Abnormal inter- and intra-hemispheric integrati on in male paranoid schizophrenia: a graph-theoreti cal analysis
Jianhuai CHEN, Zhijian YAO*, Jiaolong QIN, Rui YAN, Lingling HUA, Qing LU,4,*
•Original research article•
Abnormal inter- and intra-hemispheric integrati on in male paranoid schizophrenia: a graph-theoreti cal analysis
Jianhuai CHEN1,#, Zhijian YAO1,2,#,*, Jiaolong QIN3, Rui YAN1, Lingling HUA2, Qing LU3,4,*
paranoid schizophrenia; diffusion tensor imaging; brain mapping; characteristic path length;paxillin; alcohol dependence
1. Introduction
Schizophrenia (SZ) is a devastating psychiatric disorder characterized by a distributed brain system.[1,2]Previous neuroimaging studies have provided consistent evidence of disconnections among brain regions in SZ.[3]These disconnections lead to a reduced capacity to integrate information and, thus, may partially account for the deficits in cognition and abnormal behavior seen in SZ.[4]From this perspective, SZ can be understood as a disconnection disorder, resulting from aberrant integration of neural processes due to impaired neural connectivity between different brain regions.[5,6]
Many studies have identified altered connectivity within both left and right hemispheres in SZ.[7,8]Morphological and functional imaging studies have reported widespread intra-hemispheric disconnection in the brains of persons with SZ.[9]Aberrant functional connectivity between the frontal and temporal cortex has been associated with auditory hallucinations in SZ.[10,11]Diffusion tensor imaging (DTI) studies have also reported evidence of widespread disconnection in the brains of individuals with SZ.[12]Overall, most imaging study results suggest abnormal intra-hemispheric white matter (WM) structures in persons with SZ, particularly in the frontal lobe.[13]
Altered inter-hemispheric WM integrity has also been reported in SZ. Previous studies reported reduced inter-hemispheric connectivity involving the frontal,temporal, parietal, and occipital lobes in SZ.[14]There are also reports of reduced connections of the corpus callosum (CC) or its subregions in SZ, and CC volume and fiber integrity have been negatively correlated with auditory hallucinations.[14,15]This reduced interhemispheric connectivity may play a major role in the disturbed hemispheric co-operation in SZ.[16]
Recent studies show that the brain network of SZ has preserved small-world topological properties characterized by a high level of segregation and global efficiency.[17]However, in SZ communication between specific brain regions have longer path lengths than in healthy controls, suggesting a reduced capacity to communicate with other brain regions and, thus, a less strongly integrated global network.[18]
Gender is a key factor accounting for behavioral and cognitive differences between individuals; it may cause (or be the result of) differences in the patterns of neural systems.[19]Reported differences in the severity,symptom pattern, age of onset, and other characteristics of males and females with SZ[20,21]support the hypothesis of gender-based differences in underlying patterns of anatomical connectivity of the brain.[22]However, most previous studies about neural networks in SZ include both male and female subjects, which may introduce unexplored confounding when comparing results with healthy controls or with other studies.
The principal aim of this current study was to further elaborate the abnormalities in the connectional architecture of the inter- and intra-hemispheric networks of male SZ patients. Previous studies in SZ have reported disruption of the topological organization in the whole-brain network[17,18]and reduced functional connectivity between the same regions of the left and right hemispheres, but no significant abnormalities were found for brain-wide intra-hemispheric links.[23]The hypothesis being tested in the current study is whether or not male patients with paranoid SZ also exhibit abnormal topological organization in the inter- and intra-hemispheric networks and subnetworks.
2. Methods
2.1 Subjects
The enrollment of participants in the study is shown in Figure 1. All patients invited to participate in the study were male inpatients in the acute stage of schizophrenia being treated at the Department of Psychiatry of the Nanjing Medical University-Affiliated Brain Hospital. If the patient or guardian agreed to participate in the study and signed the informed consent form, a detailed medical and psychiatric history was obtained and a formal diagnosis was determined using the Structured Clinical Interview according to DSM-IV criteria.[24]Excluded patients included those who a) did not have paranoid schizophrenia, b) had other comorbid Axis I psychiatric disorders, c) had a history of electroconvulsive therapy, d) had a history of neurological disorders or any serious medical illness (or illnesses that required surgery), e) previous traumatic brain injury, or f) were unable to undergo a magnetic resonance imaging (MRI) examination. The remaining patients underwent a MRI examination and thePositive and Negative Syndrome Scale (PANSS)[25]was administered by an experienced psychiatrist on the day of image acquisition. A control group of age, ethnicity,and education-matched healthy male subjects with no personal history of mental illness and no family history of mental illness in 1st degree relatives were recruited by advertisement from among staff members at the Nanjing Brian Hospital. Individuals who did not meet any of the exclusion criteria (above items c through f)and who signed a written informed consent underwent a MRI examination

Figure 1. Enrollment of cases and controls
The protocol was approved by the Ethics Committee of the Nanjing Medical University-Affiliated Nanjing Brain Hospital.
2.2 Image acquisition and preprocessing
MRI data were acquired using a 3.0-Tesla Siemens Verio MRI scanner. T1-weighted (spin–lattice relaxation timeweighted) images were obtained using the following specifications: repetition time (TR)=1900 ms, echo time(TE)=2.48 ms, thickness/gap=1.0/0mm, flip angle=9°,inversion time=900 ms. DTI (diffusion tensor imaging)were acquired using the following specifications:TR=6600 ms, TE=93 ms, thickness/gap=3/3mm, flip angle=90°, 30 diffusion directions with b=1000 s/mm2.An additional image without diffusion weighting [i.e.,b=0 s/mm2] was also obtained.
Data preprocessing was performed using the Functional Magnetic Resonance Imaging of the Brain Software Library-FMRIB’s Diffusion Toolbox (http://www.fmrib.ox.ac.uk/fsl/fdt/index.html). First, the DTI data were corrected for eddy current and motion artifact. Second, the diffusion tensors at each voxel were calculated. Third, the FA (fractional anisotropy)of each voxel was calculated and then the FA map was constructed.
2.3 Network construction and assessment of network integration
A network can be constructed by defining nodes and estimating edges. The definition of nodes and edges is important in the brain network construction as they are the most basic elements of a network. In this study, the procedure that we undertook to define the nodes and edges in each network were similar to those used in previous studies, which used an automated anatomical labelling (AAL) template to identify 45 cortical and subcortical regions in each hemisphere(see Appendix).[26]The steps in the construction of the network for each participant are shown in Figure 2.
The characteristic path length (CPL) is the most commonly used measure of network integration. It is estimated by averaging the length of the shortest paths between all pairs of nodes in the network.[27,28]We used the average CPL of the inter-hemispheric path lengths to assess the level of overall inter-network integration (termed LInter-H) between the left and right hemispheres and also assessed the level of intersubnetwork integration between the left and right frontal lobes (LInter-F), temporal lobes (LInter-T),parietal lobes (LInter-P), occipital lobes (LInter-O), and subcortical structures (LInter-S). Similarly, we used average CPL within each hemisphere to assess overall intra-network integration (LIntra-H) separately in the left and right hemisphere and also assessed the intra-subnetwork integration within the frontal lobes(LIntra-F), temporal lobes (LIntra-T), parietal lobes(LIntra-P), occipital lobes (LIntra-O), and subcortical structures (LIntra-S).[27]
2.4 Statistical analysis
Statistical comparisons of network metrics between the SZ and control groups were made using two-sample two-tailed t-tests. To address the problem of multiple comparisons in the lobe-network metrics, a false discovery rate (FDR) correction[29]was performed with the threshold of p=0.05. In the patient groups we also calculated the Pearson correlation coefficients between the network metrics and scores on the PANSS.
3. Results
The MRI exam was undertaken for 32 patients and 28 controls, but 8 patients and 4 controls did not complete the examination because excessive movement during the exam invalidated the results. All 48 individuals who completed the MRI (24 patients with SZ and 24 control subjects) were right-handed and of Han ethnicity. The mean (sd) age of the 24 patients with SZ who completed the MRI was 29.2 (6.8) years while that of the 24 control subjects was 31.3 (6.3) years (t=1.24, p=0.276). At the time of conducting the MRI, the 24 patients with SZ had a mean duration of illness of 6.5 (4.2) years, were using a mean chlorpromazine equivalent dose of antipsychotic medication of 400 (102) mg/day, and the mean total PANSS score and positive symptom, negative symptom,and general psychopathology PANSS subscales scores were 100.7 (11.1), 29.4 (3.9), 21.0 (3.1), and 50.3 (6.5),respectively.
3.1 Inter-hemispheric and intra-hemispheric characteristic path lengths in schizophrenia
As shown in Table 1, all characteristic path lengths(CPL) assessed were longer in the patient group than in the control group. Some of these differences were statistically significant: the overall inter-hemispheric CPL (LInter-H) and the overall left and right intrahemispheric CPL (LIntra-H) were significantly longer in the patient group than in the control group; the subnetwork inter-hemispheric CPLs for the left and right frontal lobes (LInter-F), temporal lobes (LInter-T), and subcortical structures (LInter-S) were significantly longer in patients than controls; and the subnetwork right intra-hemispheric CPL for the frontal lobe (LIntra-F) was significantly longer in patients than in controls.

Figure 2. Flowchart of brain white matter (WM) structural network construction for patients with schizophrenia and healthy controls

Table 1. Comparison of mean (sd) characteristic path lengths (CPLs) for overall and subnetwork interhemispheric and intra-hemispheric regions between 24 males with paranoid schizophrenia and 24 healthy male controls
3.2 Correlation analyses
Table 2 shows the correlational analysis (unadjusted for multiple testing) between the various CPL measures and psychotic symptoms assessed using the PANSS at the time of the MRI examination in the 24 patients with paranoid schizophrenia. Only 5 of the 72 correlation coefficients assessed exceeded 0.30, and only 1 was statistically significant (r=0.47,p=0.021) – the correlation between the PANSS negative symptoms subscale score and overall inter-hemispheric CPL.
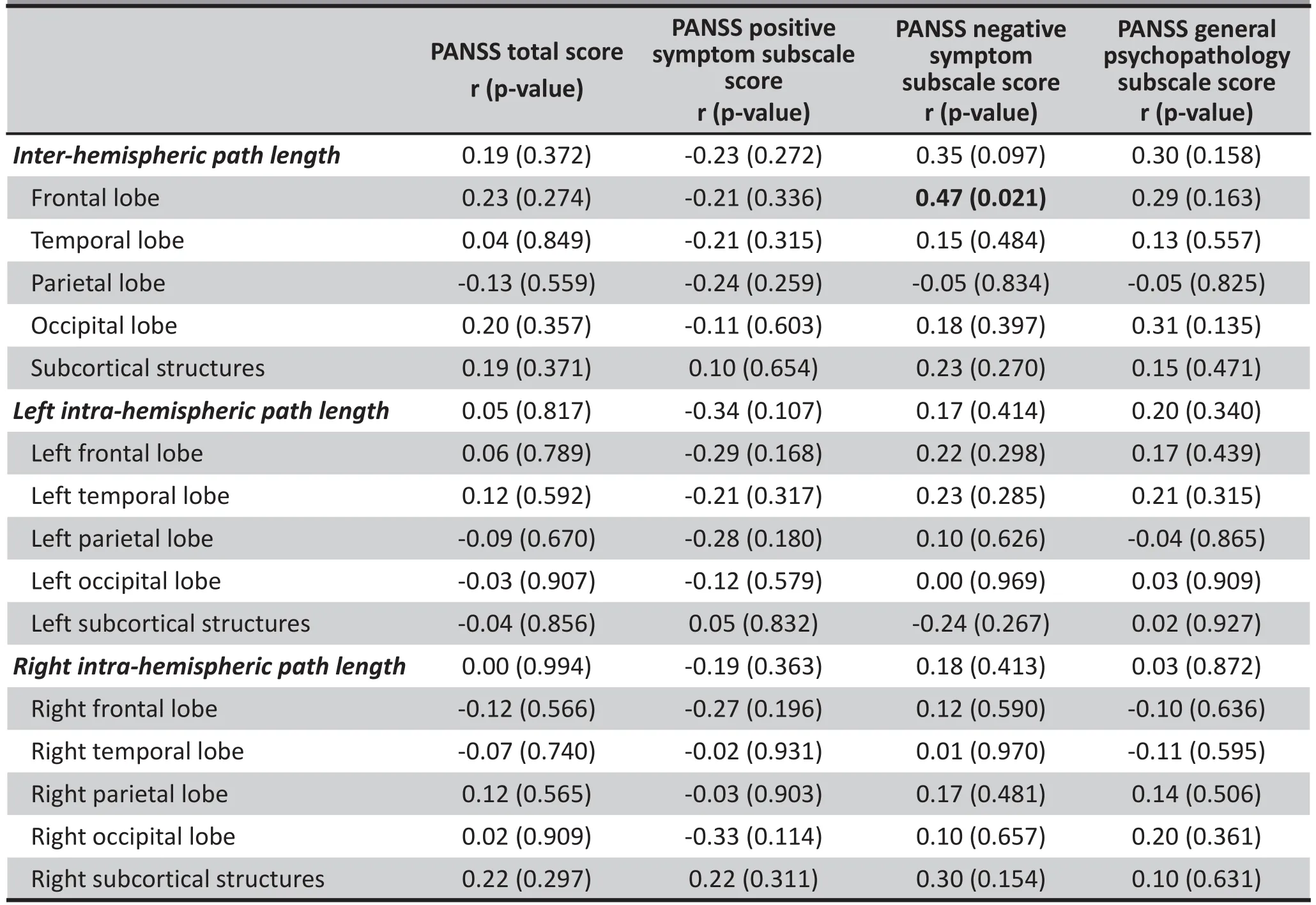
Table 2. Correlation between severity of psychotic symptoms (as assessment using the Positive and Negative Syndrome Scale [PANSS]) and the characteristic path length (CPL) for overall and subnetwork interhemispheric and intra-hemispheric regions in 24 males with paranoid schizophrenia
4. Discussion
4.1 Main findings
Overall our results show that the characteristic path lengths of both inter- and intra-hemispheric networks are longer in males with paranoid schizophrenia than in controls, suggesting reduced inter- and intrahemispheric integration.
4.1.1 Impaired Inter-Hemispheric Integration
Functional integration is a major organizational principle of the human brain.[30]An efficient brain requires shorter average path lengths (an index of global integration) of the network.[31]We found significantly longer overall inter-hemispheric characteristic path lengths (LInter-H) in schizophrenia, implying relatively sparse global connectedness and, thus, deficits in interhemispheric integration. These results provide evidence of abnormalities of structural connectivity between the left and right hemispheres in schizophrenia and, thus,support the brain network disconnection model of schizophrenia.
The connectivity of the left-right inter-subnetworks was reduced in schizophrenia in all brain regions assessed, but it was most evident in the frontal lobes,temporal lobes, and subcortical structures. Short average path length between interconnected brain regions promotes effective information exchange across the whole brain and is believed to be the basis of human cognitive processes,[28]so the longer subnetwork left-right inter-hemispheric CPL in patients with schizophrenia suggest impaired informational exchange that may be related to the deficits in cognition and abnormal behavior seen in schizophrenia.
Some previous studies suggest that altered interhemispheric connectivity in schizophrenia is related to changes in the functional connectivity, volume,and integrity of the corpus callosum (CC).[14,16,32]One structural imaging study reported smaller CC volumes in SZ (especially in the posterior genu, isthmus and splenium) and decreased fiber integrity in the CC in SZ.[14]Another diffusion tensor imaging (DTI) study reported reductions in the size of the CC in SZ.[16]Thus,the changes of the CC (the major inter-hemispheric fiber tracts) may contribute to the reduced inter-hemispheric integration in SZ.[10]This is a parallel finding to studies showing that integrity changes in the genu of the CC are associated with volume changes in the frontal lobes in SZ.[16]These results converge with those from previous studies using fMRI and structural MRI which suggested that inter-hemispheric connectivity disturbances may play a major role in hemispheric co-operation in SZ.[33,34]
4.1.2 Impaired Intra-Hemispheric Integration
We also found longer CPLs of both the left and right intra-hemispheric brain networks, suggesting impaired intra-hemispheric integration. Previous imaging studies reported that impairments in the uncinate fasciculus(UF), which connects the frontal and temporal lobes,might contribute to the slower intra-hemispheric processing in SZ.[35]However, our analysis of intrasubnetwork integration found that the reduced regional integration (i.e., the longer CPL) – though present in all subnetworks – was most prominent in the right frontal lobe. This result is consistent with previous imaging studies, which reported disruptions of tracts in the cingulo-opercular network in patients with schizophrenia.[5]
4.2 Limitations
There are several potential limitations to this study. All patients were males with paranoid schizophrenia who were taking antipsychotic medication during an active phase of their illness. We selected the sample in this manner to have a relatively homogeneous sample of patients (limiting variance in CPL between patients),but the down-side of this sampling process is that these results may not be representative of other cohorts of patients with schizophrenia; including female patients,patients with other subtypes of schizophrenia, patients in remission, and patients not taking antipsychotic medication.
The other major limitation of the study is that the sample size was relatively small, 24 individuals in each group. All 18 CPLs assessed were longer in the patient group than in the control group, but only in a few cases did these differences reach statistical significance; with a larger sample it is possible that all the differences would be statistically significant. And the correlation analysis between psychotic symptoms and the 18 different CPLs in the 24 patients only identified a single statistically significant correlation; it is probable that the correlations that exceeded 0.30 would have been statistically significant if a larger sample were assessed.
4.3 Implications
Our findings provide direct evidence of significantly longer characteristic path lengths of both overall inter-hemispheric networks and overall left and right intra-hemispheric networks in males with paranoid schizophrenia during the acute phase of their illness.Moreover, all subnetwork path lengths were also longer in patients than controls, though only some of these differences reached statistical significance. This finding supports hypotheses about the central role of reduced inter-hemispheric and intra-hemispheric integration in the etiology and course of schizophrenia. Based on this perspective, schizophrenia may be considered a ‘disconnection disorder’ of the brain network. The findings about the relationship of inter- and intrahemispheric integration and the severity of psychotic symptoms were inconclusive, probably due to the relatively small sample. All of these findings need to be confirmed with larger samples that including female patients, those with other subtypes of schizophrenia,and those not in acute phases of the illness.
Conflict of interest
The authors declare no conflict of interest related to this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81371522, 61372032);the Jiangsu Clinical Medicine Technology Foundation(BL2012052, BL2014009); and the Jiangsu Natural Science Foundation (BK2012740, BK20131074).
Ethics approval
This study was approved by the Ethics Committee of the Nanjing Medical University Affiliated Nanjing Brain Hospital.
Informed consent
All participants in the patient group or their legal guardians provided written informed consent to participate in this study and all healthy control subjects provided written informed consent to participate in the study.
1. Van Os J, Kapur S. Schizophrenia.Lancet. 2009; 374(9690):635-645. doi: http://dx.doi.org/10.1016/S0140-6736(09)60995-8
2. Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia.Curr Opin Psychiatry.2013; 26(2): 172-187. doi: http://dx.doi.org/10.1097/YCO.0b013e32835d9e6a
3. Tepest R, Schwarzbach CJ, Krug B, Klosterkotter J, Ruhrmann S, Vogeley K. Morphometry of structural disconnectivity indicators in subjects at risk and in age-matched patients with schizophrenia.Eur Arch Psychiatry Clin Neurosci.2013;263(1): 15-24. doi: http://dx.doi.org/10.1007/s00406-012-0343-6
4. Mwansisya TE, Wang Z, Tao H, Zhang H, Hu A, Guo S, et al.The diminished interhemispheric connectivity correlates with negative symptoms and cognitive impairment in first-episode schizophrenia.Schizophr Res. 2013; 150(1): 144-150. doi: http://dx.doi.org/10.1016/j.schres.2013.07.018
5. Tu PC, Hsieh JC, Li CT, Bai YM, Su TP. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study.Neuroimage.2012; 59(1): 238-247. doi: http://dx.doi.org/10.1016/j.neuroimage.2011.07.086
6. Hoptman MJ, Zuo XN, D’angelo D, Mauro CJ, Butler PD,Milham MP, et al. Decreased interhemispheric coordinati on in schizophrenia: a resting state fMRI study.Schizophr Res.2012; 141(1): 1-7. doi: http://dx.doi.org/10.1016/j.schres.2012.07.027
7. Jung WH, Jang JH, Shin NY, Kim SN, Choi CH, An SK, et al.Regional brain atrophy and functional disconnection in Broca’s area in individuals at ultra-high risk for psychosis and schizophrenia.PLoS One. 2012; 7(12): e51975. doi: http://dx.doi.org/10.1371/journal.pone.0051975
8. Sheng J, Zhu Y, Lu Z, Liu N, Huang N, Zhang Z, et al. Altered volume and lateralizati on of language-related regions in first-episode schizophrenia.Schizophr Res. 2013; 148(1-3): 168-174. doi: http://dx.doi.org/10.1016/j.schres.2013.05.021
9. Pu W, Li L, Zhang H, Ouyang X, Liu H, Zhao J, et al.Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia.Schizophr Res.2012; 141(1): 15-21. doi: http://dx.doi.org/10.1016/j.schres.2012.07.017
10. Van Den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, HulshoffPol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis.J Neurosci.2010; 30(47): 15915-15926. doi: http://dx.doi.org/10.1523/JNEUROSCI.2874-10.2010
11. Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis.Schizophr Res. 2012; 137(1-3): 169-173. doi:http://dx.doi.org/10.1016/j.schres.2012.01.038
12. Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM,Mesholam-Gately RI, et al. Extensive white matter abnormalities in patients with first-episode schizophrenia:a Diffusion Tensor Iimaging (DTI) study.Schizophr Res.2013; 143(2-3): 231-238. doi: http://dx.doi.org/10.1016/j.schres.2012.11.029
13. Quan M, Lee SH, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, et al. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia.Schizophr Res.2013; 145(1-3): 1-10.doi: http://dx.doi.org/10.1016/j.schres.2012.11.028
14. Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, Van De Ven V, Prvulovic D, et al. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaff ected relati ves.Neuroimage.2012; 59(2): 926-934. doi:http://dx.doi.org/10.1016/j.neuroimage.2011.07.088
15. Steinmann S, Leicht G, Mulert C. Interhemispheric auditory connectivity: structure and function related to auditory verbal hallucinati ons.Front Hum Neurosci.2014; 8: 55. doi:http://dx.doi.org/10.3389/fnhum.2014.00055
16. Whitford TJ, Kubicki M, Schneiderman JS, O’donnell LJ,King R, Alvarado JL, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia.Biol Psychiatry. 2010; 68(1): 70-77. doi:http://dx.doi.org/10.1016/j.biopsych.2010.03.025
17. Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, et al. Small-world networks and disturbed functional connectivity in schizophrenia.Schizophr Res.2006; 87(1-3): 60-66. doi: http://dx.doi.org/10.1016/j.schres.2006.06.028
18. Yu Q, Sui J, Rachakonda S, He H, Gruner W, Pearlson G, et al. Altered topological properties of functional network connectivity in schizophrenia during resting state: a smallworld brain network study.PLoS One. 2011; 6(9): e25423.doi: http://dx.doi.org/10.1371/journal.pone.0025423
19. Luders E, Gaser C, Narr KL, Toga AW. Why sex matters:brain size independent differences in gray matter distributions between men and women.J Neurosci.2009;29(45): 14265-14270. doi: http://dx.doi.org/10.1523/JNEUROSCI.2261-09.2009
20. Bao AM, Swaab DF. Sex differences in the brain, behavior,and neuropsychiatric disorders.Neuroscienti st. 2010; 16(5):550-565. doi: http://dx.doi.org/10.1177/1073858410377005
21. Zhang XY, Chen Da C, Xiu MH, Yang FD, Haile CN, Kosten TA,et al. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients.J Clin Psychiatry.2012; 73(7): 1025-1033. doi: http://dx.doi.org/10.4088/JCP.11m07422
22. Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, et al. A meta-analysis of sex differences in human brain structure.Neurosci Biobehav Rev.2014; 39: 34-50. doi: http://dx.doi.org/10.1016/j.neubiorev.2013.12.004
23. Guo S, Kendrick KM, Zhang J, Broome M, Yu R, Liu Z, et al.Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression.NeuroImage: Clinical.2013; 2: 818-826.doi: http://dx.doi.org/10.1016/j.nicl.2013.06.008
24. Spitzer R L, Gibbon M, Williams JB.Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition(February 1996 Final), SCID-I/P[M].Biometrics Research Department, New York State Psychiatric Institute; 1998
25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia.Schizophr Bull.1987; 13(2): 261-276
26. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain.Neuroimage. 2002; 15(1): 273-289. doi: http://dx.doi.org/10.1006/nimg.2001.0978
27. Leow A, Ajilore O, Zhan L, Arienzo D, Gadelkarim J,Zhang A, et al. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses.Biol Psychiatry.2013; 73(2): 183-193. doi: http://dx.doi.org/10.1016/j.biopsych.2012.09.014
28. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations.Neuroimage.2010; 52(3): 1059-1069. doi: http://dx.doi.org/10.1016/j.neuroimage.2009.10.003
29. Meskaldji DE, Fischi-Gomez E, Griffa A, Hagmann P,Morgenthaler S, Thiran J P. Comparing connectomes across subjects and populations at different scales.NeuroImage.2013; 80: 416-425. doi: http://dx.doi.org/10.1016/j.neuroimage.2013.04.084
30. Bullmore E, Sporns O. The economy of brain network organization.Nat Rev Neurosci.2012; 13(5): 336-349. doi:http://dx.doi.org/10.1038/nrn3214
31. Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, et al. Agerelated changes in topological organization of structural brain networks in healthy individuals.Hum Brain Mapp.2012; 33(3): 552-568. doi: http://dx.doi.org/10.1002/hbm.21232
32. Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia.Schizophr Res.2011; 129(2-3): 149-155. doi: http://dx.doi.org/10.1016/j.schres.2011.03.014
33. Guo S, Kendrick KM, Zhang J, Broome M, Yu R, Liu Z, et al.Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression.Neuroimage Clin.2013; 2: 818-826. doi:http://dx.doi.org/10.1016/j.nicl.2013.06.008
34. Bleich-Cohen M, Sharon H, Weizman R, Poyurovsky M,Faragian S, Hendler T. Diminished language lateralizati on in schizophrenia corresponds to impaired inter-hemispheric functional connectivity.Schizophr Res. 2012; 134(2-3): 131-136. doi: http://dx.doi.org/10.1016/j.schres.2011.10.011
35. Kitis O, Ozalay O, Zengin EB, Haznedaroglu D, Eker MC,Yalvac D, et al. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia.Psychiatry Clin Neurosci.2012; 66(1): 34-43.doi: http://dx.doi.org/10.1111/j.1440-1819.2011.02293.x
, 2015-03-26; accepted, 2015-06-13)

Jianhuai Chen is a postgraduate in the Brain Hospital of Nanjing Medical University majoring in psychiatry and mental health. His main research interest is the association between characteristics of brain imaging and clinical symptoms in patients with unipolar depression, bipolar depression, and schizophrenia using Diffusion Tensor Imaging and complex network theory analysis based on graph theory.

Dr. Zhijian Yao was educated in Nanjing Medical University where he obtained his bachelor’s degree,master’s degree and doctoral degree in 1993, 2003, 2009, respectively. He has been working at the Nanjing Medical University affiliated Brain Hospital since 1993. He is also the vice director of the Nanjing Neurology and Psychiatry Research Center and the director of Psychiatry Department,Nanjing Brain Hospital. He serves at the Jiangsu Medical Association as the vice chairman of Psychiatry branch and the head of the Depressive Disorders Group. Dr Yao is an editorial board member for the Clinical Psychiatry Journal, Adverse Drug Reactions Journal, and a series of other Chinese journals; he is a reviewer for the Journal of Affective Disorder, Psychiatry Research and Neuroreport. His research interests are the neuroimaging and genetics of depressive disorders.
男性偏执型精神分裂症患者半球间与半球内整合异常:一项图形理论分析
陈建淮,姚志剑,秦娇龙,阎锐,花玲玲,卢青
偏执型精神分裂症;扩散张量成像;大脑成像;特征路径长度;中国
Background: The human brain is a complex network of regions that are structurally interconnected by white matter (WM) tracts. Schizophrenia (SZ) can be conceptualized as a disconnection syndrome characterized by widespread disconnections in WM pathways.Aims: To assess whether or not anatomical disconnections are associated with disruption of the topological properties of inter- and intra-hemispheric networks in SZ.Methods: We acquired the diffusion tensor imaging data from 24 male patients with paranoid SZ during an acute phase of their illness and from 24 healthy age-matched male controls. The brain FA-weighted(fractional anisotropy-weighted) structural networks were constructed and the inter- and intra-hemispheric integration was assessed by estimating the average characteristic path lengths (CPLs) between and within the left and right hemisphere networks.Results: The mean CPLs for all 18 inter- and intra-hemispheric CPLs assessed were longer in the SZ patient group than in the control group, but only some of these differences were significantly different: the CPLs for the overall inter-hemispheric and the left and right intra-hemispheric networks; the CPLs for the interhemisphere subnetworks of the frontal lobes, temporal lobes, and subcortical structures; and the CPL for the intra- frontal subnetwork in the right hemisphere. Among the 24 patients, the CPL of the inter-frontal subnetwork was positively associated with negative symptom severity, but this was the only significant result among 72 assessed correlations, so it may be a statistical artifact.Conclusions: Our findings suggest that the integrity of intra- and inter-hemispheric WM tracts is disrupted in males with paranoid SZ, supporting the brain network disconnection model (i.e., the ‘connectivity hypothesis’) of schizophrenia. Larger studies with less narrowly defined samples of individuals with schizophrenia are needed to confirm these results.
[Shanghai Arch Psychiatry. 2015; 27(3): 158-166.
http://dx.doi.org/10.11919/j.issn.1002-0829.215036]
1Department of Psychiatry, Nanjing Medical University affiliated Nanjing Brain Hospital, Nanjing, Jiangsu Province, China
2Nanjing University Medical School, Nanjing, Jiangsu Province, China
3Research Center of Learning Science, Southeast University, Nanjing, Jiangsu Province, China
4Research Institute of Suzhou, Southeast University, Suzhou, Jiangsu Province, China
#Jianhuai CHEN and Zhijian YAO are joint first authors
*correspondence: Zhijian Yao: zjyao@njmu.edu.cn; Qing Lu: luq@seu.edu.cn
背景:人类大脑是由白质(WM)束结构性互相连结的复杂网络。精神分裂症(schizophrenia, SZ)可以被概念化为以WM路径广泛断开为特征的中断综合征。目的:评估解剖性断开是否与SZ患者的大脑半球内与大脑半球间的拓扑性质破坏有关。方法:我们采集了24例处于发病急性期的偏执型精神分裂症男性患者和24例与之年龄匹配的健康男性对照组的弥散张量成像(diffusion tensor imaging)数据。本研究构建了大脑的部分各向异性加权的(fractional anisotropy-weighted, FA-weighted)结构网络,并且通过估计平均特征路径长度(characteristic path lengths,CPLs)评估大脑内半球间与半球内的整合。结果:SZ患者组的全部18个所评估的半球间与半球内的CPL值均值均长于对照组,但这些差异中只有一些具有显著性,包括:整体半球间、左半球内部和右半球内部网络的CPL;额叶、颞叶和皮质下结构的半球间子网络的CPL;以及右半球内额叶子网络的CPL。在24例患者中,双侧额叶子网络间的CPL与阴性症状严重程度正相关,但这是72个相关性分析中唯一显著的结果,所以它可能只有统计学上的显著意义。结论:我们的研究结果表明,男性偏执型SZ患者的脑半球内和半球间白质束的完整性被破坏,这支持了精神分裂症的大脑网络中断模型(即“连通性假说”)。我们需要对更特异的精神分裂症患者样本进行更大规模的研究来证实这些结果。
本文全文中文版从2015年08月06日起在http://dx.doi.org/10.11919/j.issn.1002-0829.215036可供免费阅览下载

Appendix. Name and abbreviation of 45 cortical and sub-cortical brain regions identified by the automated anatomical labelling (AAL) template used in this study
- 上海精神医学的其它文章
- Meta-analysis for psychiatric research using free soft ware R
- Development of a checklist of short-term and long-term psychological symptoms associated with ketamine use
- Oligodendroglioma presenti ng as chronic mania
- Comparisons of family environment between homeless and non-homeless individuals with schizophrenia in Xiangtan, Hunan
- Metacognitive training for schizophrenia: a systematic review
- Addressing risk of bias in trials of cognitive behavioral therapy

