多模态磁共振成像对结节型肝细胞癌TACE+RFA术后复发灶评估的价值
吕婷婷,刘爱莲,汪禾青,李叶,陈丽华,韩铮
多模态磁共振成像对结节型肝细胞癌TACE+RFA术后复发灶评估的价值
吕婷婷,刘爱莲*,汪禾青,李叶,陈丽华,韩铮
[摘要]目的 探讨多模态磁共振成像对结节型肝细胞癌TACE+RFA术后复发灶评估的价值。材料和方法 回顾性收集我院自2009年9月至2014年9月经临床或病理证实单结节型肝细胞癌并采用TACE和RFA联合治疗的患者。筛查入组105例,男87例,女18例,年龄46~83岁,中位年龄63岁。从复发点按随诊间隔逆行追溯分为三组:复发组、可疑组和术后组。结合复发组定位观察其他两组介入灶肿瘤复发区各序列信号改变及形态学征象。采用卡方检验比较三组间的各序列信号及形态改变。根据复发组各序列信号、形态的百分比进行编号。使用ROC曲线比较复发组-术后组各序列信号的诊断阈值。使用Logistics回归计算各序列同时使用信号及形态特点诊断可疑组的灵敏度、特异度。再将序列进行联合找到约登指数最大时的序列搭配。结果 可疑组时信号特点:T1WI低信号、混杂信号;T2WI高信号、混杂信号;弥散加权成像(diffusion weighte dimaging, DWI)高信号,肝脏三维容积快速扫描(liver acquisition with volumeacceleration, LAVA)明显强化。形态特点:各序列大多以半月形为主。诊断效能:当 T1WI、T2WI、DWI、LAVA四个联合时诊断灵敏度、特异度分别为85.7%和94.3%。结论 多模态磁共振成像技术对结节型原发性肝细胞TACE+RFA介入术后复发区的观测具有一定随访价值。
[关键词]癌,肝细胞;磁共振成像;肝动脉栓塞化疗;射频消融术;弥散加权成像
作者单位:大连医科大学附属第一医院放射线科,大连 116010
接受日期:2015-12-30
吕婷婷, 刘爱莲, 汪禾青, 等. 多模态磁共振成像对结节型肝细胞癌TACE+RFA术后复发灶评估的价值.磁共振成像, 2016, 7(2): 113–120.
*Correspondence to: Liu AL, E-mail: cjr.liuailian@vip.163.com
Received 25 Oct 2015, Accepted 30 Dec 2015
我国原发性肝细胞癌(hepatocellular carcinoma,HCC)每年发病率及死亡率均居世界首位[1]。介入治疗作为能选择性使肿瘤组织缺血坏死的技术,已经广泛应用于临床,尤其对小肝癌能达到治愈的效果[2]。目前介入方法较多,其中经肝动脉栓塞化疗(transcatheter arterial chemo embolization,TACE)较为常用,但由于术后存在供血血管不能完全栓塞及侧支形成等原因导致TACE不能一次彻底杀死所有肿瘤细胞,须要进一步射频消融术(radiofrequency ablation, RFA)等补充治疗。介入术后局部坏死、出血及继发炎性反应等,导致病灶区结构复杂,对肿瘤残存或复发判断困难。如何判断介入术后肿瘤残留或复发,对于评价介入治疗效果及指导下一步治疗有重要作用。近年来,随着磁共振成像(magnetic resonance imaging,MRI)技术的提高及广泛应用,其图像质量随之提高,成为评估肿瘤病变的常规检查方法[3]。本研究通过MRI多模态序列成像比较单发结节型原发性肝细胞癌介入术后规律随诊至复发过程中的三个不同时期,来找出肿瘤早期(可疑组)的信号、形态特征是否有意义,及MRI多模态序列成像评估其对肿瘤复发灶的诊断效能。
1 材料与方法
1.1研究对象
回顾性分析2009年9月至2014年9月在我院经过活检病理学及临床诊断证实的单发 结节型HCC,获得我院伦理委员会审批并签署患者同意书经系统TACE联合RFA治疗后复发的患者149例。最后出组44例,筛选入组105例病人105个病灶,男87例,女18例,年龄46~83岁,中位年龄63岁。入组标准:无对比剂过敏;病灶未经外科手术切除;规律随诊间隔3~4个月,至目标病灶肿瘤复发为止。出组标准:有上消化道出血史者或严重凝血功能障碍者;伴严重基础疾病心功能、肝功能、肾功能严重受损;随诊非规律间隔3~4个月者;联合介入治疗前后行其他治疗。
1.2MRI检查方法
应用磁共振扫描仪为GE 1.5 T(GE Medical Systems, Signa EXCITE HD)8通道相控阵体部线圈。患者取仰卧位,足先进,腹部外加呼吸门控(补偿)。T1WI序列(TR/TE=400/8 ms, FOV=32× 32,矩阵=320×192;T2WI抑脂序列TR/TE= 4000/125 ms,FOV=32×32,矩阵=320×192。弥散加权成像(diffusion weighte dimaging, DWI)采用SEEPI序列,TR/TE=4000/70 ms,b=600 s/mm2。应用肝脏三维容积快速扫描(liver acquisition with volumeacceleration, LAVA)序列,TR/TE=3.9/ 1.9 ms,TI=7.0 ms,FOV=39×39 mm,矩阵= 272×192。对比剂为马根维显(Gd-DTPA),经肘静脉注射,注射剂量0.1 mmol/kg,速率2.5 ml/s。分别在注药后的不同时间段(16 s、32 s、48 s、64 s)进行扫描,并于延迟扫描在300 s时进行。
1.3MRI图像的分析与测量
由具有7年腹部MR诊断经验的笔者本人和一名具有13年以上腹部MR诊断经验的放射科医师共同对MRI影像进行分析和测量,两者对图像诊断不统一时,请其他参与者(两年以上诊断经验)进行共同分析达成一致意见。
1.3.1肿瘤介入术后至复发的分期
肿瘤复发组:典型肝癌影像表现,介入术后无其他(包括妊娠、生殖系胚胎源性肿瘤、活动性肝炎)引起甲胎蛋白(alpha fetoprotein, AFP)变化的情况,AFP再度升高≥400 ug/L持续1个月或≥200 ug/L持续2个月。可疑组:(1)介入灶大小:符合联合介入术后肿瘤复发mRECIST标准中既不符合部分缓解(partial response, PR)也不符合疾病进展(progressive disease, PD);(2)介入术后AFP再度轻微升高或较术后组没有明显改变;(3)各序列扫描中某一序列信号可发生轻微变化。术后组:(1)介入灶大小:要符合联合介入术后肿瘤复发mRECIST标准中较稳定(stable disease, SD); (2)介入术后AFP随诊未见明显改变;(3)各序列扫描中信号稳定。
1.3.2MRI征象分析
信号改变:分为高、等、低及混杂信号;形态学征象:(1)结节影:观测位于病灶区的孤立结节状异常信号影;(2)半月形影:观测位于病灶区局限性呈半月形增厚影,并呈明显强化或DWI明显高信号;(3)其它未见(包括环形、片形)。
1.4统计学分析
统计学采用SPSS 17.0统计软件。对三组间的单独各序列信号及形态改变进行比较采用卡方检验。根据复发组各序列组成信号百分比对信号及形态进行评分编号。使用ROC曲线比较复发组-术后组找出各序列的诊断阈值。按照可疑组中各形态的百分比对形态进行评分编号。使用Logistics回归计算T1WI、T2WI、LAVA及DWI序列单独、两两联合、三个联合、四个联合,找到约登指数最大时的序列搭配。
2 结果
复发组、可疑组、术后组单独各序列病灶信号比较见表1,由表1所示介入灶复发区三组各组信号卡方检验比较P<0.05,差异均有统计学意义。

表1 三组T1WI、T2WI、LAVA及DWI病灶信号比较Tab. 1 Comparison of T1WI, T2WI, LAVA, DWI lesions level signal
根据复发组与术后组各序列中信号的百分比依次评分编号,根据复发组中LAVA强化程度各占的百分比将复发组与术后组依次编号,编号后进行ROC的曲线分析。T1WI、T2WI、LAVA及DWI诊断复发组-术后组的ROC曲线见图1,ROC曲线结果见表2。
根据表2中评分阈值可知,诊断复发组的信号后再将可疑组和术后组依据复发组信号进行二分类评分编号,应用卡方检验对两期的信号评分诊断标准的结果进行检验,差异均有统计学意义(P<0.05),见表3。
复发组、可疑组、术后组单独各序列T1WI、T2WI、LAVA及DWI病灶形态比较结果见表4,所示介入灶复发区三期各组形态卡方检验比较,差异均有统计学意义(P<0.05)。
根据表4按照可疑组中T1WI、T2WI、DWI、LAVA序列结节状、半月形、其它未见异常(环形、片状)所占百分比将半月形、结节形、未见异常信号依次评分编号为:3、2、1。将上述信号及形态学评分编号代入Logistics回归分析可疑组诊断效能,见表5。T1WI、T2WI、DWI、LAVA四个联合使用时诊断灵敏度、特异度高。
3 讨论
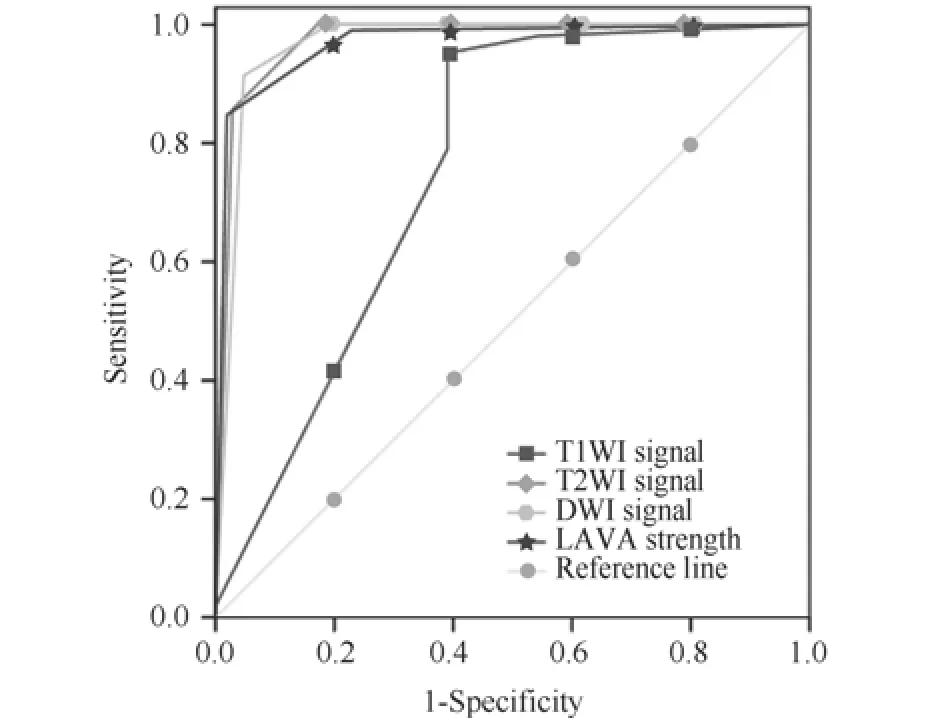
图1 T1WI、T2WI、LAVA及DWI诊断复发组-术后组的ROC曲线Fig. 1 T1WI, T2WI, LAVA grade DWI diagnosis of recurrence group - ROC curve of postoperative group

表 2 T1WI、T2WI、DWI信号及LAVA强化程度诊断复发组-术后组的诊断效能Tab. 2 T1WI, T2WI, DWI signal level to strengthen the diagnosis of recurrence and LAVA group - diagnostic efficacy of postoperative group

表 3 T1WI、T2WI、DWI及LAVA信号评分诊断标准比较Tab. 3 Comparison of T1WI, T2WI, DWI and LAVA signal rates diagnostic criteria

表4 复发组、可疑组、术后组T1WI、T2WI、LAVA及DWI病灶形态比较Tab. 4 Comparison of relapse group, suspicious group, postoperative group T1WI, T2WI, LAVA and DWI lesion morphology
原发性肝细胞癌恶性程度高,病情发展快,所以大多数HCC手术切除率仅9.0%~13.5%[4]。介入治疗具有创伤小、治疗时间短等优点,但肝癌介入术后,如何对病灶进行观测及评价疗效是临床关注的热点。TACE介入治疗随诊1年后,发现治疗效果逐渐下降[5],并且多次进行TACE手术对患者的肝功能有一定的损害,同时影响治疗疗效[6],因此TACE常需要联合其他局部治疗以改善患者生活质量。RFA是高温至60度以上后加热肿瘤组织后来达到破坏肿瘤的目的[7]。因此TACE+ RFA联合治疗能有效减少肿瘤复发及提高总体生存率和无瘤生存率[8]。有学者研究统计发现TACE +RFA联合治疗,特别对于直径>3 cm的肝癌可以治愈[9],并且联合后治疗效果明显优于TACE和RFA单独疗法。HCC介入术后48小时内由于出血及蛋白浓缩等因素,病灶T1WI多呈高信号,周围半月形低信号,T2WI以低信号为主,可有混杂信号,边缘低信号,增强后半月形强化,这与肝组织对热损伤的充血有关[10]。术后1个月复查上腹部多期增强MRI介入灶体积略缩小,周围半月形影变淡或消失,增强后动脉期强化不明显,判定为介入灶消融较完整。6个月以后半月形强化少见,且多发生在静脉期及延迟期[10]。本文通过随诊总结在TACE+RFA治疗之后三期的信号与形态改变(重点是可疑组),为临床更早的提供资料。介入灶在(术后组)T1WI序列呈等及高信号,T2WI表现为低信号,这可能是由于联合介入治疗术后肿瘤组织发生不同程度的凝固性坏死所致;DWI表现低信号可能与介入治疗后碘油及化疗药物使目标肿瘤细胞发生缺血及缺氧,致肿瘤细胞坏死凋亡,细胞膜通透性增加及破裂,细胞外间隙扩大,水分子运动自由扩散增加有关;LAVA动态增强扫描可准确地判断肿瘤无血供期,术后组病灶表现均无强化。但随时间延长,在TACE联合RFA术后可发生一系列的病理变化,包括出血、脂肪变性、液化性坏死等改变,易与复发早期相混淆。因此,病灶随访中可疑组是最值得关注的,如果此期发现异常后可以提高患者的生存率,所以本研究重点按介入灶复发区可疑组的影像特征为临床更早的提供资料。研究介入灶(可疑组)多表现T1WI呈高、混杂信号,T2WI呈多边缘出现稍高信号,早期病灶表现很细微,可以只表现为病灶边缘轻微信号改变,因此,介入术后病灶的边缘的评价也是一项参考指标[11]。本组发现可疑组时LAVA动态增强见病灶出现边缘或结节样强化及发现细小异常血管时(图2),要引起注意有存活的肿瘤细胞。DWI对病灶的残存与复发比较敏感,所以DWI呈稍高混杂信号影(图3)。鉴于HCC多是高血供的恶性肿瘤,有文献报道LAVA动态增强序列也可以发现介入术后肿瘤的变化[12],另外,TACE联合RFA术后病灶会随着时间的推移会有所缩小,随访时病灶若增大,也高度可疑局部复发的可能(图4)。肿瘤病灶可疑复发时需要再一次进行介入治疗或者加入其他的治疗方法,但有时在增强门脉期和延迟期残留病灶、纤维包膜和肿瘤内纤维间隔均可强化。有报道等[13]发现并不是所有的早期强化都意味着肿瘤的残存和复发,要注意术后还可以存在炎性及异常灌注的强化。纤维包膜在T1WI及T2WI则均是低信号,但是复发早期时不易分辨。另外有研究发现DWI受伪影的影响,也可以产生异常高信号,所以单序列观测病变时都存在一定的假阳性及假阴性[14]。有报道[15]综合使用多序列扫描对肝癌介入后疗效评价较单独使用T1WI、T2WI、动态增强和DWI的准确性高,说明联合后能提高诊断效能[16]。近年来多模态磁共振成像在腹部疾病诊断中的应用已经越来越受到关注[17]。而对TACE+RFA联合介入术后的研究报道较少,本研究将多模态磁共振成像用于肝脏介入术后复发区可疑组的诊断上,并总结了一些结果,说明序列联合后能提高肝癌介入术后肿瘤复发的诊断效能。多模态磁共振成像特别强调“联合”,由于序列稳定性等因素的存在,各序列间不可相互替代,所以联合应用可能达到更好的疾病诊断效果。

表 5 可疑组各单独序列及联合序列诊断效能Tab. 5 Sequence and joint sequences of the individual diagnostic efficacy of suspicious group

图2 男,56岁,TACE+RFA术后三个月开始入组随诊,随诊10个月发现介入灶复发,按照逆时间观察图像。A~C为T1WI三期(复发组-可疑组-术后组),D~F为T2WI三期图像,G~I为DWI三期图像,J~L为LAVE增强动脉期三期图像。可疑组时LAVA动态增强见病灶出现边缘或结节样强化及发现细小异常血管时,要引起注意有存活的肿瘤细胞Fig. 2 Male, 56 years old, TACE + RFA surgery, three months into the group followed up, 10 months follow-up found that intervention stove recurrence was observed in reverse time image. A—C is T1WI three stage images (relapse group - suspicious group - surgery group), D—F is T2WI three stage images, G—I is DWI three stage images, J—L is LAVE arterial phase three images. When the suspicious group LAVA dynamic enhanced see an edge or nodular lesions appear strengthening and found small abnormal blood vessels, to attract attention have viable tumor cells.

图3 男,68岁,TACE+RFA术后2个月为观察起点,随诊12个月发现介入灶复发,按照逆时间观察图像。A~C为T1WI三期(复发组-可疑组-术后组),D~F为T2WI三期图像,G~I为DWI三期图像,J~L为LAVE增强动脉期三期图像。DWI对病灶的残存与复发比较敏感,所以DWI呈稍高混杂信号影Fig. 3 Male, 68 years old, TACE + RFA was observed after two months starting point, 12-month follow-up found that tumor relapse intervention, the observed image in reverse time. A—C is T1WI three stage images (relapse group - suspicious group -surgery group), D—F is T2WI three stage images, G—I is DWI three stage images, J—L is LAVE arterial phase three images. DWI residual and recurrent lesions are more sensitive, so the DWI was slightly mixed signal intensity.

图4 男,81岁,TACE术后5个月发现病灶复发临床追加RFA术,术后1个月开始入组随诊,随诊15个月发现介入灶复发,按照逆时间观察图像。A~C为T1WI三期(复发组-可疑组-术后组),D~F为T2WI三期图像,G~I为DWI三期图像,J~L为LAVE增强动脉期三期图像。TACE联合RFA术后病灶会随着时间的推移会有所缩小,随访时病灶若增大,也高度可疑局部复发的可能Fig. 4 Male, 81 years old, TACE was found after five months of recurrent clinical lesions additional RFA surgery, the group began one month follow-up, 15-month follow-up found that tumor relapse intervention, in reverse time observation image. A—C is T1WI three stage images (relapse group - suspicious group - surgery group), D—F is T2WI three stage images, G—I is DWI three stage images, J—L is LAVE arterial phase three images. TACE combined with RFA after lesions over time will be reduced,if the lesions at follow-up increases, it may be highly suspicious of local recurrence.
参考文献[References]
[1]Song DS, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. ClinMolHepatol, 2012, 18(3): 258-267.
[2]Rammohan A, Sathyanesan J, Ramaswami S, et al. Embolization of liver Tumors: Past, present and future. World J Radiol, 2012, 4(9): 405-412.
[3]Guo YM, Huang YH, Wei XH, et al. 3.0 T dynamic enhanced magnetic resonance the semi-quantitative and quantitative analysis research of ovarian tumors. Chin J Magn Reson Imaging, 2015, 6(10): 786-789.郭永梅, 黄云海, 魏新华, 等. 3.0 T动态增强磁共振对卵巢肿瘤的半定量及定量分析研究. 磁共振成像, 2015, 6(10): 786-789.
[4]Peng W, Chen Y, Jiang Q, et al.Spatial analysis of hepatocellular careinoma and socioeconomic status in China from a population-based cancer registry. Cancer Epidemiol,2010, 34(1): 29-33.
[5]Chen Z, Xiao EH. Progress functional magnetic resonance imaging to evaluate the interventional treatment of liver cancer. Chinese陈柱, 肖恩华. 肝癌介入治疗的功能性磁共振成像评价研究进展. 中华临床医师杂志(电子版), 2014, 8(1): 7-13.
[6]Guo CG, Zhang JF, Xu SL. DWI after TACE combined with dynamic enhancement in the value of residual disease and liver cancer recurrence monitoring. Journal of Zhejiang University(Medical Sciences), 2014, 15(1): 77-88.过川根, 张景峰, 徐顺良. 磁共振弥散加权成像联合动态增强在肝癌患者TACE术后病灶残留及复发监测中的应用价值.浙江大学学报(医学版), 2014, 15(1): 77-88.
[7]Dong S, Ye XD, Yuan Z, et al. Relationship of apparent diffusion coefficient to survival for patients with unresectable primary hepatocellular carcinoma after chemoembolization. Eur J Radiol, 2012, 81(3): 472-477.
[8]Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with orwithouttranscatheter arterial chemoembolization in the treatment ofhepatocellular carcinoma: a prospective randomized trial. J Clinoncol, 2013, 31(4): 426-432.
[9]Chen ZG, Huang CY, Lian F, et al. Early HCC radiofrequency ablation combined with transcatheter arterial chemoembolization and Meta-analysis of the efficacy of surgical resection. Chinese Journal of Cancer Prevention, 2015,22(1): 59-65.陈志刚, 黄超源, 连芳, 等. 早期肝细胞癌射频消融联合肝动脉化疗栓塞与手术切除疗效Meta分析. 中华肿瘤防治杂志,2015, 22(1): 59-65.
[10]JeongAh Lee, Woo KyoungJeong, YongsooKim. Dual-energy CT to detect recurrent HCC after TACE: Initial experience of color-coded iodine CT imaging. European Journal of Radiology,2013, 82(4): 569.
[11]Tao R, Zhang JQ. Technology for liver cancer susceptibility envelope display. J Pract Radiol, 2012, 28(5): 699-702.陶冉, 张久权. 磁敏感技术对肝癌包膜的显示. 实用放射学杂志, 2012, 28(5): 699-702.
[12]Ming WD, Li XG, Xu HD, et al. Radiofrequency ablation dynamic enhanced magnetic resonance signals of follow-up studies after treatment. Chinese Journal of Clinicians (electronic version), 2014, 8(4): 607-610.明韦迪, 李晓光, 薛华丹, 等. 肝癌射频消融治疗后动态增强磁共振信号的随访研究. 中华临床医师杂志(电子版), 2014,8(4): 607-610.
[13]Qian T, Yin HB. Functional MRI evaluation of progress in liver cancer hepatic artery chemoembolization efficacy. Zhonghua Fang She Xue Za Zhi, 2013, 47(7): 669-670.钱亭, 尹化斌. 功能MRI评价肝癌经肝动脉灌注化疗栓塞术疗效的研究进展. 中华放射学杂志, 2013, 47(7): 669-670.
[14]Park HJ, Kim SH, Jang KM. Added value of diffusio-weighted MRI for evaluating viable tumor of hepatocellular carcinomas treated with radiotherapy in patients with chronic liver disease,AJR, 2014, 202(1): 92-101.
[15]Peeraully T, Rosenbrg ML. Spontaneous intracranial hypotension without intracranial hypotension. Neueoophthalmol, 2011, 31(3): 248-251.
[16]Qu JR, Liu JP, Liu C, et al. Comparison multislice spiral CT and 3.0 T MR imaging in hepatocellular RF evaluation role therapy. Zhonghua Fang She Xue Za Zhi, 2012, 46(8): 697-700.曲金荣, 骆俊朋, 刘翠, 等. 比较多层螺旋 CT 和 3.0 T MR 成像在肝细胞癌射频治疗疗效评价中的作用. 中华放射学杂志,2012, 46(8): 697-700.
[17]Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with Diffusion-Weighted MRI. Journal of Magnetic Resonance Imaging, 2010, 32(1): 2-16.
Follow-up value of Multi-modality imaging in the MRI assessment of recurrence after transcatheter arterial chemo embolization combined radiofrequency ablation of nodular hepatocellular carcinoma
LV Ting-ting, LIU Ai-lian*, WANG He-qing, LI Ye, CHEN Li-hua, HAN Zheng
Department of Radiology, First Affiliated Hospital of Dalian Medical University,Dalian 116010, China
Key wordsCarcinoma, hepatocellular; Magnetic resonance imaging; Transcatheter arterial chemo embolization;Radiofrequency ablation; Diffusion weighte dimaging
AbstractObjective: To study the follow-up value of Multi-modality imaging in the MRI assessment of recurrence after transcatheter arterial chemoembolization (TACE)combined radiofrequency ablation (RFA) of nodular hepatocellular carcinoma (HCC). Materials and Methods: The clinical and pathological characteristics of single nodular hepatocellular carcinoma confirmed by clinical or pathological between September 2009 and September 2014 were retrospectively collected, and the patients were treated by RFA and TACE. At last, 105 cases were screened, including 87 males and 18 females, aged 46—83 years, and median age of 63 years. The recurrence points were divided into three groups: recurrence group, suspicious group and postoperative group. Combined with the recurrence group, the changes of the serial signals and morphological signs of the other two groups were observed in the tumor recurrence region of the tumor recurrence region. Statistical analysis: using chi square test to compare the signal and morphological changes between the three groups. According to the sequence signal of the recurrence group, the percentage of the morphology of the group was number. Using the ROC curve to compare the diagnostic thresholds of the serial signals between the recurrent group and the postoperative group. Using logistics regression to calculate the sensitivity and specificity of each sequence using signal andmorphological characteristics for the diagnosis of suspicious group. Then the sequence was joint, find the maximum Youden index sequence matching. Results: Signal characteristics of suspicious group: T1WI showed low signal and mixed signal; T2WI showed high signal and mixed signal; DWI showed hyperintensity LAVA (enhancement). Morphological characteristics: the sequence mostly with half. The diagnostic efficacy: when four joint use T1WI, T2WI, DWI, LAVA and combined diagnostic sensitivity and specificity were 85.7% and 94.3%. Conclusion: Multi-modality imaging of MRI has the potential to assess the recurrence after TACE combined RFA of nodular HCCs .
通讯作者:刘爱莲,E-mail: cjr.liuailian@vip.163. com
收稿日期:2015-10-25
中图分类号:R445.2;R735.7
文献标识码:A
DOI:10.3969/issn.1674-8034.2016.02.006

