In vitro Rapid Propagation of Ficus carica L.‘Masui Dauphine’
Jinfeng LI, Lin MI, Xueping CHEN, Chunyan WAN, Hengzhi HUO, Bingyi CHEN
Zhenjiang Institute of Agricultural Sciences in Hilly Region of Jiangsu, Jurong 212400, China
Ficus carica L. ‘Masui Dauphine’,native to California,commonly known as giant fig due to its large fruit, was introduced into China from Japan. Experimental observations indicate that ‘Masui Dauphine’ is one of the most promising fig cultivars and is suitable to be cultivated in the south of the Yangtze River. In production practices, figs are mainly propagated by cutting[1]. In addition,exotic cultivar grafting is a commonly used method for fig cultivation,but it exhibits rare material sources,low propagation rate,serious seasonal restrictions, large occupied area and other issues compared with in vitro rapid tube culture method that has abundant material sources and high propagation rate, thereby failing in failing in meeting the requirements for current large-area cultivation and popularization. Moreover, from the perspective of breeding,figs have hypanthodia with small flowers hidden inside the cystiform clinanthia, which can not be improved and updated by conventional cross-breeding techniques.With the application of biotechnology in fruit tree breeding, transgenic technology, on the basis of tissue culture, has undoubtedly become the preferred method for variety improvement. Therefore, a rapid propagation system for in vitro culture of figs should be established for fig breeding using transgenic technology. In recent years, Song et al.[2-5]reported in vitro tissue culture and rapid propagation techniques of different fig varieties.However, little information is available on in vitro rapid propagation of ‘Masui Dauphine’. The success or failure in in vitro culture generally depends on genotypes. Various genotypes have different requirements for hormone types and concentrations[6-7]. In this study, an in vitro rapid propagation system of F. carica L. ‘Masui Dauphine’ was established, aiming at providing scientific basis for largescale cultivation of ‘Masui Dauphine’.
Materials and Methods
Materials
Young shoots of ‘Masui Dauphine’ with vigorous growth were collected as experimental materials.
Methods
Pretreatment of explants Tender shoots of ‘Masui Dauphine’ with vigorous growth were collected and cut into stem segments with a single bud(0.5 cm above the bud, 1-1.5 cm below the bud); terminal buds were collected separately. Subsequently, allthe materials were washed several times using detergents, rinsed with running water for 2 h,and preserved in the storage bag.
Anti-browning treatment The storage bag with a single bud was placed into a 4 ℃refrigerator for 4 h, dried with sterile paper, and soaked into 1 500 mg/L vitamin C solution for 30 min before use.
Surface disinfection After antibrowning treatment, single buds were soaked with 75% ethanol for 30 s,rinsed twice with sterile water, dried with sterile paper, disinfected using 0.1% HgCl2solution for 8 min with continuous shaking to ensure complete contact between explants and the solution. Subsequently, the explants were rinsed several times with sterile water until HgCl2was removed completely, and dried with sterile paper.
Cutting About 0.2 cm long segment was cut off from each explant.According to the polarity,the lower end of the bud was inserted into the induction medium (basal medium), and cultured for 20-30 d. Seedlings 0.4-0.5 cm in length were cut off and transferred into the propagation medium.
Screening of explants Axillary buds and terminal buds were cultured in basal medium. Each flask was inoculated with three explants,10 flasks per treatment, with three replications. The seedlings were cultured for 15-20 d before survey and statistics.
Basal medium MS, 3/4MS, 1/2MS and modified MS (MS medium containing calcium nitrate tetrahydrate instead of anhydrous calcium chloride in the macroelement, and the amount of calcium nitrate tetrahydrate was 2.12 times that of anhydrous calcium chloride according to the conversion result)were selected as basal medium, and supplemented with 1 mg/L 6-BA and 0.05 mg/LNAA.Each flask was inoculated with one seedling, 10 flasks per treatment, with three replications. The seedlings were cultured for 30 d before survey and statistics.
Screening of hormone types and concentrations Cytokinins:0.5,1.0,2.0 mg/L 6-BA; auxins: 0.05, 0.1 mg/L NAA, 0.4 mg/LIBA and 0.2, 0.4, 0.5,1.0,2.0 mg/L GA3.Each flask was inoculated with one explant, 10 flasks per treatment, with three replications.The seedlings were cultured for 30 d before survey and statistics.
Rooting and transplanting ‘Masui Dauphine’ seedlings were transferred into rooting medium(1/2 modified MS+0.5 mg/L IBA +20 mg/L sucrose + 7 mg/L agar, pH 5.8). After rooting,‘Masui Dauphine’ seedlings were transplanted.
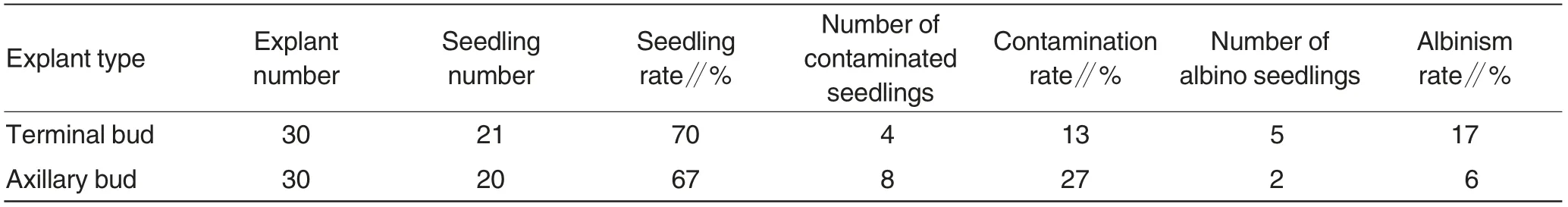
Table 1 Effects of different explants on seedling culture

Table 2 Effects of different basal medium on propagation rate of figs
Results and Analysis
Effects of explant type on seedling culture
Both axillary buds and terminal buds of ‘Masui Dauphine’ could grow into robust seedlings with substantially the same seedling rate(Table 1).After anti-browning treatment and surface disinfection, terminal buds easily grew into albino seedlings with an albinism rate of 17%, but the albinism rate of axillary buds was only 6%. Moreover,axillary buds could be easily collected with wide sources. Therefore, based on comprehensive consideration, axillary buds were better experimental materials compared with terminal buds.
Effects of basal medium on propagation rate of figs
As shown in Table 2, fig plantlets in modified MS medium exhibited robust growth and green leaves with the plant height of 7.1 cm; propagation rate reached the highest of 26.7 times;calli were white,tender and glassy with a high differentiation rate, indicating that modified MS medium was more suitable for in vitro rapid propagation of‘Masui Dauphine’.
Effects of hormone types and concentrations on in vitro rapid propagation of figs
As shown in Table 3, in modified MS medium with the addition of 1.0 mg/L 6-BA, 0.05 mg/L NAA and 1.0 mg/L GA3, fig plantlets exhibited the most robust growth with green, large,tender and fleshy leaves; propagation rate reached the highest of 28.4 times;plant height reached the highest of 6.8 cm; the maximum internode length was 1.1 cm.When 6-BA concentration reached 2.0 mg/L, fig plantlets were severely vitrificated.
Rooting and transplanting
Rooting medium was prepared(1/2 modified MS + 0.5 mg/L IBA + 20 mg/L sucrose + 7 mg/L agar, pH 5.8),in which the rooting rate reached 100%, and the survival rate of transplants reached 98%.
Conclusion and Discussion
In the in vitro culture process of figs, due to large pith and loose struc-ture, tender stems and shoot tips of figs have a large number of internal endophytes[8], resulting in difficult acquisition of aseptic seedlings. In this study, shoot tips (terminal buds) and axillary buds at the vigorous growth stage were selected as experimental materials to reduce endophyte infection. In addition, experimental materials were disinfected with ethanol and HgCl2for 8 min, which achieved good disinfection effects.

Table 3 Effects of different hormone types and concentrations on in vitro rapid propagation of figs
Browning is a common problem in the in vitro culture process of figs.Studies have shown that severe browning will seriously affect the growth of explants and even cause death.In this study,in accordance with the method proposed by Sun et al.[9],experimental materials were soaked with 1 500 mg/L vitamin C solution before culture,which greatly reduced the degree of browning and ensured normal growth of fig plantlets during the culture process.
Figs have strong vegetative propagation ability, and it is relatively easy to perform in vitro propagation and induce rooting. In this study, low concentrations of exogenous hormones led to desired results, but extremely high concentrations of exogenous hormones were not conducive to the growth of figs. According to the results, the most suitable medium for in vitro rapid propagation of ‘Masui Dauphine’ was modified MS + 1.0 mg/L 6-BA + 0. 05 mg/L NAA + 1.0 mg/L GA3+20 mg/L sucrose+7 mg/L agar, pH 5.8, which was different from the reports of Duan et al.[3,10-11], suggesting that experimental materials with different genotypes should be cultured with different medium.
[1]LI YL (李艳玲).Hardwood cutting technology of figs in summer(无花果春季硬枝扦插技术)[J]. Modern Agricultural Science and Technology (现代农业科技),2007,(2):22.
[2]SONG YN (宋仪农),WU QL (吴钦林),DU RL(杜启兰),et al.In vitro rapid propagation technology of fig stems(无花果的茎段离体快速繁殖技术) [J].Forestry Science Technology (林业科技),2002,27(6):50-51.
[3]DUAN XL (段新玲),REN DS (任东岁),ZHAO SZ (赵书珍).Tissue culture and regenerative system of Ficus carica (无花果组织培养再生系统的研究) [J].Forest Research ( 林业科学研究),2001,14(6):621-627.
[4]LI K (李康),CHEN JH (陈聚恒),SONG FH (宋锋惠), et al. Tissue culture and rapid propagation of Ficus carica L.(无花果组织培养及快速繁殖技术研究)[J].Acta Horticulturae Sinica (园艺学报),1997,24(1):90-91.
[5]ZHU JH (朱建华),GUAN LX (关丽霞).The study of tissue culture in Ficus carica(无花果的组织培养研究)[J]. Northern Fruits(北方果树),2002(3):9-10.
[6]JIANG LM (姜灵敏),XU YM (徐有明),ZHANG DM (张冬梅), et al. Establishment of regeneration system for Rosa multiflora Thunb. var. cathayensis through calli induction(红刺玫愈伤组织诱导再生体系的建立)[J].Jiangsu Journal of Agricultural Sciences (江苏农业学报),2012,28(4):914-916.
[7]ZHOU J (周杰), CAO QH (曹清河),ZHOU ZL(周志林),et al.Study on differences in tissue culture of different varieties of leaf-vegetable sweet potato (菜用型甘薯不同品种组织培养差异研究)[J]. Jiangsu Agricultural Sciences (江苏农业科学),2012,40(1):60,62.
[8]ZHANG HC(张弘弛), MA YM(马养民),LIU R(刘瑞),et al.Study on endophytic fungi in Ficus carice Ⅰ. Screening of antifungal activities of endophytic fungi(无花果内生真菌的研究Ⅰ. 抗植物病原真菌活性的筛选)[J]. Acta Agriculturae Boreali-Occidentalis Sinica (西北农业学报),2007,16(2):232-236.
[9]SUN LJ (孙丽娟),GUAN HB (关洪斌),ZHAO J (赵晶),et al.Preliminary study on preventing explant from browning in tissue culture of Ficus carica(无花果组织培养中防止外植体褐化的研究)[J].Journal of Anhui Agricultural Sciences(安徽农业科学),2009,37(2):535-536.
[10]HU JG(胡建刚),GUO JS(郭继善).Tissue culture of Ficus carica L. (无花果的组织培养)[J]. Journal of Nanjing Forestry University (南京林业大学学报),1994,18(3):73-76.
[11]WANG L (王亮),WANG CH (王彩虹),TIAN YK (田义轲), et al. Propagation and conservation of ‘Zhongguoziguo’(Ficus carica L.) (无花果品种“中国紫果” 离体培养与保存研究)[J]. Hebei Journal of Forestry and Orchard Research (河北林果研究), 2009, 24(1):81-83.
 Agricultural Science & Technology2015年7期
Agricultural Science & Technology2015年7期
- Agricultural Science & Technology的其它文章
- Variation in Enzymes Activities of Rhizospheric Substrate and Influencing Factors during Nursing of Watermelon Seedlings
- Determination of Iprobenfos Residue in Rice by GC-FTD using Two-dim Ensional Purification
- Screening and Taxonomic Status of a Highly Efficient Antifungal Strain against Cytospora chrysosperma
- Antioxidant Activity of Polysaccharides in Yam Bulbils and Their Hypoglycemic Effect in Diabetic Mice
- Adsorption Kinetics of NH4+by Purple Soils with Different pH Values
- Inhibition of Chlamydospore Germination and Mycelial Growth of Trichoderma spp.by Chemical Fungicides
