发性甲状旁腺功能亢进(SHPT)是终末期肾病(ESRD)患者严重的并发症,其发生机制与肾小球率过滤降低、尿磷排泄降低、活性维生素(Vit)D合成减少密切相关,是慢性肾脏病矿物质和骨代谢异常(CKD-MBD)的表现[1]。SHPT不仅导致骨代谢异常,也引起心血管钙化,使心血管疾病发病率和病死率增加。因此,需要控制CKD-MBD的相关指标,包括血清钙、磷和甲状旁腺素(PTH)水平。对ESRD合并SHPT患者的起始药物治疗通常包括磷结合剂、活性维生素D及其类似物。西那卡塞通常用于早期治疗效果不佳的SHPT患者,它是一种拟钙剂,可以活化甲状旁腺和其他组织中的细胞外钙敏感受体(CaSR),增加CaSR对细胞外钙离子的敏感性,参与维持钙离子的动态平衡,还可以通过活化CaSR直接抑制PTH的分泌和1,25(OH)2D的合成,降低血清钙和磷水平,有效控制SHPT和CKD-MBD[2],在治疗SHPT方面具有广泛的应用前景。本研究旨在系统性评价西那卡塞对ESRD合并SHPT患者的有效性和安全性。
1 资料与方法
1.1检索策略计算机检索MEDLINE(1966.1—2014.9)、OVID(1963.1—2014.9)、中文万方数据库(1996.1—2014.9)、CNKI(1979.1—2014.9)、Cochrane图书馆临床对照试验资料库,手工检索已发表或未发表的相关文献。中文检索词:西那卡塞、拟钙剂、终末期肾病、透析、继发性甲状旁腺功能亢进。英文检索词:cinacalcet,calcimimetic,end stage renal disease,dialysis,secondary hyperparathyroidism。中文文献检索方案:(西那卡塞OR拟钙剂)AND(终末期肾病OR透析)AND继发性甲状旁腺功能亢进。英文文献检索方案:(cinacalcet OR calcimimetic)AND(end-stage renal disease OR dialysis)AND secondary hyperparathroidism。
1.2文献筛选
1.2.1入选标准(1)研究类型:随机对照试验,无论是否采用盲法,纳入汉语和英语文献。(2)研究对象:慢性肾功能不全尿毒症期行规律血液透析或腹膜透析的患者,透析时间超过1个月,PTH>300 ng/L。(3)干预措施:西那卡塞组服用西那卡塞,对照组服用安慰剂或维生素D类似物(帕立骨化醇)。(4)结局测量指标:①治疗后血清PTH水平。②治疗后血清PTH达标率,达标范围以KDOQI指南为标准[3]。③治疗后血钙、血磷水平。④治疗相关的不良反应。⑤全因病死率。
1.2.2排除标准严重的肝脏、心脏、消化道疾病,合并其他严重的慢性疾病,严重感染,哺乳、妊娠,使用其他可能影响钙磷代谢的药物。重复研究及相关数据。
1.3资料提取及文献质量评价
1.3.1资料提取2位研究者独立阅读所获文献题目和摘要,在排除明显不符合纳入标准的试验后,对可能符合纳入标准的试验阅读全文,以确定是否符合纳入标准。2位研究者交叉核对纳入试验的结果,对有分歧而难以确定其是否纳入的试验通过讨论或由第3位研究者决定其是否纳入[4]。提取资料主要包括:(1)一般资料:题目、作者姓名、发表日期和文献来源。(2)研究特征:研究对象的一般情况、各组患者的基线可比性、干预措施。(3)结局测量指标:治疗后血清PTH、血钙、血磷水平,治疗后血清PTH达标率,不良反应发生率,全因病死率。
1.3.2质量评价纳入研究的方法学质量依据Cochrane评价手册[5],对随机对照试验的质量进行评估。包括6个方面:随机方法、分配隐藏、盲法、不完整资料偏倚、选择性报告结果、其他潜在影响真实性的因素。针对每个纳入研究,对上述6条分别作出低偏倚风险、高偏倚风险、不清楚3种评价[4]。
1.4统计学方法由2名评价员将西那卡塞组与对照组数据独立输入RevMan 5.2进行Meta分析。计量资料采用加权均数差(WMD)为疗效分析统计量,计数资料以危险比(risk ratio,RR)为疗效分析统计量。各效应量均以95%可信区间(CI)表示。各纳入研究结果间的异质性采用I2和Q检验[6]。当各研究间无统计学异质性(I250%,P 0.1),采用固定效应模型进行Meta分析;如各研究间有统计学异质性(I2>50%,P<0.1),分析其异质性来源,对可能导致异质性的因素进行亚组分析,若两个研究组之间存在统计学异质性而无临床异质性或差异无统计学意义时,采用随机效应模型进行分析。若异质性源于低质量研究,进行敏感性分析。如两组间异质性过大或无法找寻数据来源时,采用描述性分析[4]。
2 结果
2.1纳入研究的一般特征和质量评价根据检索词共检索到文献521篇。通过阅读题目和摘要,初筛获得27篇文献,进一步阅读全文,最终纳入19项研究[7-25],见图1。包括7 702例患者,其中西那卡塞组4 076例,对照组3 626例,见表1。依据Cochrane评价手册对随机对照试验的质量评价标准进行评估,8项研究[13-14,16,18-21,25](42.1%)报告了随机序列的产生,6项研究[10,14-16,18,24](31.6%)报告了分配隐藏,13项研究[7-16,18-19,21](68.4%)中实施者和参与者均双盲,2项研究[15,19](10.5%)在结局评估中使用盲法,9项研究[8,10,13,15-16,20-21,23,25](47.4%)报告了不完整结局数据,9项研究[8,12-13,15,17,19,21,23-24](47.4%)无明显的发表偏倚,所有研究均不能除外其他偏倚。
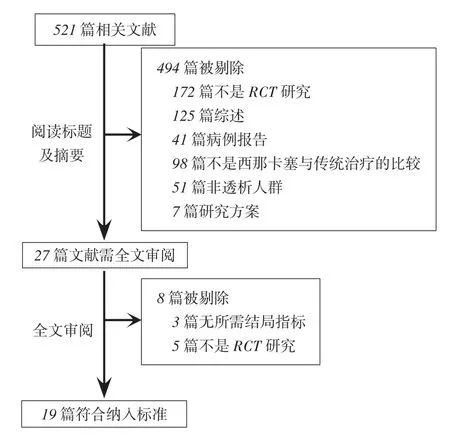
Fig. 1 Flow chart of literature screeningfor the meta-analysis图1 文献筛选流程图
2.2 Meta分析结果
2.2.1疗效指标(1)对PTH的影响:共有9项研究[9-10,12-14,16-17,19-20]比较了西那卡塞和对照组对PTH的影响,其中西那卡塞组1 479例,对照组1 084例。各研究间有统计学异质性(χ2=14.09,P=0.08,I2=43%),采用随机效应模型进行效应量的合并。Meta分析结果显示西那卡塞组PTH低于对照组(WMD=-301.54µg/L,95%CI:-344.38~-258.7µg/L,P<0.05),见图2。11项研究[9-10,12,14,16-17,20-21,23-25](包括2 879例患者)比较了西那卡塞组和对照组PTH达标率。Meta分析结果显示西那卡塞组PTH达标率明显高于对照组(RR=2.51,95%CI:1.52~4.14,P<0.05),见图3。(2)对血钙、血磷水平、Ca×P的影响:10项研究[10,12-14,16-19,21,23](包括2 683例患者)分析了西那卡塞和对照组对血钙、血磷水平的影响,结果显示西那卡塞组血钙和血磷水平均低于对照组(血钙:WMD=-8.3 mg/L,95%CI:-9.1~-7.4 mg/L,P<0.05;血磷:WMD=-3.4 mg/L,95%CI -4.6~-2.3 mg/L,P<0.05)。9项研究[10,12,14,16,17-19,21,23](包括2 273例患者)比较了西那卡塞和对照组对Ca×P的影响,结果显示西那卡塞组Ca×P水平低于对照组(WMD=-806 mg2/L2,95%CI:-925~-687 mg2/L2,P<0.05),其中Malluche等[18]中的对照组包含VitD,其他研究的对照组均为安慰剂,剔除该研究后分析结果无明显变化。
2.2.2安全性指标(1)总体不良反应发生率的比较:10项研究[12,14,15,17-21,24-25](包括6 413例患者)报道了治疗相关的不良反应。2组总体不良反应发生率差异无统计学意义(RR=1.03,95%CI:0.98~1.09,P>0.05),见图4。但西那卡塞组的一些不良反应发生率高于对照组,包括恶心、呕吐、腹泻和无症状的低钙血症,见表2。其他不良反应包括呼吸困难、头痛、腹痛、上呼吸道感染、低血压、消化不良、瘙痒、鼻咽炎、肌肉痉挛、发热等,但2组发病率均低,且考虑和药物治疗无明显相关性。(2)全因病死率比较:14项研究[7-9,12,14-15,17-23,25](包括6 834例患者)比较了西那卡塞和对照组全因病死率。分析显示,西那卡塞组与对照组全因病死率差异无统计学意义(RR= 0.97,95%CI:0.89~1.05,P>0.05),见图5。
2.2.3偏倚评估纳入文献在各组间偏倚漏斗图提示大致对称,无明显发表偏倚,见图6。
3 讨论
SHPT是ESRD患者的常见并发症,其机制与钙、磷和1,25(OH)2D代谢紊乱,维生素D受体(VDR)、CaSR、klotho表达减少,PTH mRNA降解减少等因素相关,这些因素共同促进了PTH合成、释放增加,并诱发甲状旁腺细胞增生、肥大,甚至导致PTH自主性分泌[26];超生理剂量PTH使内皮细胞表达炎症因子白细胞介素-6和终末糖基化产物的受体增加,因而促进动脉斑块形成和血管钙化[27],增加心血管死亡和全因死亡风险[28]。拟钙剂西那卡塞可以降低PTH,同时降低血钙和血磷,减轻血管和心脏瓣膜钙化[22]。MBD-5D研究提出,西那卡塞联合维生素D受体激活剂(VDRA)可有效降低PTH,使血钙和血磷水平达标,这种联合治疗方案优于VDRA的单一治疗[29]。
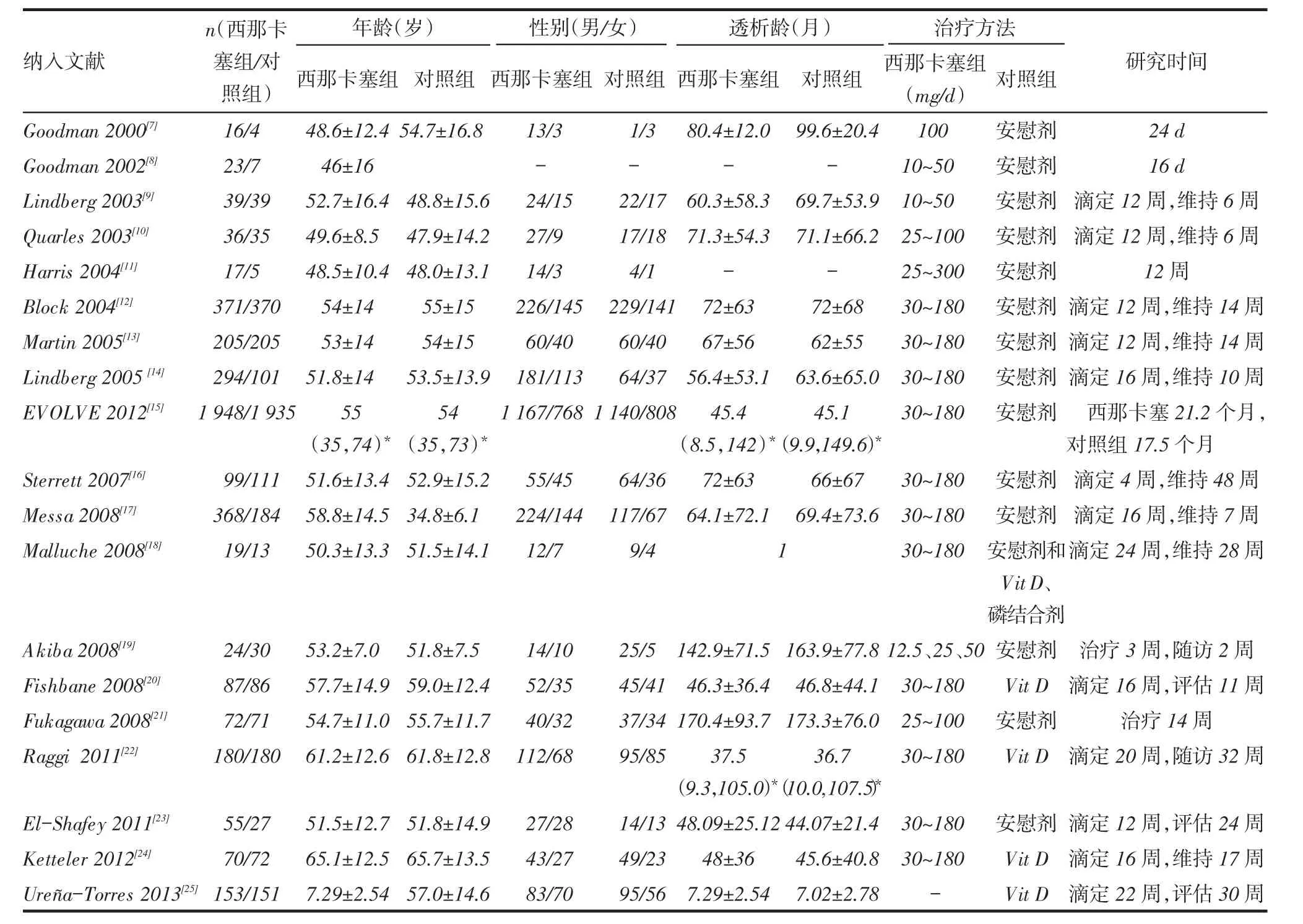
Tab.1 Characteristics of included literature表1 纳入文献情况

Fig. 2 Comparison of PTH levels between cinacalcet group and control group图2 西那卡塞和对照组对PTH作用比较
CaSR存在于甲状旁腺、肾脏以及骨骼中,其作用主要是增强对血Ca2+水平变化的感知并产生相应的反应,从而维持血中Ca2+水平的相对稳定[30]。高Ca2+可以通过活化CaSR直接抑制PTH的分泌以及1,25(OH)2D的合成,通过减少PTH的分泌间接减少1,25(OH)2D的合成并刺激降钙素的分泌,减少破骨细胞的形成和骨质吸收的反应,使血Ca2+向骨内转移,减少肾皮质髓袢升支粗段和远曲小管对Ca2+的重吸收,最终可使Ca2+水平恢复正常[31]。拟钙剂是G-蛋白偶联受体的变构激活剂,它可以活化甲状旁腺和其他组织中的细胞外CaSR,参与维持钙离子的动态平衡,有效控制SHPT[14]。
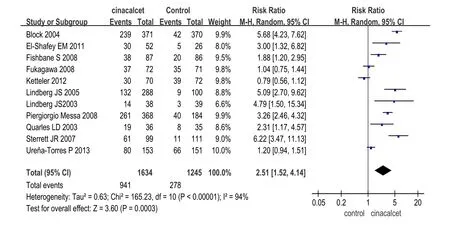
Fig. 3 Comparison of qualified rate of PTH between cinacalcet group and control group图3 西那卡塞和对照组PTH达标率比较
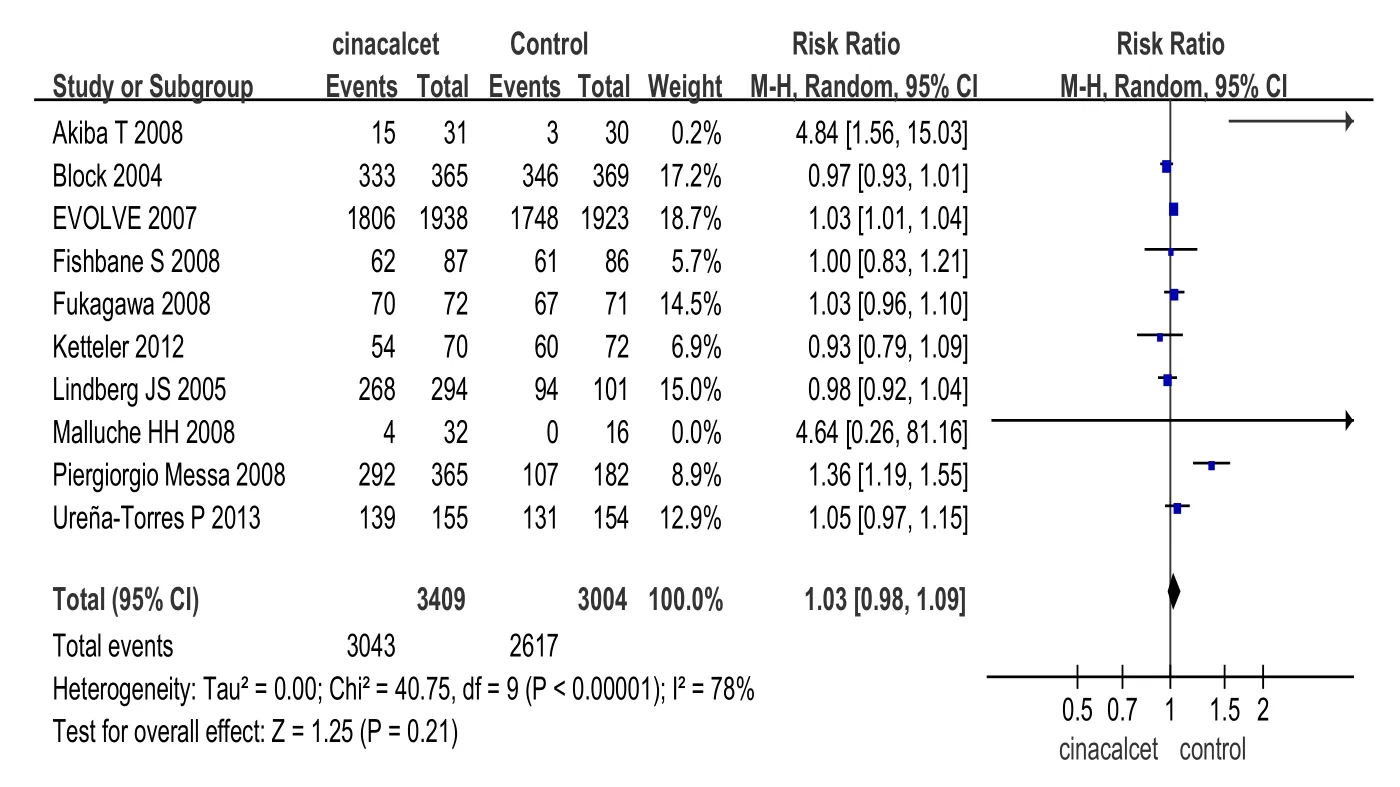
Fig. 4 Comparison of all adverse events between cinacalcet group and control group图4 西那卡塞和对照组总体不良反应发生率比较
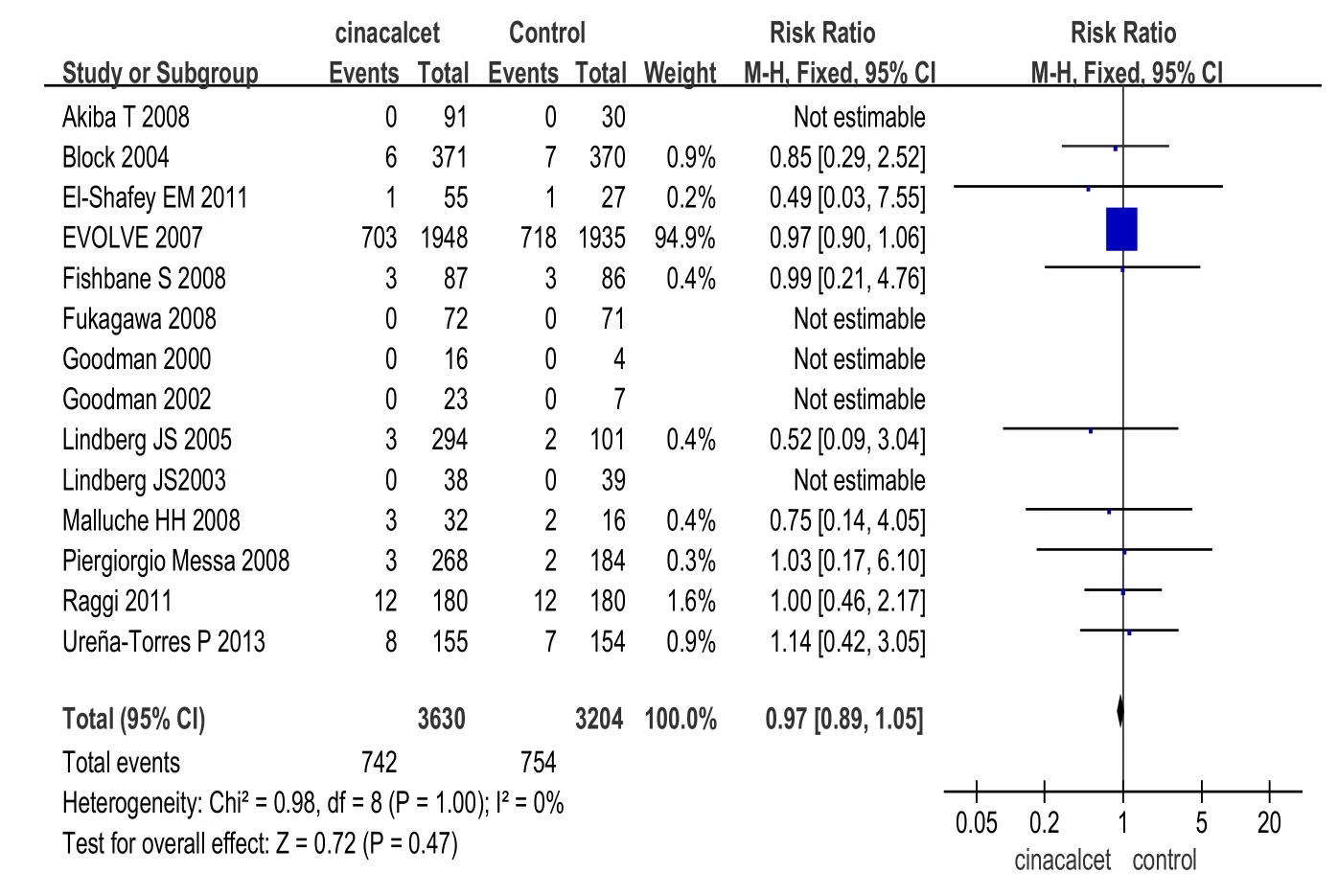
Fig. 5 Comparison of all-cause mortality between cinacalcet group and control group图5 西那卡塞和对照组全因病死率比较
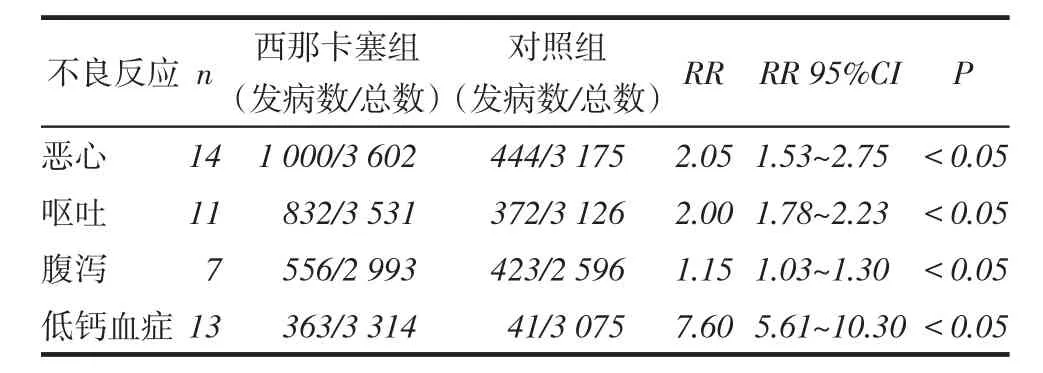
Tab. 2 Comparison of incidence of adverse reactions between cinacalcet group and control group表2 西那卡塞组和对照组不良反应发生率比较
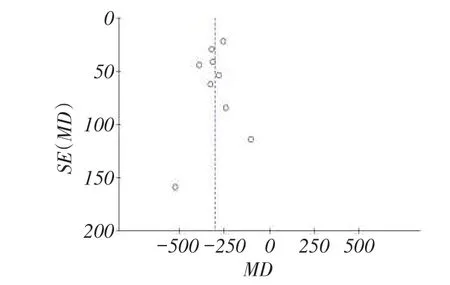
Fig. 6 Funnel graph for the assessment of potential publication bias in PTH图6 文献发表偏倚漏斗图
低钙血症、高磷血症、肾脏合成1,25(OH)2D减少长期刺激甲状旁腺,使PTH分泌增加,导致甲状旁腺细胞增殖。Gogusev等[32]观察发现,CaSR的表达可以下调CKD患者增生的甲状旁腺的mRNA和蛋白水平,提示CaSR受体和甲状旁腺增生的关系。Mendoza等[33]指出,拟钙剂可以使尿毒症小鼠甲状旁腺CaSR mRNA和VDR mRNA升高,从而抑制细胞增殖,这一发现支持拟钙剂对甲状旁腺细胞CaSR和VDR表达的直接作用。应用西那卡塞可以使甲状旁腺切除率降低一半以上[15]。西那卡塞联合维生素D或磷结合剂可以有效控制SHPT,使血钙和血磷水平达标[23,25]。本文通过Meta分析证实西那卡塞可以有效降低透析患者PTH水平,同时降低血钙和血磷,提高患者PTH达标率。
心血管疾病是CKD患者死亡的主要原因,其风险比普通人群高20~30倍[34]。血管钙化已成为CKD患者心血管事件增加的独立危险因素[35]。Brandenburg等[36]发现,血液透析患者中65%存在冠状动脉钙化,40%存在主动脉瓣钙化。ADVANCE研究首次证实以西那卡塞为基础的SHPT治疗方案可以降低冠脉钙化积分,减少瓣膜局部钙化[29]。尽管本研究未探讨西那卡塞对心血管系统钙化的影响,但研究发现,西那卡塞组全因病死率低于对照组。
本研究发现,西那卡塞抑制PTH分泌,降低血钙、血磷,不增加病死率,增加恶心、呕吐、腹泻、低钙血症的发生率,但是这些不良反应并不严重,并且持续时间短暂。西那卡塞对胃肠道功能紊乱的作用机制还不清楚,需要进一步研究。
尽管本研究通过Meta分析证实了西那卡塞对SHPT的作用,推荐其作为ESRD伴SHPT患者的治疗方法。但是,本研究也存在一些局限性。首先,纳入研究的观察时间较短,不足以评价西那卡塞对SHPT和心血管系统的长期影响。另外,纳入研究的异质性也会影响Meta分析的结果,异质性可能来源于各检测指标的基线水平、药物剂量、给药方式和检测方法。
总之,西那卡塞为ESRD合并SHPT患者的治疗开辟了新的途径,为重度甲状旁腺功能亢进的患者提供了非手术治疗的机会。但是,其临床应用时间较短,尤其在我国临床应用尚处于初级阶段,需要通过实践累积更多的数据和经验进一步评价其有效性和安全性。
[1]Kidney Disease:Improving Global Outcomes(KDIGO)CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis,evaluation,prevention,and treatment of Chronic Kidney Disease-Mineral and Bone Disorder(CKD-MBD)[J]. Kidney Int,2009,(113):S1-130. doi:10.1038/ki.2009.188.
[2]Brown EM. Clinical lessons from the calcium-sensing receptor[J]. Nat Clin Pract Endocrinol Metab,2007,3(2):122- 133. doi:10.1038/ncpendmet0388.
[3]Eknoyan G,Levin A,Levin N. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease[J]. Am JKidney Dis,2003,,42(Suppl 3):S1-201.
[4]Wang Z,Wei F,Chen HY,et al. Meta analysis on effect of parathyroid hormone elimination through hemoperfusion and hemodiafiltration[J]. Tianjin Med J,2015,43(6):684-689.[王喆,魏芳,陈海燕,等.血液灌流和血液透析滤过对甲状旁腺素清除效果的Meta分析[J].天津医药,2015,43(6):684-689]. doi:10.11958/j. issn.0253-9896. 2015.06.029.
[5]Higgins JP,Green S(editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0[updated March 2011]. The Cochrane Collaboration 2011.Available from www.cochrane-handbook.org. doi:10.1002/14651858.CD008223. pub2.
[6]Higgins JP,Thompson SG,Deeks JJ,et al. Measuring inconsistency in meta-analysis[J]. BMJ,2003,327(7417):557-560.
[7]Goodman WG,Frazao JM,Goodkin DA,et al. A calcimimetic agent lowers plasma parathyroid hormone levels in patients with secondary hyperparathyroidism[J]. Kidney International,2000,58(1):436-445.
[8]Goodman WG,Hladik GA,Turner SA,et al. The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism[J]. J Am Soc Nephrol,2002,13(4):1017-1024.
[9]Lingdberg JS,Moe SM,Goodman WG,et al. The calcimimetic AMG073 reduces parathyroid hormone and calcium×phosphorus in secondary hyperparathyroidism[J]. Kidney Int,2003,63(1):248-254.
[10]Quarles LD,Sherrard DJ,Adler S,et al. The calcimimetic AMG 073 as apotential treatment for secondary hyperparathyroidism of end-stage renal disease[J]. JAm Soc Nephrol,2003,14(3):575-583.
[11]Harris RZ,Padhi D,Marbury TC,et al. Pharmacokinetics,pharmacodynamics,and safety of cinacalcet hydrochloride in hemodialysis patients at doses up to 200 mgonce daily[J]. Am JKidney Dis,2004,44(6):1070-1076.
[12]Block GA,Martin KJ,de Francisco AL,et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis[J]. N Engl J Med,2004,350(15):1516-1525. doi:10.1056/NEJMoa031633.
[13]Martin KJ,Juppner H,Sherrard DJ,et al. First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl[J]. Kidney Int,2005,68(3):1236-1243. doi:10.1111/j.1523-1755.2005.00517.x.
[14]Lindberg JS,Culleton B,Wong G,et al. Cinacalcet HCl,an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis:a randomized,doubleblind,multicenter study[J]. J Am Soc Nephrol,2005,16(3):800-807. doi:10.1681/ASN.2004060512.
[15]The EVOLVE Trial Investigators. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis[J]. N Engl J Med,2012,367(26):2482-2494. doi:10.1056/NEJMoa1205624.
[16]Sterrett JR,Strom J,Stummvoll HK,et al. Cinacalcet HCI(Sensipar/ Mimpara)is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism[J]. Clin Nephrol,2007,68(1):10-17.
[17]Messa P,Maca´rio F,Yaqoob M,et al. The OPTIMA study:assessing a new cinacalcet(sensipar/mimpara)treatment algorithm for secondary hyperparathyroidism[J]. Clin J Am Soc Nephrol,2008,3 (1):36-45. doi:10.2215/CJN.03591006.
[18]Malluche HH,Monier-Faugere MC,Wang G,et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism[J]. Clinical Nephrology,2008,69 (4):269-277. doi:10.5414/ CNP69269.
[19]Akiba T,Akizawa T,Tsukamoto Y,et al. Dose determination of cinacalcet hydrochloride in Japanese hemodialysis patients with secondary hyperparathyroidism[J]. Ther Apher Dial,2008,12(2):117-125. doi:10.1111/j.1744 -9987.2008.00556.x.
[20]Fishbane S,Shapiro WB,Corry DB,et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone:the ACHIEVE study results[J]. Clin J Am Soc Nephrol,2008,3 (6):1718-1725. doi:10.2215/CJN.01040308.
[21]Fukagawa M,Yumita S,Akizawa T,et al. Cinacalcet(KRN1493)effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients[J]. Nephrol Dial Transplant,2008,23(1):328-335.
[22]Raggi P,Chertow GM,Torres PU,et al. The ADVANCE study:a randomizedstudytoevaluate the effects ofcinacalcetplus low-dose vitamin Don vascular calcification in patients on hemodialysis[J]. Nephrol Dial Transplant,2011,26(4):1327-1339.doi:10.1093/ndt/gfq725.
[23]El-Shafey EM,Alsahow AE,Alsaran K,et al. Cinacalcet hydrochloride therapy for secondary hyperparathyroidism in hemodialysis patients[J]. Ther Apher Dial,2011,15(6):547-555.
[24]Ketteler M,Martin KJ,Wolf M,et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis:results of the IMPACT SHPT study[J]. Nephrol Dial Transplant,2012,27 (8):3270-3278. doi:10.1093/ndt/gfs018.
[25]Ureña-Torres P,Bridges I,Christiano C,et al. Efficacy of cinacalcet with low- dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism[J]. Nephrol Dial Transplant,2013,28(5):1241-1254. doi:10.1093/ndt/gfs568.
[26]Lin S,Jia JY. Relationship between secondary hyperparathyroidism and cardiovascular calcification in chronic kidney disease patients and its clinical implication[J]. Chin JKidney Dis Invest(Electronic Edition),2013,2(2):76-79.[林珊,贾俊亚.慢性肾脏病患者继发性甲状旁腺功能亢进与心血管钙化的联系及意义[J].中华肾病研究电子杂志,2013,2(2):76-79]. doi:10.3877/cma.j. issn.2095-3216.2013.02.005.
[27]Rashid G,Bernheim J,Green J,et al. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways[J]. Am JPhysiol Renal Physiol,2007,292 (4):F1215-1218. doi:10.1152/ajprenal.00406.2006.
[28]Jean G,Bresson E,Lorriaux C,et al. Increased levels of serum parathyroid hormone and fibroblast growth factor-23 are the main factors associated with the progression of vascular calcification in long-hour hemodialysis patients[J]. Nephron Clin Pract,2012,120 (3):c132-c138. doi:10.1159/000334424.
[29]Fukagawa M,Fukuma S,Onishi Y,et al. Prescription patterns and mineral metabolism abnormalities in the cinacalcet era:results from the MBD-5D study[J]. Clin J Am Soc Nephrol,2012,7(9):1473-1480. doi:10.2215/CJN.13081211.
[30]Brown EM,Gamba G,Riccardi D,et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid[J]. Nature,1993,366(6455):575-580.
[31]Yan Y. Clinical applications and research progress of cinacalcet [J]. Chinese Journal of Blood Purification,2012,11(8):460-463.[燕宇.西那卡塞的临床应用以及研究进展[J].中国血液净化,2012,11(8):460-463].
[32]Gogusev J,Duchambon P,Hory B,etal.Depressedexpressionofcalcium receptorinparathyroidglandtissue ofpatients withhyperparathyroidism [J].Kidney Int,1997,51(1):328-336.doi:10.1038/ki.1997.41.
[33]Mendoza FJ,Lopez I,Canalejo R,et al. Direct upregulation of parathyroid calcium-sensing receptor and vitamin D receptor by calcimimetics in uremic rats[J]. Am J Physiol Renal Physiol,2009,296 (3):F605-613. doi:10.1152/ajprenal.90272.2008.
[34]Noordzij M,Cranenburg EM,Engelsman LF,et al. Progression of aortic calcification is associated with disorders of mineral metabolism and mortality in chronic dialysis patients[J]. Nephrol Dial Transplant,2011,26(5):1662-1669. doi:10.1093/ndt/gfq582.
[35]Chen NX,Moe SM. Vascular calcification:pathophysiology and risk factors[J]. Curr Hypertens Rep,2012,14(3):228-237.
[36]Brandenburg VM,Kramann R,Koos R,et al. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients:across-sectional study[J]. BMC Nephrol,2013,14:219-228.
(2015-07-24收稿2015-11-10修回)
(本文编辑魏杰)
Efficacy of cinacalcet for end-stage renal disease patients with secondary hyperparathyroidism:a Meta-analysis
WANG Zhe,WEI Fang,CHEN Haiyan,JIANG Aili
The Second Hospital of Tianjin Medical University,Tianjin 300211,China
Objective To evaluate the efficacy and safety of cinacalcet on secondary hyperparathyroidism(SHPT)in patients with end-stage renal disease(ESRD). Methods Patients with ESRD and SHPT for the treatment with calcimimeticagents were included in this study. MEDLINE(1996.1- 2014.9),OVID(1963.1- 2014.9),Chinese Wanfang database (1996.1- 2014.9),CNKI(1996.1- 2014.9)and the clinical control test database of Cochrane Library were searched.Related literature,including published or unpublished papers,and meeting procedding were hand- searched. Quality assessment and data extraction were conducted by two independent investigators. Meta-analysis was conducted by RevMan 5.2. Results Nineteen randomized controlled trials involving 7 702 patients were included. The meta-analysis showed that compared with conventional therapy,cinacalcet can significantly decrease serum parathyroid hormone in dialysis patients[WMD=-301.54µg/L,95%CI:(-344.38)-(-258.7)µg/L,P<0.05],decrease serum level of calcium[WMD=-8.3 mg/L,95%CI:(-9.1)-(-7.4)mg/L,P<0.05],and decrease serum level of phosphorus[WMD=-3.4 mg/L,95%CI:(-4.6)-(-2.3)mg/L,P<0.05]. The total incidence of adverse events was similar(RR=1.03,95%CI:0.98-1.09,P>0.05). Cinacalcet increased nausea(RR =2.05,95%CI:1.53-2.75,P<0.05),vomiting(RR =2.00,95%CI:1.78-2.23,P<0.05),diarrhea (RR =1.15,95%CI:1.03-1.30,P<0.05),and asymptomatic hypocalcaemia(RR =7.60,95%CI:5.61-10.30,P<0.05),but they were usually transient,and mild to moderate in severity. The mortality was similar(RR =0.97,95%CI:0.89-1.05,P>0.05). Conclusion Results confirm that cinacalcet suppresses parathyroid hormone and decreases calcium and phosphorus in secondary hyperparathyroidism patients receiving dialysis. Cinacalcet increases risks of nausea,vomiting,diarrhea and hypocalcaemia,without increasingmortality.
receptors,calcium-sensing;renal dialysis;hyperparathyroidism,secondary;Meta-analysis;cinacalcet;calcimimetic;end-stage renal disease
R582.1,R692
A
10.11958/20150067
天津医科大学第二医院肾脏病血液净化科(邮编300211)
王喆(1983),女,主治医师,博士在读,主要从事肾脏病血液净化研究

