IgA肾病肾小球内C4d沉积与临床病理的联系
张婷,陈波,李凡凡,陈孝倩,黄朝兴
(温州医科大学附属第一医院 肾内科,浙江 温州 325015)
·临 床 经 验·
IgA肾病肾小球内C4d沉积与临床病理的联系
张婷,陈波,李凡凡,陈孝倩,黄朝兴
(温州医科大学附属第一医院 肾内科,浙江 温州 325015)
目的:探讨肾小球内补体C4d沉积是否为IgA肾病(IgAN)预后判断的新标志。方法:于2011年1月-2014年5月我院肾活检资料库中,选取78例原发性IgAN患者,排除合并高血压、糖尿病等系统疾病。对上述病例行肾活检组织免疫组织化学C4d染色。按肾小球内C4d沉积结果分为阳性组和阴性组,回顾性比较2组病例的临床和病理学参数。结果:78例IgAN,C4d阳性组26例(占33.3%),阴性组52例(占66.7%);所有病例的免疫荧光检查补体C1q均阴性。与C4d阴性组相比,阳性组IgAN患者较少发生肉眼血尿,高血尿酸(男>488 μmol/L、女>363 μmol/L)、低估算肾小球滤过率[eGFR<60 mL·min-1·(1.73 m2)-1]和低白蛋白血症(Alb<30 g/L)的发生率较高,血肌酐和24 h尿蛋白定量水平较高,且伴系膜增生(M1)、节段硬化(S1)、肾小管萎缩/间质纤维化(T1)损伤的发生率较高(P<0.05)。而2组间高血IgA(>400 mg/dL)、低血补体C3(<70 mg/dL)比例及病理上肾小球内皮细胞增生病变(E1)差异均无统计学意义(P>0.05)。logistic回归单因素分析显示,肾小球内C4d沉积与肉眼血尿、24 h尿蛋白定量,低eGFR、低血白蛋白和高血尿酸的比例,及M1、S1和T1相关(均P<0.05)。结论:肾小球内C4d沉积阳性的IgAN患者临床病理表现较重,提示IgAN患者疾病的严重程度可能与通过甘露糖凝集素(MBL)途径激活补体相关。
IgA肾病;补体C4d;甘露糖凝集素途径
IgA肾病(IgA nephropathy,IgAN)是世界范围内最常见的原发性肾小球疾病[1],约30%患者10~20年后进展到终末期肾病(end stage renal disease,ESRD)[2]。因IgAN患者病情变异较大,故如何正确识别和评价IgAN的疾病状态,寻找与疾病活动性、进展或预后相关的指标,对延缓该病进展具有重要意义。
一般认为,IgAN主要通过旁路途径激活补体。但近年有研究表明,部分IgAN患者还存在甘露糖凝集素(MBL)途径激活补体,且预后较差[3-4]。故本研究通过对78例IgAN患者进行肾活检组织C4d免疫组织化学检查,探讨肾脏C4d的沉积在IgAN临床病理中的意义。
1 资料和方法
1.1 一般资料 于2011年1月至2014年5月我院肾内科肾脏病理室IgAN肾活检资料库中,选取78例原发性IgAN患者,男38例,女40例,年龄18~60岁,平均(31.79±0.99)岁。所有病例均排除过敏性紫癜、肝硬化、系统性红斑狼疮、乙肝病毒相关性肾炎等引起的继发性IgAN,及合并高血压、糖尿病等全身系统疾病。
1.2 方法
1.2.1 肾脏病临床及病理的判断标准:①高血压:2次以上收缩压>140 mmHg和/或舒张压>90 mmHg;②估算的肾小球滤过率(eGFR):根据MDRD公式[8]eGFR[mL·min-1·(1.73 m2)-1]=186×[Pcr]-1·154×[年龄]-0.203×0.742(女性);③依据IgAN Oxford[9]分型病理指标评价病理改变程度。
1.2.2 肾活检:经皮肾穿刺取得肾组织,石蜡包埋切片厚度2~3 μm,常规HE、PAS、PASM和Masson染色;冰冻切片厚度4~5 μm,直接免疫荧光法检查IgG、IgA、IgM、补体C3和C1q;间接免疫荧光法检查HBsAg和HBcAg;部分行电镜检查。
1.2.3 主要试剂:兔抗人补体片段C4d多克隆抗体购于奥地利Biomedia公司,免疫组织化学试剂盒为福州迈新公司的Elivision plus广谱试剂盒(PV 9901)。
1.2.4 免疫组织化学染色:石蜡切片脱蜡水化,浸入1∶50的EDTA修复液中高温高压修复2 min;先后滴加3% H2O2及1% BSA,室温静置20 min;加入1∶400兔抗人C4d抗体4 ℃冰箱孵育过夜;聚合物增强剂37 ℃孵育20 min,滴加辣根过氧化物酶标记二抗37 ℃孵育30 min,DAB显色。各步骤间均用0.01 mol/L PBS(pH 7.4)洗涤切片。PBS代替第一抗体作为阴性对照;选择肾脏肿瘤全肾切除病例,取距离肿瘤6 cm外的正常肾脏组织作为C4d染色阴性对照片;选择发生急性加速排斥反应的移植肾组织作为C4d染色阳性对照片。
1.2.5 C4d阳性的判断标准[6]:肾活检穿刺组织含非球性硬化肾小球>3个;在非硬化的肾小球中,有大于25%的肾小球C4d节段性或球性系膜区沉积,伴或不伴肾小球毛细血管壁沉积。
1.3 统计学处理方法 采用SPSS 16.0统计软件进行统计学分析。正态检验采用正态性检验法(K-S检验)。计量资料采用正态分布)或M(全距)(非正态分布)来表示,两样本均数的比较则分别采用两独立样本t检验或秩和检验;计数资料用频数和率表示,2组间率的比较采用卡方检验或Fisher’s精确概率法。采用logistic回归分析相关危险因素。P<0.05为差异有统计学意义。
2 结果
2.1 C4d沉积形态 原发性IgAN 78例患者中,肾小球C4d沉积阳性组26例,C4d阴性组52例,所有病例的免疫荧光检查C1q均阴性。肾小球C4d沉积主要表现为弥漫性或局灶性及球性或节段系膜区沉积,形态多呈颗粒状或团块状;伴或不伴毛细血管壁沉积,形态似假线状或粗绳状。阳性对照片可见肾小球弥漫球性毛细血管壁及管周毛细血管壁C4d沉积;而阴性对照片C4d均阴性,见图1。
2.2 C4d沉积阳性组和阴性组2组的临床病理比较 C4d沉积阳性及阴性2组性别、年龄和病程均相似。与C4d阴性组相比,阳性组IgAN患者肉眼血尿发作较少,高血尿酸(男>488 μmol/L、女>363 μmol/L)、eGFR<60 mL·min-1·(1.73 m2)-1和低白蛋白血症(Alb<30 g/L)的发生率较高,血肌酐和24 h尿蛋白定量水平较高,且伴系膜细胞增生(M1)、节段肾小球硬化(S1)、肾小管萎缩/间质纤维化(T1)病变的发生率较高(P<0.05);高血IgA(>400 mg/dL)、低血C3(<70 mg/dL)比例及E1病变的发生率在2组间的差异均无统计学意义(P>0.05),见表1。
2.3 影响C4d沉积的因素分析 logistic回归分析显示,肾小球内C4d沉积与肉眼血尿、24 h尿蛋白定量,低eGFR、低血白蛋白和高血尿酸的比例,及M1、S1和T1相关(P<0.05),见表2。
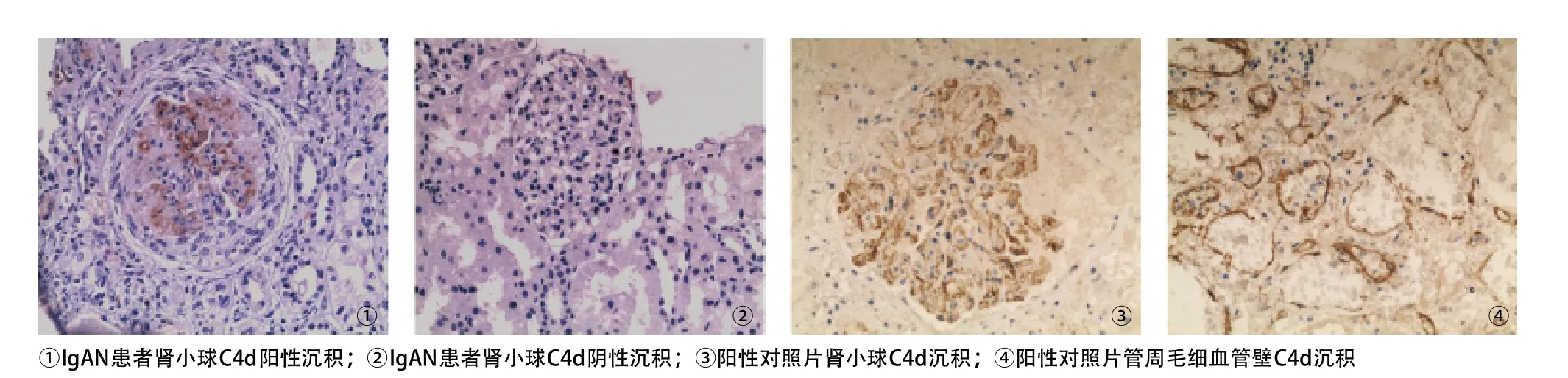
图1 C4d沉积形态(EliVision法,×200)
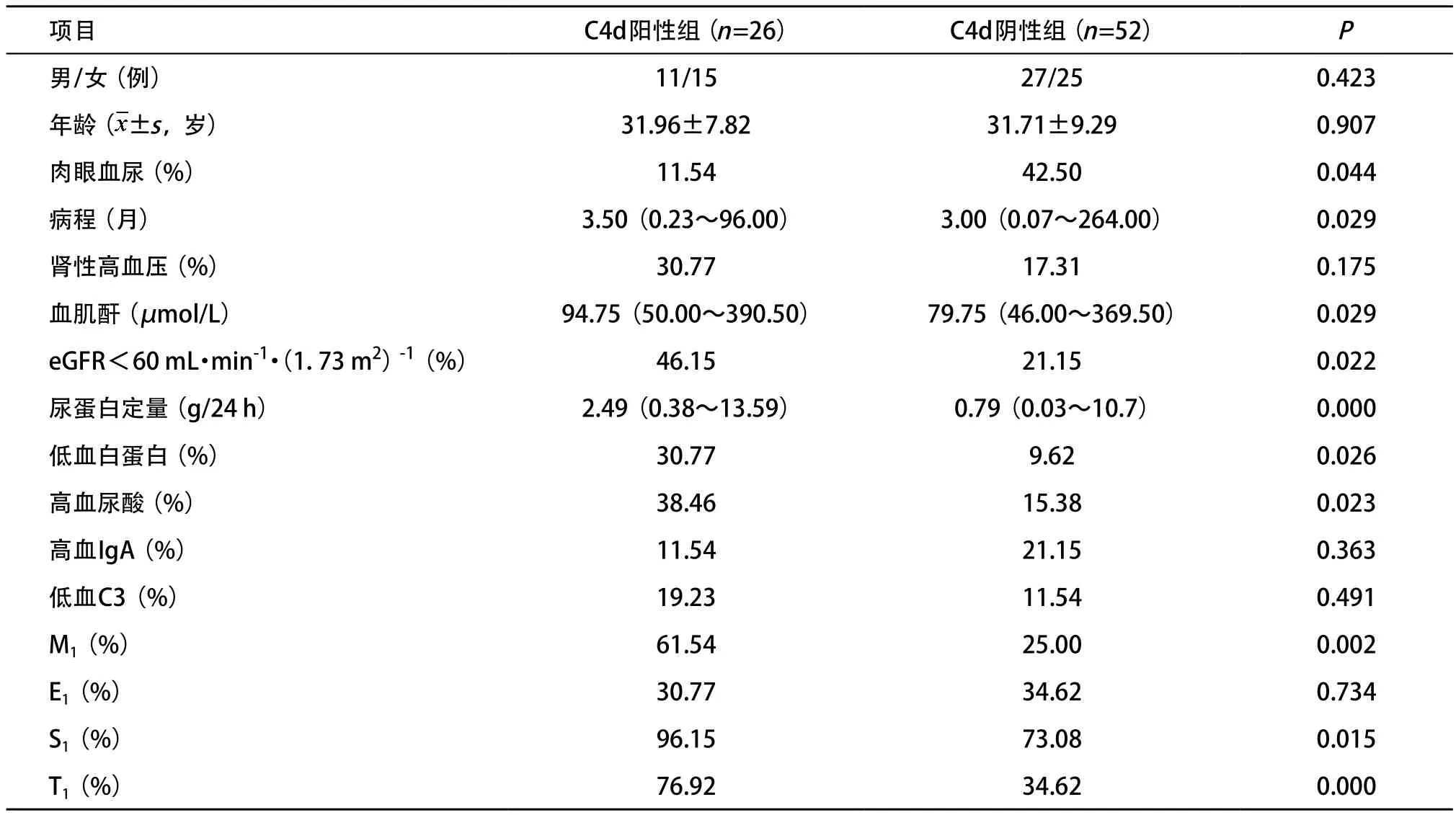
表1 C4d沉积阳性组和阴性组临床病理参数的比较
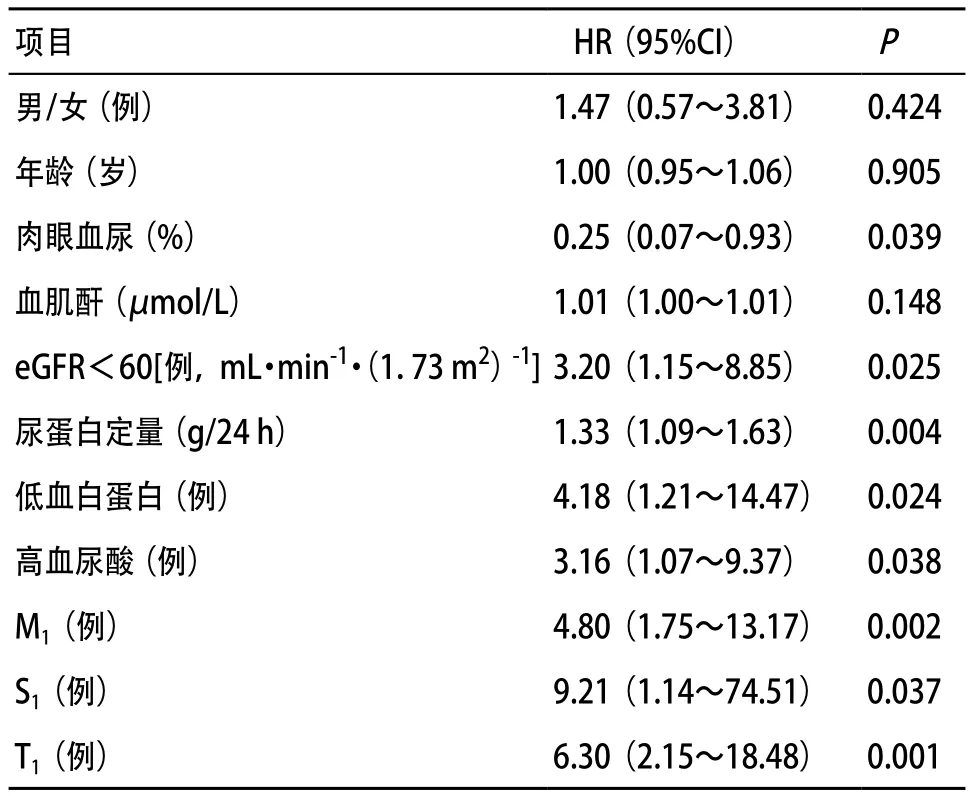
表2 影响C4d沉积的logistic回归分析
3 讨论
一般认为,IgAN患者肾活检时已有的血压升高、eGFR及持续性尿蛋白>1 g/d是预后不良的高危因素[10-11]。在病理方面,IgAN牛津分型提出,M1、E1、S1、T1是IgAN病变进展的重要形态学指标,而T1的作用得到了较多的证实。
Roos等[3]的研究结果显示,60例IgAN患者中,有25%的患者其肾小球系膜区可检测到MBL、纤维胶凝蛋白(L-ficolin)、MBL相关的丝氨酸蛋白酶(MASP)与C4d共沉积,且这些病例伴有更明显的组织损伤,如更多肾小球系膜细胞及毛细血管内皮细胞增生、肾小球节段性硬化和肾小管间质病变增多和更严重的蛋白尿,说明有一部分的IgAN患者通过MBL途径激活补体。Espinosa等[4]在此基础上进一步予以证实,283例IgAN,肾小球系膜C4d阳性沉积109例(占38.5%),其肾脏20年存活率明显低于C4d阴性的患者(P<0.001),认为肾小球系膜区伴有C4d沉积是IgAN预后较差的独立危险因素。
本研究基本证实了上述观点。肾小球内C4d阳性表达的IgAN患者,其临床病理表现均较重。本研究的所有对象肾活检组织免疫荧光结果显示C1q均为阴性,可排除补体激活的经典途径,故可推测本组C4d阳性的病例存在MBL途径激活补体。但由于本研究样本量较少,且没有同时做MBL和MASP等与补体MBL途径激活相关的分子,故需进一步研究验证。
虽然IgA沉积在肾小球的系膜区是触发IgAN的关键,但补体C4d在肾脏沉积阳性为何会带来更严重的临床表现和病理损伤,其具体致病机制尚不清楚。C4d的检测方法主要是通过免疫荧光法或免疫组织化学法。事实上,肾组织C4d染色已是移植肾肾活检的常规项目,成为反映体液免疫排斥的一项标志[12-13]。在IgAN肾活检开展此项检测项目可能有利于患者预后的早期判断。
[1] Wyatt R, Julian B. IgA nephropathy[J]. N Engl J Med, 2013, 368(2): 2402-2414.
[2] Le W, Liang S, Hu Y, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population[J]. Nephrol Dial Transplant, 2012, 27(4): 1479-1485.
[3] Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease[J]. J Am Soc Nephrol, 2006, 17(6): 1724-1734.
[4] Espinosa M, Ortega R, Gómez-Carrasco JM, et al. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy[J]. Nephrol Dial Transpl, 2009, 24(3): 886-891.
[5] Maeng YI, Kim MK, Park JB, et al. Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria[J]. Int J Clin Exp Patho, 2013, 6(5): 904-910.
[6] Espinosa M, Ortega R, Sánchez M, et al. Association of C4d deposition with clinical outcomes in IgA nephropathy[J]. Clin J Am Soc Nephrol, 2014, 9(5): 897-904.
[7] Sahin OZ, Yavas H, Taslı F, et al. Prognostic value of glomerular C4d staining in patients with IgA nephritis[J]. Int Clin Exp Pathol, 2014, 7(6): 3299-3304.
[8] Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group[J]. Ann Intern Med, 1999, 130(6): 461-470.
[9] Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility[J]. Kidney Int, 2009, 76(5): 546-556.
[10] Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy[J]. Am J Kidney Dis, 2001, 38(4): 728-735.
[11] Berthoux F, Mohey H, Laurent B, et al. Predicting the risk for dialysis or death in IgA nephropathy[J]. J Am Soc Nephrol, 2011, 22(4): 752-761.
[12] Vargha R, Mueller T, Arbeiter K, et al. C4d in pediatric renal allograft biopsies: a marker for negative outcome in steroidresistant rejection[J]. Pediatr Transplant, 2006, 10(4): 449-453.
[13] Herzenberg AM, Gill JS, Djurdjev O, et al. C4d deposition in acute rejection: an independent long-term prognostic factor[J]. J Am Soc of Nephrol, 2002, 13(1): 234-241.
(本文编辑:胡苗苗)
Correlation between Glomerular C4d deposition in IgA nephropathy and clinical pathology
ZHANG Ting, CHEN Bo, LI Fanfan, CHEN Xiaoqian, HUANG Zhaoxing. Department of Nephrology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325015
Objective: To explore glomerular C4d deposition whether it is a new prognostic factor in IgA nephropathy. Methods: Seventy-eigth patients with the primary IgAN who underwent renal biopsy at our centre were enrolled from January 2011 to May 2014. Patients with diabetes, hypertension, systemic disease, and any type of secondary IgAN were excluded. C4d was detected with immunohistochemical staining in the paraffin embedded renal tissues, using a polyclonal antibody. The cases were divided into positive group and negative group according to the staining of C4d. Then the clinical and pathological parameters were compared between these two groups respectively. Results: Of 78 cases with the primary IgAN, 26 cases (33.3%) were defined as positive group and 52 cases (66.7%) were defined as negative group. C1q detections were negative by immunofluorescence in all these cases. Compared with negative group, C4d-positive patients had gross hematuria in less proportion, had higher incidence of hyperuricemia (male>488 μmol/L; female>363 μmol/L), low estimated glomerular filtration rate [eGFR<60 mL • min-1• (1.73 m2)-1] and hypoalbuminemia (Alb<30 g/L) at the time of renal biopsy. C4d-positive patients also had higher levels of serum creatinine and 24-hour urine protein. The proportion of patients with mesangial proliferation (M1), segmental sclerosis (S1), tubular atrophy or interstitial fibrosis (T1) was higher in C4d-positive group according to the Oxford pathologic classification criteria. Between positive group and negative group, there were no significant differences in gender, age, the course of disease, the incidence of high levels of serum IgA (>400 mg/dl), low levels serum complement C3 (<70 mg/dl) and the glomerular endothelial cell hyperplasia (E1) (P>0.05). Logistic regression univariate analysis showed that the glomerular deposition of complement C4d was correlated with gross hematuria, 24-hour urine protein, low eGFR, hypoalbuminemia, hyperuricemia, M1, S1, T1(P<0.05). Conclusion: The patients with positive glomerular C4d staining with IgAN has heavier clinical injuries and pathological lesions, which suggests that glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease.
IgA nephropathy; complement C4d; the mannose binding lectin pathway
R692
B
10.3969/j.issn.2095-9400.2015.07.016
2014-10-17
张婷(1989-),女,浙江温州人,硕士生。
黄朝兴,主任医师,Email:huangzhaoxing@medmail.com.cn。

