Determination of Iprobenfos Residue in Rice by GC-FTD using Two-dim Ensional Purification
Yu WAN , Xiaobo ZHANG, Jun WANG Hong JIANG
1. Anhui Research Institute of Chemical Industry, Hefei 230041, China;
2. Hefei University of Technology, Hefei 230009, China
Iprobenfos is a systemic organic phosphorus fungicide,as a substitute for kitazine. The toxicity of iprobenfos is moderate. The main mechanism of iprobenfos is to interfere with cell membrane permeability and to block the package of certain lipophilic chitins through cell membrane,inhibiting the growth of cell wall.Iprobenfos is absorbed by plants mainly through underwater roots and leaf sheaths, and then distributed to various parts of the plants. It has certain control effects on rice stem rot, Rhizoctonia solani and rice leaf scald.Compared with kitazine, iprobenfos has higher efficiency and longer persistence. In addition, iprobenfos also has control effects on planthoppers and leafhoppers and anti-lodging effect,so it is widely used in production.
In both China and Japan, it has been reported that the application of kitazine causes undesirable flavor odor in rice grains. Iprobenfos, as an organic phosphorus substitute for kitazine, has naturally attracted much attention. Certainly, the determination of iprobenfos in rice samples has also aroused widespread attention. Currently, there have been some reports on iprobenfos residue analysis methods at home and abroad, including GC-FPD for environment and soil[1-2],GC-MS-SIM for tea leaves[3], GC-FID for water bodies[4]and LC-MS/MS for mangos[5]. Different methods are established and different apparatuses are adopted for determination of iprobenfos in different types of samples. The pigments, organic acids,lipids, sterols and other impurities in rice samples can sometimes cause greatly reduced sensitivity in mass de-tector,so it is very important to choose good purification devices, as well as specific detectors. Referring to previous literature, a GC-FTD method, with two-dimensional purification, was established in this study for determination of iprobenfos residue in rice samples. The recovery and sensitivity of established method all met the requirements by residual analysis.Moreover, the established method was characterized by simple reagents,conventional instruments, less interference impurities and high sensitivity,which all met the requirements by residual analysis. This will provide a theoretical basis for rational use of iprobenfos in rice cultivation and development of Standards for Safe Use of Pesticides.
Materials and Methods
Equipment and instruments
The used equipment and instruments in this study included gas chromatograph equipped with flame thermionic detector(FTD)(GC-2014C,Shimadzu), electronic balance (XA-105DU, Mettler-Toledo), rotary evaporator (RN-1001, Zhengzhou Great Wall Machinery Manufacture Co.,Ltd.), vortex mixer (SA8, STUART),solid phase extraction instrument(Supelco)and pipettes(Eppendorf).
Drugs and reagents
The used drugs and reagents in this study included iprobenfos standard with purity higher than 99.8%(Sigma-Adrich), acetone, ethyl acetate, methanol, n-hexane, sodium chloride, fumaric acid, ammonia (of analytical grade), anhydrous sodium sulfate (dried at 575 ℃for 4 h), distilled water, Oasis HLB solid phase extraction column (3 ml/500 mg, WATERS), PSA solid phase extraction column, C18 solid phase extraction column and Florisil SPE solid phase extraction column (3 ml/500 mg,Agela).
Analysis methods Sample preparation
Rice straw A certain amount (10 g)of rice straw powder was mixed with a certain volume (80 ml) of acetoneethyl acetate mixed solvent(v/v,1/4)in a conical flask. The conical flask was then placed on a shaker for 1.5 h.Subsequently, the solution was filtered through a funnel containing anhydrous sodium sulfate and magnesium sulfate(10 g). The residue was then rinsed with a certain volume (15 ml) of mixed solvent. The filtrates were mixed together and concentrated on a rotary evaporator at 40 ℃for purification.
Husked rice A certain amount(20 g)of husked rice powder was mixed with a certain volume(2 ml)of distilled water and a certain volume (60 ml) of acetone-ethyl acetate mixed solvent(v/v, 1/4) in a conical flask. The flask was placed on a shaker for 1.5 h.Subsequently, the solution was filtered through a funnel containing anhydrous sodium sulfate (10 g). The residue was rinsed with a certain volume(15 ml)of mixed solvent. The filtrates were mixed together and concentrated on a rotary evaporator at 40℃for purification.
Sample purification The SPE C18column was pre-eluted with methanol(5 ml) and water (5 ml). The sample that was about to be purified was dissolved in methanol aqueous solution(v/v,1/8)and then loaded in the equilibrated SPE C18column, which was eluted with water (2 ml), 0.5% aqueous ammonia methanol solution (2 ml;v/v, 1/4) and 0.5% aqueous famarate methanol solution(2 ml; v/v,1/4)successively. Subsequently, the column was vacuumized for 5 min and eluted with methanol-ethyl acetate mixed solvent(2 ml).After blow-dried with nitrogen gas, the column was eluted with 40% ethyl acetate in n-hexane (5 ml)and n-hexane solution (5 ml)successively. For the sample in which the iprobenfos residue was about to be determined, it was dissolved in a certain volume (2 ml) of n-hexane solution,and the column was first eluted with n-hexane solution (2 ml) and then eluted with 40%ethyl acetate in n-hexane(5 ml).
Chromatographic conditions The chromatographic conditions were as follows: detector, FTD; column, DB-17(30 m ×0.32 mm ×0.25 μm); injector temperature,245 ℃;detector temperature,265 ℃.The temperature raising procedure was as follows: start at 100℃, hold 1 min; 20 ℃/min to 200 ℃,hold 1 min;20 ℃/min to 245 ℃,hold 5 min. The carrier gas was high-purity nitrogen with total flow of 120 kPa.The flows of hydrogen, air and make-up gas were 52, 90 and 50 kPa, respectively. The range was 0, and the current was 5 pA. The splitless injection was adopted,and the split was started 0.5 min later.The injection volume was 1 μl. The relative retention time was 8.5 min(Fig.1).
Drawing of standard working curve for iprobenfos A certain amount(0.010 0 g)of iprobenfos standard was weighed accurately and dissolved in n-hexane to 100 ml.Thus the iprobenfos standard solution with concentration of 100 mg/L was prepared. It was then diluted gradiently into standard solutions with concentrations of 0.005,0.01, 0.05, 0.5, 1.0 and 5.0 mg/L. As describe above,the determination was performed. The regression analysis between injection volume(x)and peak area (y) was carried out, and the regression equation was obtained:
y=669 431x-175.94, r = 0.999 8(Fig.2).
Added recovery test In certain amounts of rice straw and husked rice samples, different-level standard solutions were added. The iprobenfos contents in the samples were determined by GC-FTD as described above. Then the added recoveries were calculated.
Results and Discussion
Selection of extraction solvents
The extraction effects of acetone,ethyl acetate, acetone-n-hexane (v/v,1/1),acetone-methanol(v/v,1/1),acetone-ethyl acetate (v/v, 1/1), acetoneethyl acetate (v/v, 1/4) and methanol were compared. The results showed that the extraction efficiencies of all the extraction solvents were all higher than 75%,except that(<60%)of acetone-methanol (v/v, 1/1). The extraction solvents containing n-hexane or methanol might introduce non-polar and polar impurities.At the same time,the moisture content was high in rice straw but low in husked rice. The iprobenfos could be sufficiently extracted by acetone-ethyl acetate (v/v,1/4), so it was selected as the extraction solvent.
Sensitivity and accuracy of established method
In the control rice straw and husked rice samples that had not been applied with iprobenfos,different-level iprobenfos standard was added. The samples were extracted, purified and determined as described above. The results showed the minimum detectable quantity was 5×10-12g; the minimum detectable concentrations in rice straw and husked rice were 2 and 0.5 μg/kg, respectively, which were one order of magnitude lower than in SN/T0148-2011[6]. The average added recoveries of iprobenfos in rice straw samples ranged from 72.6% to 99.7%with RSD ranging from 5.65% to 8.48%; the average added recoveries of iprobenfos in husked rice samples ranged from 81.6% to 97.6% with RSD ranging from 3.74% to 7.63%(Table 1).The relative standard deviations of added recoveries all met the requirements by Guideline on Pesticide Residue Trial.
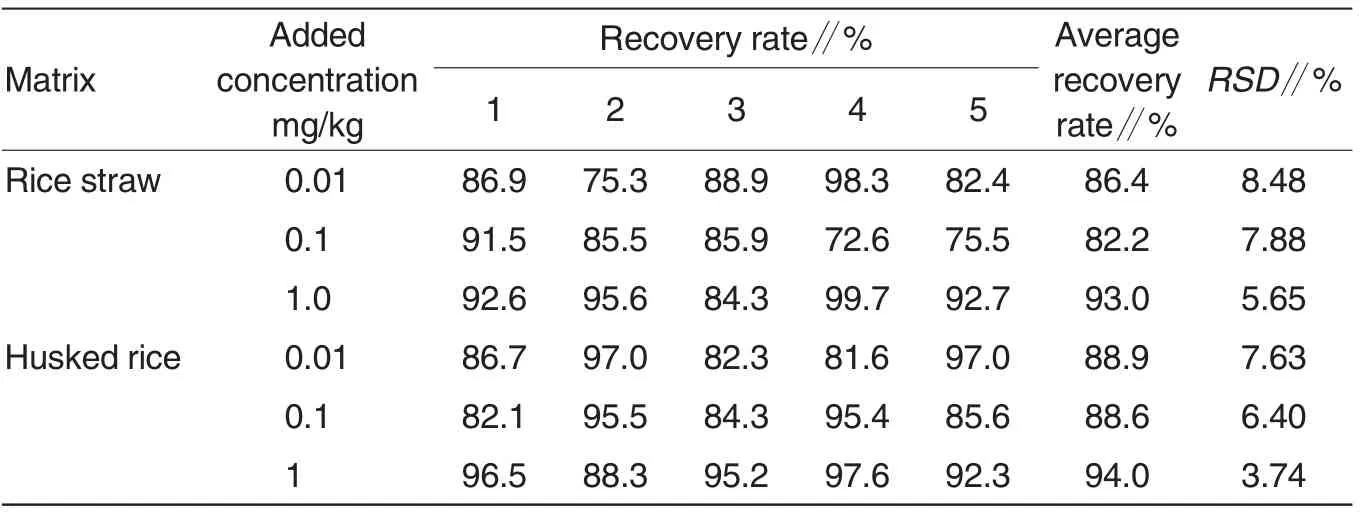
Table 1 Added recoveries of iprobenfos standard in rice samples
Selection of chromatographic conditions
FTD is a kind of detector with high sensitivity but low stability. So the flow rates of various gases and ratios between heating currents all needed to be optimized so as to achieve the best performance of the FTD detector.
Considering the selection of heating current, the baseline flow and components signals of FTD were all increased with the increase in heating current[7]. In this study, the effects of heating current size (1, 5, 10, 20, 50 and 100 PA)on response signals were investigated. The results showed that when the heating current was higher than 10 PA, the baseline flow was increased rapidly, but the response signal size of iprobenfos was changed slightly. In the premise of reaching detection limits, the heating current should be lower. Therefore, the 5 PA of heating current was finally selected.
In terms of selection of flow rates of carrier gases, the gases that enter FTD and their flow rates determine the composition of air surrounding the ionization source, thereby greatly affecting the sensitivity and specificity ofFTD detector[8-10].FTD is a quality-type detector. Its baseline flow and response values are all increased with the increase in flow rate of carrier gas.But in the FTD detector with constant heating current, carrier gases also have a function of cooling the surface of ionization source. In this study, the ratios between carrier gases were also optimized. Among the carrier gases,when the flow rates of the other carrier gases were fixed, the increased flow rate of air caused decreased baseline flow and response values. When the air pressure was increased from 50 kPa to 60 kPa, the voltage of baseline flow was reduced from 80 MV to -30 MV(Fig.3).When the flow rates of the other carrier gases were fixed, the increased hydrogen pressure (from 50 kPa to 100 kPa) resulted in great increase in voltage of baseline flow(from -10 MV to 700 MV) (Fig.4).Moreover, the response values were also changed greatly. Therefore, appropriate flow rates of air and hydrogen are extremely essential for sensitivity and specificity of FTD detector.For determination iprobenfos, the flow rates of carrier gases were finally optimized as follows: nitrogen, 120 kPa;hydrogen, 90 kPa; air, 52 kPa; make up,35 ml/min.
Selection of purification method
In this study, the purification effects of HLB, PSA, NH2, GCB, C18,Florisil and other 10 kinds of solid phase extraction columns were compared. Although their recoveries were all higher than 70%, the purification effects were not the same. Even worse,the non-polar and polar impurities and weak organic acids and bases could not be removed simultaneously.Referring to chemical and chromatographic principles, the selectivity and retention effect all can be greatly improved through regulating ratio between organic solvent to water and acidity of environment and changing the mode of phase so as to get a very clean chromatogram.
In this study, two-dimensional purification mode was developed. On the basis of one-dimensional purification by C18column, the processes of acid and alkaline elution were added,and the reversed phase extraction was changed into two-dimensional normal phase extraction. Using the strong retention property of iprobenfos in C18column, the strongly polar, weakly alkaline and weakly acidic organic impurities were removed by eluting the column with water (2 ml), 0.5%aqueous ammonia methanol solution(2 ml; v/v, 1/4) and 0.5% aqueous famarate methanol solution (2 ml;v/v,1/4)successively (Fig.5,Fig.6);using the retention property of iprobenfos in NH2column,the non-polar and weakly polar organic impurities were removed,and the strongly polar normal organic impurities were retained in the normal phase NH2column. The iprobenfos was eluted out with n-hexane-ethyl acetate (v/v, 7/3) (Fig.7).
Discussion
This study develops a GC-FTD determination method for iprobenfos residue in rice straw and husked rice.The sensitivity, specificity and recovery of the established method all meet the requirements by pesticide residue analysis.In terms of selection of purification conditions,two-dimensional purification can effectively purify and enrich iprobenfos in rice straw and avoid the interference of pigments, organic acids, lipids, sterols, sugar and other impurities, thereby improving the sensitivity of established method and weakening matrix effects.The used equipment and instruments and reagents are all conventional.The established method has high applicability and reproducibility, which all meet the requirements by Guideline on Pesticide Residue Trials of the Ministry of Agriculture, China. In a word, the established method is fully applicable to iprobenfos residue analysis[11].
[1]Environmental Protection Institute of Ministry of Agriculture(农业部环境保护科研监测所). GB/TI4552-2003 Method of gas chromatographic for determination of organophosphorus pesticides in water and soil(水、土中有机磷农药测定的气相色谱法)[S].Beijing:China Standard Press (北京: 中国标准出版社),2004.
[2]DENG YH (邓延慧).To detect trace or-ganic phosphorus pesticide using GCPulse FPD(气相色谱-脉冲火焰光度检测器测定痕量有机磷农药)[J].Administration and Technique of Environmental Monitoring(环境监测管理与技术),2003,3:20-21.
[3]LI YJ,DAI H,YI WL,et al.Determination of iprobenfos residue in tea by GC-MSSIM [J]. Agrochemicals, 2007, 46(4):263-264.
[4]LIU DC(刘德仓),HE J(何娟).Analysis of pesticide residues in water using selfmade acrylic polymer based solidphase micro-extraction and gas chromatography (自制丙烯酸酯类共聚物固相微萃取气相色谱对水中农药残留的分析) [J]. Journal of Instrumental Analysis(分析测试学报),2008,27(9):977-979.
[5]M BUJAGENDRA RAJU, C NARASIMHA RAO. Determination of multiclass pesticide residues in mangoes by liquid chromatography-tandem mass spectrometry[J].International Journal of Applied Biology and Pharmaceutical Technology,2011,62(2):279-289.
[6]Xiamen Inspection and Quarantine Bureau(厦门检验检疫局).SN/T0148-2011 Determination of organophosphorus pesticides multiresidues in tea for import and export-GC-FPD and GC-MS methods (进出口水果蔬菜中有机磷农药残留量检测方法气相色谱和气相色谱-质谱法)[S].Beijing:Standard Press of China(北京: 中国标准出版社),2011.
[7]The US Hewlett-Packard Company (美国惠普公司). HP6890 Series GC Operation Manual(Volume III)(HP6890 系列气相色谱仪操作手册(第三卷)).1997.
[8]B KOLB,J BISCHOFF.A new design of a thermionic nitrogen and phosphorus detector for GC[J].Journal of Chromatographic Science,1974,12(11):625-629.
[9]PATTERSON PL, ROBERT L HOWE.Thermionic nitrogen-phosphorus detection with an alkali-ceramic bead [J].Journal of Chromatographic Science,1978,16(7):275-280.
[10]PATTERSON PL.Detectors for Capillary Chromatography.Hill HH,Mcminn DG. New York: John Wiley & Sons,Inc.,1992,139.
[11]Institute for the Control of Agrochemicals,Ministry of Agriculture (农业部农药检定所). NY788-2004 Guideline on pesticide trials(农药残留试验准则)[S].Beijing:China Agriculture Press(北京:中国农业出版社),2004.
 Agricultural Science & Technology2015年7期
Agricultural Science & Technology2015年7期
- Agricultural Science & Technology的其它文章
- Variation in Enzymes Activities of Rhizospheric Substrate and Influencing Factors during Nursing of Watermelon Seedlings
- Antioxidant Activity of Polysaccharides in Yam Bulbils and Their Hypoglycemic Effect in Diabetic Mice
- In vitro Rapid Propagation of Ficus carica L.‘Masui Dauphine’
- Adsorption Kinetics of NH4+by Purple Soils with Different pH Values
- Study on Multiplication,Rooting and Transplanting of Tissue Culture Plantlets of Rhododendron chrysanthum Pall
- Inhibition of Chlamydospore Germination and Mycelial Growth of Trichoderma spp.by Chemical Fungicides
