瑞芬太尼诱发大鼠切口痛觉过敏时脊髓CXCL10及其配体CXCR3的表达变化及意义
雷鹏飞, 高 杉, 薛然荣, 王慧玲
河南省新乡市中心医院麻醉科,新乡 453000
瑞芬太尼诱发大鼠切口痛觉过敏时脊髓CXCL10及其配体CXCR3的表达变化及意义
雷鹏飞,高杉△,薛然荣,王慧玲
河南省新乡市中心医院麻醉科,新乡453000
摘要:目的探讨瑞芬太尼诱发大鼠切口痛觉过敏时脊髓CXC类趋化因子10(CXCL10)及其配体CXC趋化因子受体3(CXCR3)表达变化及意义。方法30只健康雌性SD大鼠随机分为假手术组、切口痛模型组、瑞芬太尼+切口痛模型组,每组10只。制备切口痛模型,瑞芬太尼+切口痛模型组以1 μg/(kg·min)速率从尾静脉输注瑞芬太尼60 min,假手术组和切口痛组以同样的速率输注生理盐水。分别于建模前12 h(T0)、输注瑞芬太尼4 h(T1)、12 h(T2)、24 h(T3)和48 h(T4),测定大鼠机械缩足反应阈(PWT)和热缩足潜伏期(TWL),于T4时测定完PWT和TWL后,处死动物并取骨髓,利用实时荧光定量PCR测定大鼠脊髓组织中CXCL10和CXCR3基因表达,Western blot法检测大鼠脊髓组织中CXCL10和CXCR3蛋白表达。免疫组织荧光染色检测脊髓组织中小胶质细胞标志物Iba1的表达。结果与T0时相比,切口痛模型组和瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均降低(均P<0.05);瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均低于切口痛模型组和假手术组,差异均有统计学意义(均P<0.05);与假手术组相比,切口痛模型组和瑞芬太尼+切口痛模型组大鼠脊髓组织中CXCL10和CXCR3基因和蛋白表达量均升高,差异均有统计学意义(均P<0.05),与切口痛模型组相比,瑞芬太尼+切口痛模型组大鼠脊髓组织中CXCL10和CXCR3基因和蛋白表达量均升高,差异均有统计学意义(均P<0.05)。免疫荧光染色结果显示,与假手术组和切口痛模型组相比,瑞芬太尼+切口痛模型组Ibal表达量显著增加,小胶质细胞体积增大,分支增多。结论瑞芬太尼诱发大鼠切口痛觉过敏时脊髓中CXCL10及其配体CXCR3表达水平升高,可能与CXCL10作用于神经元表面CXCR3而间接诱导小胶质细胞活化有关。
关键词:瑞芬太尼;痛觉过敏;CXC类趋化因子10;CXC趋化因子受体3
瑞芬太尼作为临床上常用的芬太尼类μ型阿片受体激动剂,具有起效快、持续时间短等特点,常用于全麻诱导及维持镇痛[1]。但在全麻术后常诱发痛觉过敏,使术后疼痛出现较早且需镇痛药剂量增加[2]。目前,瑞芬太尼诱发痛觉过敏有关机制尚不清楚。研究表明[3],痛觉过敏的发生与神经系统小胶质或星形胶质细胞异常活化有关。近年来研究发现[4],趋化因子异常表达是痛觉过敏发生的必要因素。CXC类趋化因子10(CXC chemokine 10,CXCL10)在中枢神经系统主要表达于胶质细胞,有研究指出[5],正常大鼠鞘内注射CXCL10蛋白可诱发痛觉过敏。CXC趋化因子受体3(CXC chemokine receptor 3,CXCR3)亦可表达于神经胶质细胞,CXCL10/CXCR3通路活化可诱导痛觉中枢敏化[6]。本研究通过分析瑞芬太尼诱发大鼠切口痛觉过敏时脊髓中CXCL10及其配体CXCR3表达的变化,探讨瑞芬太尼诱发大鼠切口痛觉过敏的可能机制,以期为临床防治提供基础资料。
1材料与方法
1.1实验动物及分组
健康雌性SD大鼠30只,由河南省实验动物中心提供,2~3月龄,体重250~270 g,饲养于标准环境下,温度24℃(上下不超过2℃),相对湿度50%~60%,自由饮水、饮食。利用随机数字表将大鼠随机分为3组:假手术组、切口痛模型组、瑞芬太尼+切口痛模型组,每组10只。
1.2方法
1.2.1实验动物处理及切口痛模型制备所有大鼠均于腹腔注射戊巴比妥钠40 mg/kg麻醉后,将尾部鳞片刮除,用24号静脉留置针从鼠尾中上1/3穿刺,成功置管后退出针芯,将留置针固定后,用含肝素生理盐水进行冲洗。
参照文献[7]方法制备大鼠切口痛模型:对右后足进行消毒后,从足底近端0.5 cm处开始用11号刀片向趾部做1 cm长纵行切口,皮肤切开后,将足底肌肉挑起并纵行切割到骨膜,并使肌肉起止及附着保持完整。进行按压止血,对皮肤进行缝合。术后对切口进行消毒,涂抹少量红霉素软膏以防止感染。假手术组大鼠仅于麻醉后穿刺固定留置针。大鼠切口痛模型制备的同时,瑞芬太尼+切口痛模型组以1 μg/(kg·min)速率从尾静脉输注瑞芬太尼(批号:100501,宜昌人福药业),输注60 min,假手术组和切口痛组以同样的速率输注生理盐水。
1.2.2机械缩足反应阈(PWT)和热缩足潜伏期(TWL)测定分别于建模前12 h(T0)、输注瑞芬太尼4 h(T1)、12 h(T2)、24 h(T3)和48 h(T4),利用美国Harvard Apparatus公司生产的电子Von Frey纤维丝测定PWT。大鼠置于专用金属笼中10 min,在鼠右后足2和3趾骨间用Von Frey纤维丝垂直施压,当大鼠出现嘶叫、快速缩足或舔舐右足任一反应时,记录压力值,连续进行5次,每次间隔15 min,取均值。利用上海精密仪器仪表有限公司生产的YLS-6 B智能热板仪测定TWL,将大鼠右后足接触热板,当大鼠出现嘶叫、快速缩足或舔舐右足任一反应时,记录时间,连续进行5次,每次间隔15 min,取均值。
1.2.3实时荧光定量PCR测定大鼠脊髓组织中CXCL10和CXCR3基因表达于T4时测定完PWT和TWL后,将大鼠麻醉后处死,取L4-5节段脊髓,研磨后,用Trizol总RNA提取试剂盒(购自美国Invitrogen公司)对总RNA进行提取,用紫外分光光度计(购自北京普析通用仪器有限责任公司)对RNA纯度进行测定,取A260/A280≥1.80标本完成后续实验。用逆转录试剂盒(购自美国ABI公司)进行逆转录为cDNA,用PCR试剂盒[购自宝生物工程(大连)有限公司]完成PCR。CXCL10、CXCR3及内参引物均由上海生工公司设计合成。CXCL10引物:上游5′-GGGCCATAGGAAAACTTGAAATC-3′,下游5′-CATTGTGGCAATGATC-TCAACAT-3′;CXCR3引物:上游5′-TCGGCTCTGGTCTCTGCAA-3′,下游5′-GCTTATACAGGCCAGCAGGAA-3′;GAPDH引物:上游5′-GACA-GTCAGCCGCATCTTCT-3′,下游5′-GCGCCCA-ATACGACCAAATC-3′。PCR反应条件:94℃ 1 min,94℃ 30 s,56℃ 15 s,74℃ 15 s,连续进行36次循环。用2-ΔΔCt法计算脊髓组织中CXCL10和CXCR3基因相对表达量。
1.2.4Western blot法检测大鼠脊髓组织中CXCL10和CXCR3蛋白表达取麻醉处死大鼠L5-6节段脊髓,加入蛋白裂解液,进行研磨,于4℃下3 500 r/min离心10 min后,取上清,用总蛋白测定试剂盒对总蛋白含量进行测定。用SDS凝胶电泳进行电泳后转膜到PVDF膜上,加入5%脱脂奶粉,室温下封闭120 min,洗膜后,将1∶500稀释的CXCL10单克隆抗体(购自上海瑞齐生物技术有限公司)或1∶500稀释的鼠抗人CXCR3单克隆抗体(购自上海恪敏生物科技有限公司)一抗加入,将1∶1 000稀释二抗加入后室温孵育60 min,用TBST冲洗3次,于暗室中用发光试剂曝光并扫描成像。用Image J图像分析软件进行分析,以内参为对照,获得大鼠脊髓组织中CXCL10和CXCR3蛋白表达量。
1.2.5免疫组织荧光染色检测脊髓组织中小胶质细胞标志物Iba1的表达留取L5-6节段脊髓用多聚甲醛固定5 h,转入蔗糖溶液(质量分数15%),等组织沉淀后再转入30%的蔗糖溶液中静置48 h。OCT包埋,冰冻切片,厚度约30 μm。PBST洗涤3次,用5%血清封闭120 min,将山羊抗大鼠Iba1抗体(1∶200稀释,购自美国Abcam公司)加入,于4℃条件下过夜孵育,PBST洗涤3次,将荧光标记的二抗(1∶800稀释)加入,于室温下避光孵育120 min,PBST洗涤3次,避光条件下贴片,甘油封片,于显微镜下观察。
1.3统计学处理

2结果
2.13组大鼠不同时点PWT和TWL变化情况
与T0时相比,假手术组大鼠T1~4时PWT和TWL差异无统计学意义(均P>0.05),切口痛模型组和瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均降低,差异均有统计学意义(均P<0.05)。与假手术组相比,切口痛模型组和瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均降低,差异均有统计学意义(均P<0.05);与切口痛模型组相比,瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均降低,差异均有统计学意义(均P<0.05),详见表1。
2.23组大鼠脊髓组织中CXCL10和CXCR3基因表达
3组大鼠脊髓组织中CXCL10 mRNA和CXCR3 mRNA相对表达量差异均有统计学意义(均P<0.05)。与假手术组相比,切口痛模型组和瑞芬太尼+切口痛模型组大鼠脊髓组织中CXCL10 mRNA和CXCR3 mRNA相对表达量均升高,差异均有统计学意义(均P<0.05);与切口痛模型组相比,瑞芬太尼+切口痛模型组大鼠脊髓组织中CXCL10 mRNA和CXCR3 mRNA相对表达量均升高,差异均有统计学意义(均P<0.05),见表2。
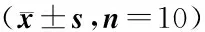
Table 1Changes of PWT and TWL at different time in


组别PWT(g)TWL(s)假手术组T022.1±0.717.5±0.4T121.5±0.417.9±0.5T221.6±0.517.6±0.6T320.9±0.317.2±0.3T421.8±0.617.4±0.7切口痛模型组T021.8±0.517.8±0.8T114.2±0.7ab13.1±0.5abT210.5±0.6ab9.8±0.7abT39.4±0.4ab8.4±0.6abT49.2±0.3ab8.2±0.5ab瑞芬太尼+切口痛模型组T021.7±0.417.6±0.7T111.2±0.5abc11.1±0.4abcT29.5±0.6abc8.4±0.6abcT37.1±0.7abc6.5±0.5abcT46.9±0.5abc6.2±0.3abc
与T0时比较,aP<0.05;与假手术组比较,bP<0.05;与切口痛模型组比较,cP<0.05
表23组大鼠脊髓组织中CXCL10和CXCR3

Table 2Expression of CXCL10 and CXCR3 mRNA in spinal


组别CXCL10mRNACXCR3mRNA假手术组1.02±0.151.05±0.12切口痛模型组1.37±0.26b1.41±0.23b瑞芬太尼+切口痛模型组2.14±0.37bc2.19±0.42bcF45.45120.701P0.0000.000
与假手术组比较,bP<0.05;与切口痛模型组比较,cP<0.05
2.33组大鼠脊髓组织中CXCL10和CXCR3蛋白表达
3组大鼠脊髓组织中CXCL10蛋白和CXCR3蛋白表达量差异均有统计学意义(均P<0.05)。与假手术组相比,切口痛模型组和瑞芬太尼+切口痛模型组大鼠脊髓组织中CXCL10蛋白和CXCR3蛋白表达量均高于假手术组,差异均有统计学意义(均P<0.05);与切口痛模型组相比,瑞芬太尼+切口痛模型组大鼠脊髓组织中CXCL10蛋白和CXCR3蛋白表达量均高于切口痛模型组,差异均有统计学意义(均P<0.05),见表3和图1。

Table 3Expression of CXCL10 and CXCR3 proteins in spinal cord


组别CXCL10蛋白CXCR3蛋白假手术组0.24±0.070.19±0.08切口痛模型组0.62±0.11b0.58±0.10b瑞芬太尼+切口痛模型组0.93±0.14bc0.89±0.12bcF59.65878.181P0.0000.000
与假手术组比较,bP<0.05;与切口痛模型组比较,cP<0.05
2.43组大鼠脊髓背角小胶质细胞标记物Iba1表达情况
免疫荧光染色结果显示,与假手术组和切口痛模型组相比,瑞芬太尼+切口痛模型组Ibal表达量显著增加,小胶质细胞体积增大,分支增多,见图2。
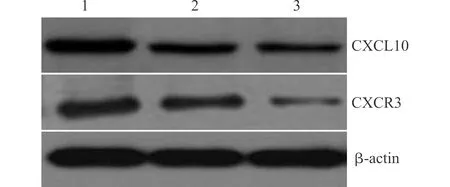
1:瑞芬太尼+切口痛模型组;2:切口痛模型组;3:假手术组图1 Western blot法检测大鼠脊髓组织中CXCL10和CXCR3蛋白表达Fig.1 Western blot analysis of the expression of CXCL10 and CXCR3 proteins in spinal cord tissues of rats

A:假手术组;B:切口痛模型组;C:瑞芬太尼+切口痛模型组图2 3组大鼠脊髓背角小胶质细胞标记物Iba1表达情况(×400)Fig.2 Expression of Iba1,a marker of microglia in the dorsal horn of spinal cord,in rats in the three groups (×400)
3讨论
瑞芬太尼作为常用的超短效μ型阿片受体激动剂,其临床效果已得到认可,但临床实践发现[8],瑞芬太尼用于全麻术后易发生切口痛觉过敏,不利于患者术后恢复。本研究为进一步探讨瑞芬太尼导致切口痛觉过敏的可能机制,在构建大鼠切口痛模型基础上,参考有关文献[9],以1 μg/(kg·min)速率从尾静脉输注瑞芬太尼60 min,并于不同时点测定PWT和TWL,以观察痛觉变化。本研究显示,切口痛模型组和瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均降低,且切口痛模型组和瑞芬太尼+切口痛模型组大鼠出现自主活动减少,患肢抬高,出现频繁舔舐患肢,说明切口痛模型构建成功。同时,本研究显示瑞芬太尼+切口痛模型组大鼠T1~4时PWT和TWL均低于假手术组和切口痛模型组,提示瑞芬太尼可诱发切口痛模型大鼠出现痛觉过敏,且处理后48 h达到最大峰值,与李楠等[10]研究结论相同。
切口痛的发生机制主要与小胶质细胞活化有关,小胶质细胞活化可使趋化因子、细胞因子等分泌加速,诱发脊髓及脊髓上神经中枢痛觉敏化[11]。本研究免疫荧光染色结果显示,瑞芬太尼+切口痛模型组Ibal表达量显著增加,小胶质细胞体积增大,分支增多,Ibal作为小胶质细胞特异性标志物,提示瑞芬太尼可诱发切口痛模型脊髓背角中小胶质细胞活化,从而引发痛觉过敏。小胶质细胞中CXCL10可被IFN-γ诱导表达而参与神经系统感染性疾病的发生[12],其受体CXCR3主要存在于神经元上,可通过与CXCL10结合而影响小胶质细胞的活化[13]。本研究显示,切口痛和瑞芬太尼+切口痛大鼠均出现脊髓组织中CXCL10和CXCR3表达上调,且瑞芬太尼+切口痛大鼠升高更为明显,提示瑞芬太尼可能通过诱导脊髓组织中CXCL10表达上调,使其作用于神经元表面CXCR3而间接诱导小胶质细胞活化,从而诱发切口痛觉过敏发生。
综上所述,瑞芬太尼诱发大鼠切口痛觉过敏时脊髓CXCL10及其配体CXCR3表达水平升高,可能与CXCL10作用于神经元表面CXCR3而间接诱导小胶质细胞活化有关。
参考文献
[1]Rivosecchi R M,Rice M J,Smithburger P L,et al.An evidence based systematic review of remifentanil associated opioid-induced hyperalgesia[J].Expert Opin Drug Saf,2014,13(5):587-603.
[2]Choi E,Lee H,Park H S,et al.Effect of intraoperative infusion of ketamine on remifentanil-induced hyperalgesia[J].Korean J Anesthesiol,2015,68(5):476-480.
[3]Schwaller F,Beggs S,Walker S M.Targeting p38 mitogen-activated protein kinase to reduce the impact of neonatal microglial priming on incision-induced hyperalgesia in the adult rat[J].Anesthesiology,2015,122(6):1377-1390.
[4]Pevida M,González-Rodríguez S,Lastra A,et al.Involvement of spinal chemokine CCL2 in the hyperalgesia evoked by bone cancer in mice:a role for astroglia and microglia[J].Cell Mol Neurobiol,2014,34(1):143-156.
[5]Zhang X,Shen J,Man K,et al.CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis[J].J Hepatol,2014,61(6):1365-1375.
[6]Ye D,Bu H,Guo G,et al.Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia:involvement of Gi protein[J].J Mol Neurosci,2014,53(4):571-579.
[7]Liu Y,Hou B,Zhang W,et al.The activation of spinal astrocytes contributes to preoperative anxiety-induced persistent post-operative pain in a rat model of incisional pain[J].Eur J Pain,2015,19(5):733-740.
[8]Xia W S,Peng Y N,Tang L H,et al.Spinal ephrinB/EphB signalling contributed to remifentanil-induced hyperalgesia via NMDA receptor[J].Eur J Pain,2014,18(9):1231-1239.
[9]Jiang M,Zhang W,Cheng C,et al.Intrathecal injection of KN93 attenuates paradoxical remifentanil-induced postoperative hyperalgesia by inhibiting spinal CaMKII phosphorylation in rats[J].Pharmacol Biochem Behav,2015,134(7):35-41.
[10]李楠,张麟临,舒瑞辰,等.瑞芬太尼诱发切口痛大鼠痛觉过敏时脊髓CCL3和CCR5表达水平的变化[J].中华麻醉学杂志,2015,35(3):326-329.
[11]Huang C T,Chiang R P,Chen C L,et al.Sleep deprivation aggravates median nerve injury-induced neuropathic pain and enhances microglial activation by suppressing melatonin secretion[J].Sleep,2014,37(9):1513-1523.
[12]Bu H,Shu B,Gao F,et al.Spinal IFN-γ-induced protein-10(CXCL10)mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models[J].Breast Cancer Res Treat,2014,143(2):255-263.
[13]Yue C,Shen S,Deng J,et al.STAT3 in CD8+T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis[J].Cancer Immunol Res,2015,3(8):864-870.
(2015-12-03收稿)
Changes of the Expression of CXCL10 and Its Ligand CXCR3 in Spinal Cord during Remifentanil-induced Hyperalgesia in Rats with Incisional Pain
Lei Pengfei,Gao Shan△,Xue Ranrongetal
DepartmentofAnesthesiology,XinxiangCentralHospital,Xinxiang453000,China
AbstractObjectiveTo investigate the changes of the expression of CXC chemokine 10(CXCL10)and its ligand CXC chemokine receptor 3(CXCR3)in spinal cord during remifentanil-induced hyperalgesia in rats with incisional pain.MethodsThirty healthy female SD rats were randomly divided into sham group,incisional pain model group,remifentanil+incisional pain model group,each group having 10 rats.Incisional pain models were established.In remifentanil+incisional pain model group,remifentanil was infused at 1 μg/(kg·min) for 60 min via the tail vein,and in sham group and incision pain model group,normal saline was given in the same manner.The mechanical paw withdrawal threshold(PWT)and thermal withdrawal latency(TWL)were measured 12 h before modeling(T0),4 h(T1),12 h(T2),24 h(T3)and 48 h(T4)after infusion of remifentanil.The expression of CXCL10 and CXCR3 in rat spinal cord tissues was detected at mRNA level by real-time quantitative PCR assay and at protein level by Western blotting at T4 after detection of the PWT and TWL.ResultsThe PWT and TWL of rats were significantly decreased at T1-4when compared with T0 in incisional pain model group and remifentanil+incisional pain model group(P<0.05),and they were lower in remifentanil+incisional pain model group than in the incisional pain model group and the sham group at T1-4,with the difference being statistically significant(P<0.05).Compared with the sham group,the expressions levels of CXCL10 and CXCR3 mRNA and protein in the rat spinal cord tissues in incisional pain model group and remifentanil+incisional pain model group were increased,and the differences were statistically significant(P<0.05).The expressions levels of CXCL10 and CXCR3 mRNA and protein were markedly increased in the rat spinal cord tissues in remifentanil+incisional pain model group relative to the incisional pain model group(P<0.05).Immunofluorescence staining showed that the expression levels of Ibal,a marker of microglial cells,were significantly increased in remifentanil+incisional pain model group compared with the sham group and incisional pain model group.Microglia became large cells and had more processes.ConclusionThe expression levels of CXCL10 and its ligand CXCR3 were elevated in the spinal cord during remifentanil-induced hyperalgesia in rats with incisional pain,which may be related to the action of CXCL10 on CXCR3 on the surface of neurons to indirectly induce the microglial activation.
Key wordsremifentanil;hyperalgesia;CXC chemokine 10;CXC chemokine receptor 3
中图分类号:R614.2
DOI:10.3870/j.issn.1672-0741.2016.03.014
雷鹏飞,男,1979年生,医学硕士,主治医师,E-mail:694826581@qq.com
△通讯作者,Corresponding author,E-mail:694826581@qq.com

