Changes of Physiological Characteristics and Yield in Peanut Varieties with Different Resistance Effected by Peanut Scab
Shengyao GUO, Jianhong CHEN, Yu XIAO, Jinxian WANG, Ruyan CHEN, Yongshui CHEN
Quanzhou Institute of Agricultural Sciences, Quanzhou 362212, China
Scab disease is one of the main diseases in peanut, which has been epidemic in most peanut production areas. The disease has caused peanut production great loss by reducing yield of diseased field by 10%-30% generally, or even 50% seriously in epidemic years, and it thus became an important replanting factor[1-2]. Therefore, researching the resistance mechanisms of different resistant peanut varieties to scab is of great importance in scab prevention and variety utilization. Peanut scab is a kind of fungal disease, the pathogen of which is Sphaceloma arachidis Bitaucourt et Jenkins, belonging to Deuteromycotina.The disease can infect both spring peanut and autumn peanut. Some researches indicated that a series of Metabolic changes would happen in crops infected with the pathogen as well as variation of the kinds, contents and activities of components including proteins, enzymes and carbohydrates, and these changes were related to the disease resistance of hosts to certain extent[3].In recent years, scholars at home and abroad have done some researches on pathogen isolation, sporulating characters, variety resistance selection and control effect[4-9]. Fang et al.[6]performed screening on 19 antisepticsthrough trials for indoor anti-pathogen effect and field plot control efficacy,obtaining good effects with Topsin,Difenoconazole, procymidone and Duoliu Chaowei Duojunling, and they considered that Topsin and Difenoconazole had better effects. Wang et al.[7]tested 218 peanut cultivars to screen and identify their resistance to Sphaceloma arachidis in disease field naturally and artificial inoculated, obtaining 20 cultivars including P9032-40 and ICG1376, and considered that Cultivars exhibited MR or R resistance to this disease can be used as the antidisease parents in sexual hybridization. However, there is less study on the change of leaf physiological property of infected peanuts. In this paper,resistant and susceptible peanuts were selected as materials, and after inoculation, the contents of leaf protein,soluble sugar and malonaldehyde(MDA), the activities of catalase(CAT),peroxidase (POD)and superoxide dismutase (SOD) and yields were determined to understand the differences between physiological and biochemical changes of resistant and susceptible peanuts during interaction with scab, thus revealing the resistance mechanism of peanuts to scab,aiming at providing scientific basis for utilization of resistant varieties and breeding of new scab-resistant variety.
Materials and Methods
Test materials and pathogen inoculation
The test materials were peanut varieties ‘Heyou 13’ and ‘Quanhua10’. The test was carried out at the test field of Quanzhou Institute of Agriculture Science during April to August 2013. The soil was sandy soil with medium fertility and used for cropping rice previously. The test were provided with four treatments as follows: ‘Quanhua10’ with inoculation(sprayed with pathogen solution, TR),‘Quanhua10’ without inoculation (not sprayed with pathogen solution, CK),‘Heyou 13’ with inoculation (sprayed with pathogen solution, TR), ‘Heyou 13’ without inoculation (not sprayed with pathogen solution,CK).Randomized block arrangement was adopted for the test, with three repetitions. A textural research region was further established. The plot had an area of 6.67 m2with a length of 6.67 m and a width (including furrows) of 1 m, and plant spacing was set as 19.8 cm×19.8 cm. Surround the test field were provided protection rows. The test began with sowing on April 1; from May 17,diseased stems and diseased leaves were collected from local infected fields for cultivating peanuts, and sheared into fragments; and 30 g of the fragments were added into each of multiple conical flasks (500 ml),which was then added with 200 ml of distilled water and oscillated on an oscillator for 4 h to extract pathogen solution, and on May 18, inoculation was performed using backpack type manual sprayer on stems and leaves of peanut varieties in the afternoon, and the above operation was repeated once every 5 days, until the susceptible ‘Quanhua 10’ was slightly diseased after three times of inoculation. In whole growth process, pests were controlled without curing the disease. Other management measures were the same as local land for growing field crops.
Sampling method
Sampling was performed once before inoculation and once every 14 days after inoculation, with a total number of 5 times(obtaining functional leaves at the same parts each time,i.e., upper third leaves), for the determination of each physiological index,and the determination results were obtained as the averages of three repetitions.
Determination of physiological indexes
The content of soluble sugar was determinate by anthrone colorimetry[10].Extraction of enzyme solution was performed by adding accurately weighed sample (0.5 g) and normal saline at a weight(g)∶volume(ml)ratio of 1∶4 into a mortar,grinding under ice bath condition to get 20%homogenate to be transferred into a centrifuge tube (10 ml), and centrifuging for 10 min (4 000 r/min, 4 ℃) after mixing to get supernatant as the enzyme extract to be stored at 4 ℃for later use. Protein content, activities of peroxidase(POD),catalase(CAT)and superoxide dismutase(SOD)were determined according to the instruction of detection kit provided by Nanjing Jiancheng Bioengineering Institute.
Checking of agronomic characters
In order to check main agronomic characters, ten plants were obtained among representative plants to perform indoor textural research before harvest. After harvest, pod yield of each plot was determined after drying.
Data analysis
Data analysis was performed using DPS and Excel.
Results and Analysis
Changes of soluble sugar content after inoculation
It showed in Fig.1 that the content of soluble sugar in leaves of resistant variety ‘Heyou 13’ was higher than that in leaves of susceptible variety‘Quanhua10’before inoculation.After inoculation,the variation trends of soluble sugar content in leaves of the resistant and susceptible varieties were in accordance with each other, i.e.,with the growth process,the content of soluble sugar increased gradually to a maximum until the 56thday, and then decreased. For the resistant variety‘Heyou 13’, the content of soluble sugar was higher than its control at each stage only lower than its control at the14thday; while the susceptible variety ‘Quanhua10’ had a content of soluble sugar higher than its control from inoculation.
Changes of soluble protein content
As shown in Fig.2, the content of soluble protein in leaves of resistant variety ‘Heyou 13’ was higher than that in leaves of susceptible variety‘Quanhua10’before inoculation.After inoculation, the variation of soluble protein content in leaves of the resistant and susceptible varieties were in accordance with each other, i.e., the content of soluble protein increased firstly and was then on the decrease,but the peak values of the resistant and susceptible varieties presented at different time. After inoculation, for the susceptible variety ‘Quanhua10’, the content of soluble protein in its leaves showed a maximum at the 14thday,and the maximum of its control appeared at the 28thday; and for the resistant variety ‘Heyou 13’, the trend accorded with its control, with a maximum presented at the 42thday and then decreased. It thus clear that thedegradation of soluble protein in susceptible variety ‘Quanhua10’ was earlier and faster that in the resistant variety‘Heyou 13’.
Influences on activity of protective enzymes
In Fig.3, SOD activity in the leaves of both the two varieties presented a single-peak variation trend which increased at first and then decreased after inoculation. After inoculation, the SOD activity increased gradually to a maximum at the 42thday and then decreased.For the susceptible variety ‘Quanhua10’,the SOD activity in its leaves decreased by 20.34%, 7.23%, 12.12%, 30.17% and 9.64% respectively in comparison with its control; while for the resistant variety ‘Heyou 13’,the SOD activity in its leaves decreased by 7.46%, 9.21%,-10.71%, 6.74% and 12.66% respectively in comparison with its control.Overall, it could be seen that the decrease of the resistant variety was lower that of the susceptible variety.
Fig.4 showed the variation of CAT activity of peanut leaves of each treatment. It could be seen from Fig.4 that with the growth process the CAT activity of peanut leaves decreased at first, then increased and again decreased overall. As to the susceptible variety ‘Quanhua10’,the CAT activity in the leaves thereof accorded with that of the control thereof substantially after inoculation, and the CAT activity in its leaves was lower than that of its control only higher than that of the control at the 28thday and the 42thday.As to ‘Heyou 13’,in comparison with the control thereof, the variation of both their leaves was flatter after inoculation, with a slowly increasing or decreasing trend, and its activity was higher than that of the control after the 28thday.
In Fig.5, the POD activity of peanut leaves showed a trend accorded with the variation trend of CAT activity substantially, i.e., a trend of decreasing at first,then increasing and again decreasing with the growth process, and its peak value appeared at the 56thday.After inoculation,the POD activity of the susceptible variety‘Quanhua10’ was lower than that of its control, while the resistant variety‘Heyou 13’ was on the contrary, with a POD activity value higher than that of its control.
Influence on malondialdehyde content
Fig.6 was the variation in MDA contents of the 4 treatments of the resistant and susceptible varieties. It was showed that MDA contents in the leaves of both the two varieties presented a single-peak variation trend which increased at first and then decreased. However, the peak values of different varieties appeared at different time. The treatments of the two varieties sprayed with pathogen solution both had a peak value at the 28thday,while the corresponding control treatments had a peak value at the 42thday. After inoculation, the MDA contents of the two varieties were higher than their corresponding controls at most time except that the MDA content of ‘Heyou 13’was slightly lower than its control at the 14thday. For the resistant variety ‘Heyou 13’, the MDA contents determined at each time period in comparison with its control increased by-14.93%,22.78%, 4.76%,18.34%and 21.30%respectively,all of which were lower than the increases of the susceptible variety ‘Quanhua10’,which were 9.42%, 92.59%, 5.53%,39.61%and 21.91%respectively.
Influences on yield and yield components
Table 1 showed the yield of each peanut treatment and the perfor-mances of main agronomic characters. In Table 1, both the resistant and susceptible varieties had a decrease in yield. The susceptible variety had a greater output reduction by 16.81%, while the output reduction of the resistant variety was only of 5.43%which was relatively lower. The two varieties both had their plant heights and dry weights of overground parts of single plants decreased to different extents. From yield components,the main reason for output reductions of the resistant and susceptible varieties was reduction in single-plant productivity due to decreased kernel percent resulted from reduction in single-plant plump pod, size decreases of pods and seed kernels and empty pods. However,the influence on resistant variety ‘Heyou 13’after inoculation was relatively lesser.
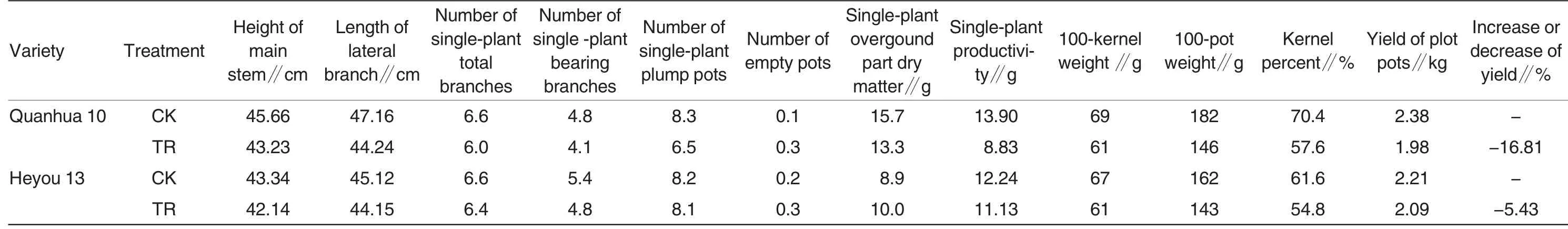
Table 1 Influences on agronomic characters and pod yields of peanut varieties inoculated with scab
Conclusions and discussion
Saccharides are the basis for metabolism, so the higher the saccharides in plants, the more vigorous the metabolism, resulting in stronger vitality as well as higher resistance to pathogens.Some researches deemed that the increase of sugar content could act positively on resistance of plants to diseases, and pathogens could infect varieties with lower soluble sugar contents easier[11]while some other researches reported that saccharides were essential nutritional substances for growth and reproduction of pathogens, and higher sugar content was an important factor facilitating infection of plants by diseases[12];and furthermore,there were studies indicating that sugar contents were different between resistant and susceptible materials, but the difference changed with growth process and whether plants were infected, and there was no inevitable relation between sugar content and variety resistance[13].The research results of this paper indicated that, whether for the resistant variety or the susceptible variety, the content of soluble sugar increased and then decreased until a maximum reached at the 56thday with the growth progress under inoculation or no inoculation. The resistant variety ‘Heyou 13’ had a soluble sugar content higher than that of the control after inoculation at most time,while the susceptible variety ‘Quanhua 10’ had a soluble sugar content lower than that of the control all the determination time, indicating that there was certain relationship between soluble sugar content in peanut leaves and plant disease resistance.
Soluble protein plays an important role in the resistance of plants to adversity stress. It was reported that soluble protein content in cells of infected plant tissues decreased[14].There were also reports that due to the difference between the resistance genes contained in vivo, the stimulation from pathogens could result in soluble protein content in plant showing an induced or non-induced increase[15]. The results of this study showed that the soluble protein in peanut leaves increased to different extents at different time in comparison with the controls, which was similar to the research result from the research on rice by Li et al.[15]; but the soluble protein content in leaves of the susceptible variety ‘Quanhua 10’had a peak value presented earlier than that of the resistant variety ‘Heyou 13’,this might because the susceptible variety was more sensitive to the infection by pathogens than the resistant variety, and the disease resistance of varieties was in negative correlation with the infection by pathogens.
The infection by pathogens could induce plants to produce some physiologically active substances, which participate in many physiological metabolism processes,such as oxidation, lignification and reaction with pathogen toxins, in which defensive enzyme system plays an important role. In this study, the SOD of the resistant and susceptible varieties both decreased after inoculation, while that of the susceptible variety decreased by a larger amplitude, indicating that the resistant variety had a strong ability in removing active oxygen than the susceptible variety; and the CAT activity presented a trend of decreasing at first, then increasing and again decreasing in the overall growth process of peanuts,and in comparison with the controls,the resistant variety ‘Heyou 13’had a CAT activity higher than the control, while the susceptible variety ‘Quanhua 10’had that lower than the control at most time except that at the 28thday and the 42thday on the contrary, indicating that the CAT activity had a relationship with the resistance of peanuts to scab, and a higher CAT activity after infection resulted in stronger disease resistance. After scab inoculation, the resistant variety ‘Heyou 13’had a POD activity in its leaves higher than that in its control, while the susceptible variety ‘Quanhua 10’ has that lower than its control,indicating that the resistant variety had a stronger epidemic prevention system while the susceptiblevariety could not resist the toxicity by pathogens. From the study on MDA in peanut leaves, it was considered that after inoculation, the MAD contents in the leaves of the resistant and susceptible varieties were higher those in the controls, while the increase amplitude of the susceptible variety was greater than that of the resistant variety, indicating that the resistant and susceptible varieties were subjected to membrane lipid peroxidation to different extents after being infected as well as damage to membranes, in which the damage to the resistant variety was lighter than that to the susceptible variety. It was thus considered that pathogens were resisted by hosts in propagation through increasing contents of soluble sugar and soluble protein and enhancing activities of SOD,CAT and POD in vivo to remove harmful substances, protecting cell from damage and finally delaying pathological change. However, the susceptible variety could not withstand the toxicity from the pathogen,harmful substances including MDA increased continuously, resulting in aggravation of the disease.
In this study, the yields of the resistant and susceptible varieties were both reduced,while the susceptible variety ‘Quanhua 10’ had its yield reduced by a large amplitude. The output reduction was mainly due to reduction of single-plant productivity caused by decreased kernel percent resulted from reduction of plump pot number of single plant, smaller pots and kernels,and decreases of 100-pot weight, 100-kernel weight and plumpness of pots; and compared with the susceptible ‘Quanhua 10’,the resistant variety ‘Heyou 13’ was diseased lighter, and subjected to less influence by the disease.
This study preliminarily discussed changes of contents and activities of soluble sugar,soluble protein and partial defensive enzymes after scab inoculation, while the relationship between other disease resistant substances including phenolic substances, phytoalexins, and chitinase with resistive or sensitive phenotype of varieties was left to be studied in future.
[1]HU M (胡淼),QIAN FC (钱方才).Outbreak and prevention of peanut scab disease[J].Plant Protection(植物保护),2000,26(1):21-22.
[2]CAI XQ(蔡学清), HU FP(胡方平). New disease of peanuts in Fujian provincepreliminary study of investigation of scab disease[J]. Fujian Agricrltural Science and Technology (福建农业科技),2000(2):23.
[3]HE CY (何晨阳), WANG JS (王金生).Physiological and biochemical changes during hypersensitive response in plants(植物过敏反应中的生理生化变化)[J]. Plant Physiology Communications(植物生理学通讯), 1996, 32 (2): 150-154.
[4]LI F(李峰),ZHU S(朱旭),YANG TQ(杨廷勤), et al. outbreak regularity and prevention measures for peanut scab in Nanyang basin(南阳盆地花生疮痂病的发生规律及防治措施)[J].Bulletin of Agricultural Science and Technology (农业科技通讯),2012(6):195-238.
[5]YAN Z (鄢铮), WANG ZR (王正荣),FANG SM (方树民),et al.Investigation and control of peanut scab(花生疮痂病的调查与防治)[J]. Fujian Agricultural Science and Technology (福建农业科技),2010(6):58-59.
[6]FANG SM(方树民) , WANG ZHR(王正荣),GUO JM(郭建铭),et al.Fungicides selection for peanut scab disease(花生疮痂病药剂防治试验)[J].Chinese Journal of Oil Crop Sciences(中国油料作物学报),2006,28(2):220-223.
[7]WANG ZR (王正荣),WANG KC (王开春),FANG SM(方树民),et al.Screening and evaluation of peanut germplasm resistant to Sphaceloma arachidis(花生种质资源抗疮痂病筛选鉴定)[J]. Fujian Journal of Agricultural Sciences(福建农业学报),2006,21(4):313-316.
[8]FANG SM (方树民),WANG ZHR (王正荣),KE YQ(柯玉琴),et al.The Evaluation of Resistance and Resistant Mechanisms of Peanut Varieties to Scab Disease (花生品种对疮痂病抗性及其机制的研究) [J].Scientia Agricultura Sinica(中国农业科学),2007,40(2):291-297.
[9]GUO SY (郭陞垚),CHEN YS (陈永水),CHEN JH (陈剑洪), et al. Preliminary report on identification of peanut germplasm resources resistant to scab disease(花生品种资源抗疮痂病鉴定研究初报)[J]. Fujian Agricultural Science and Technology (福建农业科技),2014(2):11-14.
[10]LI HS(李合生).The principle and technology of plant physiology and biochemistry experiments(植物生理生化实验原理和技术)[M]. Beijing: Higher Education Press (北京:高等教育出版社),2001.
[11]YAN HJ (闫慧娟), HAN YJ (韩玉杰),ZHOU XIM(周小梅),et al.Physiological and biochemical changes of different resistant corn infected with maize dwarf mosaic virus(不同抗性玉米接种矮花叶病毒后的生理生化变化研究)[J].Journal of Shanxi University: Natural Science Edition(山西大学学报:自然科学版),2010,33(3):458-462.
[12]LIU SP (刘素萍),WANG RX (王淑贤),ZHANG R (张荣) , et al. Effects of Sugar and Amino Acid in Root Exudation of Different Resistant Cotton Cultivars on Cotton Fusarium Wilt Pathogen (根系分泌物中糖和氨基酸对棉花枯萎病菌的影响)[J].The journal of northwest agricultural university (西北农业大学学报),1998,26(6):30-35.
[13]JIANG DW (蒋道伟),SI LT (司龙亭).Changes of Physiological Characteristics in Different Cucumber Breeding Lines Infected by Sphaerotheca fuliginea(不同抗性黄瓜自交系接种白粉病原菌后生理特性的变化)[J].Acta Agriculturae Boreali-occidentalis Sinica(西北农业学报),2010,19(8):161-165.
[14]ZHU LM (朱丽梅),LUO FX (罗凤霞).The relationship between contents of soluble protein,chlorophyll and soluble sugar in leaves of lily and resistance to Botrytis(百合叶片中可溶性蛋白、 叶绿素、可溶性糖含量与灰霉病抗性的关系)[J].Jiangsu Agricultural Sciences(江苏农业科学),2011,39(5):134-136.
[15]LI ZT (李佐同), JIN XH (靳学慧),ZHANG YL (张亚玲),et al.The Relationship between soluble protein,soluble sugar content and rice blast resistance of rice seedlings (水稻幼苗可溶性糖及可溶性蛋白含量与抗瘟性的关系)[J].Reclaiming and Rice Cultivation(北方水稻),2009,39(4):6-9.
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Effects of Specific Gravity-based Seed Grading on Seed Germination,Seedling Emergence and Grain Yield of Hybrid Rice
- Effects of NaCl Stress on Seed Germination of Four Canavium album Raeuseh Cultivars
- Application Effects of Ultra-fine Powder Shaped Maize Seed Coating Agent in Spring Sowing areas in northeast China
- Breeding and Application of a Japonic Rice Cytoplasmic Male Sterility Line,E-Jing A
- Effect of Low Temperature and Sparse Light Conditions on Cold Tolerance of Different Rice Lines at Seedling Stage
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
