抗坏血酸对暗诱导拟南芥叶片衰老的影响
李 媚, 陈晓锋, 彭长连
(华南师范大学生命科学学院,广州 510631)
抗坏血酸对暗诱导拟南芥叶片衰老的影响
李媚, 陈晓锋, 彭长连*
(华南师范大学生命科学学院,广州 510631)
分析黑暗诱导衰老条件下3种内源抗坏血酸含量不同的拟南芥(GLDH基因超表达植株gldh236OE、哥伦比亚野生型Col和拟南芥抗坏血酸缺乏突变体vtc2-1)表型、抗坏血酸含量及叶绿素荧光参数等生理指标的变化,研究抗坏血酸对暗诱导拟南芥叶片衰老的影响.结果表明,黑暗胁迫后3种拟南芥抗坏血酸含量均呈下降趋势,与Col相比,vtc2-1的抗坏血酸含量显著降低(P<0.05),叶片黄化速度明显较快;gldh236OE叶片黄化速度较慢;黑暗均引起了PSII最大光能转化效率(Fv/Fm)和PSII实际光化学量子产量(Yield)降低,PSII的光合效率受到阻碍;也减少了vtc2-1非光化学猝灭(NPQ),外施抗坏血酸对植株热耗散能力显著增强(P<0.05).研究表明,内源抗坏血酸含量的缺失会导致植株对黑暗胁迫的适应性降低,外施1 mmol/L抗坏血酸能部分缓解植物的黑暗胁迫.
拟南芥; 抗坏血酸; 黑暗处理; 叶绿素荧光参数; 抗逆性
植物衰老是指一个器官或整个植物生命功能逐渐衰退的过程,植物衰老的普遍特征:叶绿素、蛋白质迅速丧失及RNA水解等称为“衰老综合征”,并认为衰老是由内部因素控制的导致死亡的衰退过程.已有报道提出衰老原因的假说,其中生物自由基伤害学说受到较多关注[1].植物在正常代谢过程中会产生活性氧自由基(reactive oxygen species, ROS),在细胞中活性氧的产生及清除处于动态平衡,当植物在衰老或受到外界环境胁迫过程中产生并积累过量的活性氧[2].
抗坏血酸(ascorbicacid, AsA)也称维生素C,是植物细胞中大量存在的有机小分子,在活性氧清除系统中起着重要作用,参与抗氧化、光保护、调节生长发育、衰老调控以及胁迫响应等多种生理调控过程[3-6].在合成抗坏血酸主要途径L-半乳糖途径[7]中,L-半乳糖-1,4-内酯脱氢酶(L-galactono-1,4-lactonedehydrogenase, GLDH)是调节最后一步的关键酶[8],定位于植物细胞线粒体内膜上[9],相对分子量约为56×103[8],专一地以细胞色素C(Cytc)作为酶促反应的电子受体,催化还原Cytc并直接氧化L-半乳糖内酯(Gal)生成AsA[10].VTC2基因位于拟南芥的4号染色体[11],与VTC5基因编码GDP-L-半乳糖磷酸酶(GGPase),是L-半乳糖途径的第六步的关键酶[12-13],催化GDP-L-半乳糖转化成L-半乳糖-1-磷酸[14].有研究报道指出VTC2与GLDH基因控制植物体内AsA的含量,在植物生长与发育以及抗逆性等方面起重要作用[15-16].
光照是影响植物衰老的一个重要外源因素,黑暗胁迫被认为是最有效、最简便的诱导衰老的信号,用于研究叶片衰老的机制[17-18].当植株在黑暗下生长时,叶绿素加速降解伴随着光合功能被破坏的现象被称为黑暗诱导衰老.Weaver等[19]指出拟南芥成熟连体叶片被整株置于黑暗环境下时不能引发衰老,但黑暗胁迫会诱导离体叶片的衰老.当整株拟南芥置于黑暗环境中,成熟连体叶片的衰老不是被诱发而是部分被抑制,但是当单个叶片处于黑暗而植株其余部分处于光照环境时,黑暗条件极大地加速了叶片的衰老[17].一般而言,抗坏血酸与植物的抗逆性呈正相关,即植物细胞内的抗坏血酸的含量增加,植物耐炎热、寒冷和盐碱环境等逆境的能力也会增强[20]. 如果植物体内AsA缺失往往会诱发氧化胁迫,进而触发细胞提前衰老[21-22],而外施AsA能够延缓细胞衰老[23].为了解抗坏血酸对拟南芥叶片衰老的生理生化变化,本文采用黑暗诱导的方法研究叶片衰老过程中抗坏血酸含量、表型以及叶绿素荧光参数的变化,为认识拟南芥抵御外界环境胁迫提供理论依据.
1 材料与方法
1.1植物材料
所用植物材料拟南芥(ArabidopsisthalianaL.)为哥伦比亚野生型(Col);gldh突变体株系SALK_008236(经鉴定为GLDH基因超表达植株),购于美国拟南芥突变体种子库(http://www.arabidopsis.org/),本文用gldh236OE表达;抗坏血酸合成缺失植株vtc2-1,由Patricia Müller-Moulé教授实验室(Developmental and Molecular Plant Biology, Heinrich-Heine-University, Düsseldorf, Germany)赠予.
拟南芥种子置于4 ℃冰箱春化2~3 d;经10%次氯酸钠消毒10 min、70%乙醇表面消毒30 s、无菌水冲洗4~5次后,均匀播种于MS培养基上.10~12 d后移至土壤中, 在光周期为16 h光/8 h暗,培养温度为22±2 ℃,光照强度为70~80 μmol/(m2·s)条件下培养,2~3 d浇水1次;将苗龄4周的幼苗转移至完全黑暗条件下(其它生长条件不变)培养.
1.2抗坏血酸含量测定
参照Gillespie等[24]的方法并改进,取拟南芥新鲜莲座叶的成熟叶片约0.05 g,用2 mL预冷的6%三氯乙酸充分研磨、震荡混匀后置于冰上.待所有样品研磨完毕,4 ℃、13 000 r/min离心5 min.取上清液加入2 mL反应体系中,37 ℃水浴1 h,用分光分光计检测525 nm的吸光值.
1.3叶绿素荧光参数的测定
参照柯展鸿等[25]的方法并改进,叶绿素荧光参数Fv/Fm和Yield和NPQ均由便携式荧光测定仪(PAM—2100,Germany)测定和计算得出.测定前先将植株暗适应30 min,测定初始荧光Fo和暗下最大荧光Fm.经光活化后,测定光下最大荧光F′m和稳态荧光Fs,光化光强度为80 μmol/(m2·s).最大原初光化学量子效率Fv/Fm、实际光化学效率Yield和非光化学淬灭NPQ的计算公式如下:
Fv/Fm=(Fm-Fo)/Fm,Yield=l-Fs/Fm,NPQ=(Fm-F′m)/F′m.
1.4数据统计分析
实验数据用Microsoft Excel 2010进行整理,用SPSS 12.0 One Way ANOVA 对数据进行统计分析,求出平均值及标准误,对同一测定指标在不同处理间的差异进行方差分析,并用LSD法检验各处理与对照组的差异显著性,获得的数据利用SigmaPlot12.0进行绘图.统计分析采用T检验.
2 结果与分析
2.1黑暗条件下不同GLDH基因型植株AsA含量与表型的变化
经黑暗处理的不同GLDH基因型5周龄幼苗总抗坏血酸含量变化趋势相同(图1).黑暗处理3 d,Col、gldh236OE和vtc2-1的总抗坏血酸含量分别为处理前的54%、53%和29%,显著降低(P<0.01),其中vtc2-1降幅最明显;黑暗处理10 d,总AsA达到最大值.与Col相比,除了处理前,vtc2-1无显著差异;gldh236OE的总抗坏血酸含量一直处于同时期的最大值.
Col、gldh236OE和vtc2-1植株在黑暗处理7 d后开始衰老(图2).与对照Col相比,抗坏血酸缺失突变体vtc2-1在黑暗处理后黄化较严重且出现卷曲,甚至枯死的表征,而抗坏血酸超表达突变体gldh236OE受损较轻微,从水处理组分析,叶片黄化速度vtc2-1>Col>gldh236OE;同时期同基因型外源施加1 mol/L AsA处理,拟南芥黄化速度没有显著缓解,所有植株在黑暗处理15 d时完全死亡.
2.2黑暗条件下不同GLDH基因型植株光合作用的变化
图3显示了黑暗胁迫处理条件下,抗坏血酸含量不同的拟南芥幼苗叶片叶绿素荧光参数Fv/Fm、Yield、NPQ的变化.其中叶绿素荧光参数Fv/Fm代表PSII光化学反应的最大量子产率,反映PSII反应中心捕获激发能的最大量子效率与利用能力,是植物PSII受害程度的重要指标[26].PSII实际光化学量子产量Yield,能反映PSII对吸收光能的利用情况和光合电子传递链的电子传递的真实速率,能更好地反应PSII的功能[27].黑暗处理后,不同基因型植株的叶片Fv/Fm与Yield均呈下降趋势,说明幼苗叶片的PSII均受到损伤,随着胁迫时间延长损伤加剧;三者间无显著性差异,外施抗坏血酸组与对照组差异不显著. 非光化学淬灭系数NPQ反映的是PSII天线色素吸收的光能不能用于光合电子传递而以热的形式耗散掉的光能部分.当PSII反应中心天线色素吸收了过量的光能时,如不能及时地耗散将对光合机构造成损伤,所以非光化学淬灭是光合机构的一种保护机制[28].图3E表示,抗坏血酸外施组的不同基因型幼苗的NPQ呈先上升后下降的趋势.黑暗处理3 d,3种基因型幼苗NPQ均增加;黑暗处理3 d之后,NPQ均不同程度下降;黑暗处理10 d,gldh236OE的NPQ有部分回升;不同基因型幼苗无显著性差异.图3F表示,对照组的Col与gldh236OE的NPQ呈先上升后下降的趋势,而vtc2-1则呈相反的趋势.黑暗胁迫处理3 d后,抗坏血酸外施组Col与vtc2-1的NPQ分别是对照组的123.64%、138.68%,显著高于对照组(P<0.05).
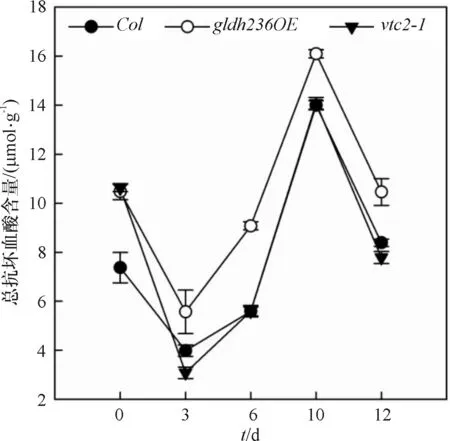
图1暗诱导衰老条件下Col、gldh236OE和vtc2-1植株总抗坏血酸含量变化(平均值±标准误,n=4)
Figure 1Changes of total AsA contents in leaves of 5-week-oldCol,gldh236OEandvtc2-1 plants under dark-induced senescence condition (Mean±SE,n=4)
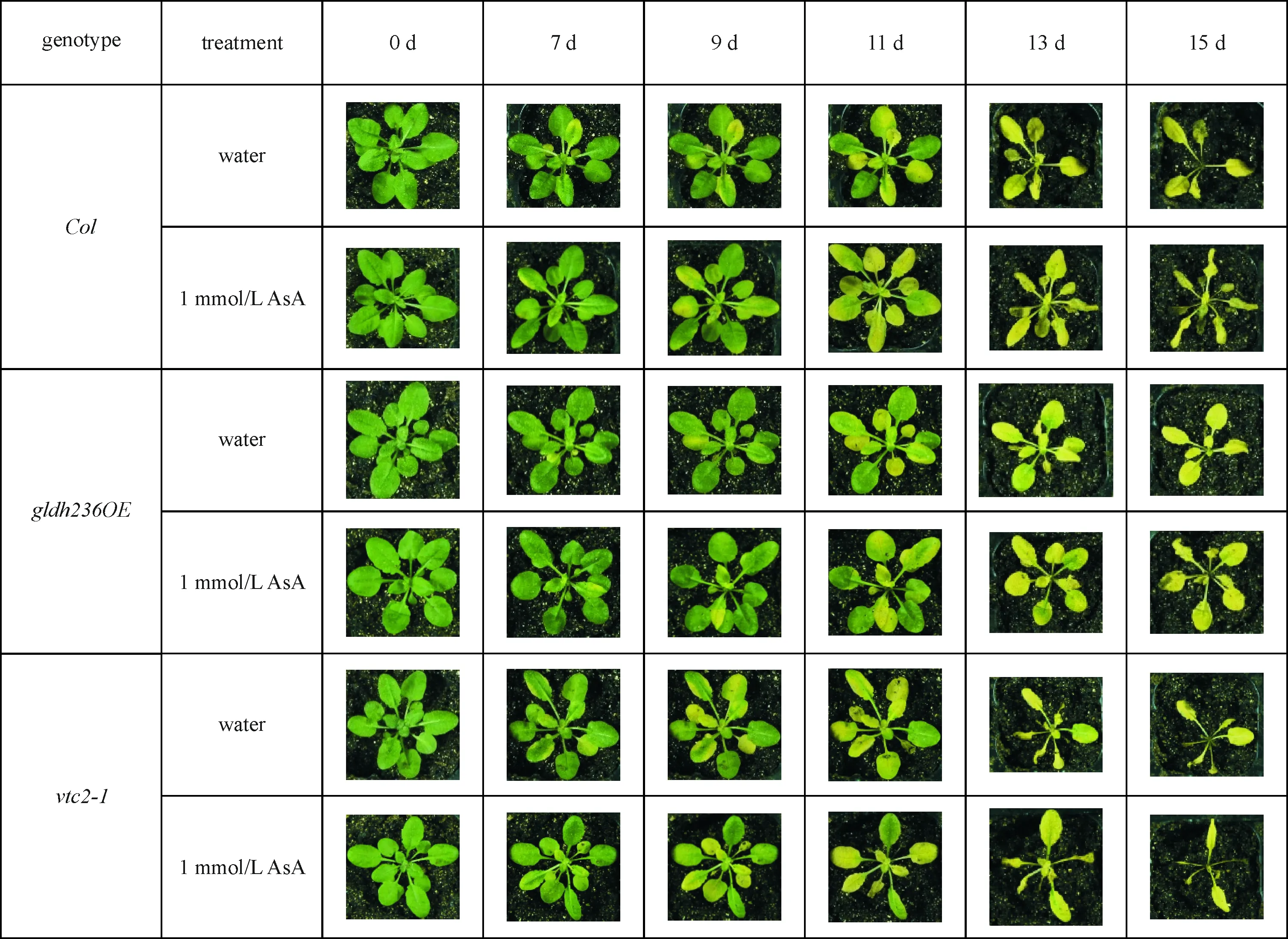
图2 抗坏血酸含量对暗诱导衰老条件下植株表型的影响
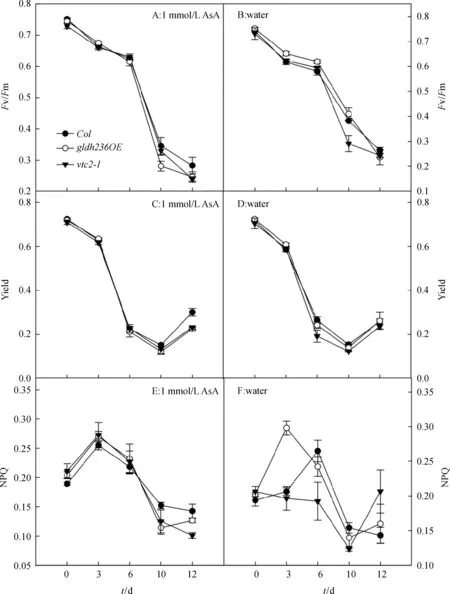
图3 暗条件下苗龄4周Col、gldh236OE和vtc2-1植株叶绿素荧光参数指标变化(平均值±标准误,n=4).
Figure 3Changes of chlorophyll fluorescence parameters in 4-week-oldCol,gldh236OEandvtc2-1 plants under dark condition (Mean±SE,n=4). Student’s t-test was used to calculate statistical significance.
3 讨论
本研究中,抗坏血酸合成超表达突变体gldh236OE与抗坏血酸合成缺失突变体vtc2-1的AsA含量,分别比野生型增加约32%或减少70%~80%[11],实现了从内源调控抗坏血酸合成的含量.有报道指出,通过超表达水稻GLDH基因可提高抗坏血酸的合成量,延缓水稻衰老,提高结实率,而干涉水稻GLDH基因表达使抗坏血酸合成量减少,加速水稻衰老,结实率降低[29].过表达VTC2基因可提高植物体内抗坏血酸含量[12-13,30],而vtc2-1突变体与野生型相比,抗坏血酸含量更低且株型更小[12].
VTC2与GLDH基因的转录具有光诱导特性,其特性与光诱导植物细胞中AsA含量增加一致.Tamaoki等[31]发现,拟南芥GLDH基因的表达呈现出昼夜变化并且受光调控,它在调控AsA库的大小上可能起作用.Dowdle等[12]发现,在强光条件下,诱导VTC2基因表达,GDP-L-半乳糖磷酸酶活性增加,同时植物叶片中AsA含量上升.超表达GLDH基因植物体内AsA含量提高,一定程度提高植株对强光的抗性[15].
黑暗诱导的叶片衰老具有个体与基因型之间的同步性,因此被作为模型应用到很多叶片衰老的研究中,然而其具体的调控机制仍不清楚.在本研究中,黑暗胁迫处理后,3种基因型幼苗的AsA含量均显著下降(P<0.05);在黑暗胁迫处理6 d后,与处理前相比,抗坏血酸缺失突变体vtc2-1的抗坏血酸含量显著降低(P<0.05),进一步印证了VTC2与GLDH基因具有光诱导特性.黑暗胁迫处理后,不同基因型幼苗开始出现不同程度的黄化、卷曲,甚至枯死的表征.与对照Col相比,gldh236OE衰老特征较轻,抗坏血酸缺失突变体vtc2-1衰老更为明显.推测可能在拟南芥幼苗受到黑暗胁迫过程中,较高含量的抗坏血酸能够提高植株对黑暗的适应性,维持正常的生理状态.但是,外施AsA除了显著提高抗坏血酸缺失突变体vtc2-1叶绿素荧光参数的非光化学淬灭系数NPQ(P<0.05),对于其他部分暗诱导的衰老并没有得到显著性缓解.
生物或非生物胁迫对植物光合作用过程产生的影响都可通过叶片叶绿素荧光诱导动力学参数的变化反映出来[32].Fv/Fm的降低是光抑制的显著特征,说明植物可能遭遇逆境,受到光抑制,光抑制还会引起PSII反应中心的破坏或失活,导致PSII的下降[33].本实验中研究叶片衰老的结果显示,与黑暗胁迫处理前相比,3种基因型拟南芥Fv/Fm显著降低,并且随着胁迫时间延长而加剧,说明黑暗胁迫使幼苗产生了光抑制,可能造成了PSII反应中心的失活,使光能转化效率降低. Yield呈下降趋势,而后期有部分上升,进一步说明PSII反应中心的功能受到可逆的损伤.与黑暗处理前相比,Col与gldh236OE幼苗的NPQ随黑暗处理时间的延长呈先上升后下降的趋势,说明Col与gldh236OE幼苗热耗散能力是先增强后减弱,推测拟南芥幼苗在遭受胁迫后采取的一种应激反应抵抗外界环境胁迫,但是NPQ的形成受到了抑制.而vtc2-1幼苗的NPQ则无显著变化,推测抗坏血酸缺失会降低幼苗对外界环境胁迫的响应.而内源AsA的增加和外施AsA均减缓了黑暗胁迫对幼苗叶绿素荧光参数NPQ的影响,说明AsA有利于幼苗在黑暗胁迫的生理响应,可能是内源AsA对黑暗胁迫下幼苗的PSII反应中心有一定的保护作用,增加其热耗散能力,以增强幼苗对黑暗胁迫的适应性,而外施AsA可补充内源AsA不足,在一定程度缓解植物受到响应胁迫.
众所周知,衰老与抗氧化能力的下降和ROS的增加有密切关系[34].VTC2与GLDH基因表达量降低导致抗坏血酸含量减少,低水平的抗坏血酸可以导致ROS的积累,光合器官的损害将会增加,导致缺少抗坏血酸的光合组织的光合能力下降,衰老也会因此被加速.AsA不仅能作为电子供体维持光合作用, 还在保护光合器官方面起到重要作用[35].AsA通过参与水循环、谷胱甘肽-抗坏血酸循环或者作为APX的辅助因子消除光呼吸等过程产生的过剩ROS,保护光合功能[36].本研究中抗坏血酸含量最高的gldh236OE在黑暗诱导的衰老中能够维持更高的叶片光合能力也证实了AsA的功能[33].
[1]McCord J M, Fridovich I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein)[J]. Journal of Biological Chemistry, 1969, 244(22):6049-6104.
[2]王宇涛,陈志勇,曾琬淋,等. 拟南芥对镉胁迫的生理响应[J]. 华南师范大学学报:自然科学版, 2014, 46(2):99-107.
Wang Y T, Chen Z Y, Zeng W L, et al.Physiological responses ofArabidopsisthalianato cadmium stress[J].Journal of South China Normal University:Natural Science Edition, 2014, 46(2):99-107
[3]Smirnoff N. Botanical briefing: The function and metabolism of acid in plants[J]. Annals of Botany, 1996, 78(6):661-669.
[4]Fotopoulos V, Kanellis A K. Altered apoplastic ascorbate redox state in tobacco plants via ascorbate oxidase overexpression results in delayed dark-induced senescence in detached leaves[J]. Plant Physiology and Biochemistry, 2013, 73(6):154-160.
[5]Ivanov N B. Role of ascorbic acid in photosynthesis[J]. Biochemistry (Moscow), 2014, 79(3):282-289.
[6]Gallie D R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth[J]. Journal of Experimental Botany, 2013, 64(2): 433-443.
[7]Wheeler G L, Jones M A, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants[J]. Nature, 1998, 393(6683):365-369.
[8]Oba K, Ishikawa S, Nishikawa M, et al. Purification and properties of L-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis,from sweet potato roots[J].Journal of Biochemistry, 1995, 117(1): 120-124.
[9]Siendones E, Gonzalez-Reyes J A, Santos-Ocana C, et al. Biosynthesis of ascorbic acid in kidney bean: L-galactono-gamma-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane[J]. Plant Physiology, 1999, 120(3):907-912.
[10]Imai T, Karita S, Shiratori G I, et al. L-galactono-γ-lactone dehydrogenase from sweet potato: Purification and cDNA sequence analysis[J]. Plant & Cell Physiology, 1998, 39(12):1350-1358.
[11]Conklin P L, Saracco S A, Norris S R, et al. Identification of ascorbic acid-deficient Arabidopsis thalian mutants[J]. Genetics, 2000, 154(2):847-856.
[12]Dowdle J, Ishikawa T, Gatzek S, et al. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability[J]. Plant Journal, 2007, 52(4):673-689.
[13]Linster C L. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants[J]. Journal of Biological Chemistry, 2007, 282(26):18879-18885.
[14]Linster C L, Adler L N, Webb K, et al. A second GDP-L-galactose phosphorylase in Arabidopsis en route to vitamin C[J]. Journal of Biological Chemistry, 2008, 283(27):18483-18492.
[15]Tokunaga T, Miyahara1 K, Tabata K, et al. Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for L-galactono-1,4-lactone dehydrogenase[J]. Planta, 2005, 220(6): 854-863.
[16]Alhagdow M, Mounet F, Gilbert L, et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato[J]. Plant Physiology, 2007, 145(4): 1408-1422.
[17]Weaver L M, Amasino R M. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants[J]. Plant Physiology, 2001, 127(3):876-886.
[18]Wada S, Ishida H, Izumi M, et al. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves[J]. Plant Physiology, 2009, 149(2):885-893
[19]Weaver L M, Gan S, Quirino B, et al. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment[J]. Plant Molecular Biology, 1998, 37(3): 455-469.
[20]邹礼平, 陈锦华. 植物抗坏血酸的合成和代谢以及相关酶基因的调控[J]. 植物生理学报,2009,45(9):925-930.
Zou L P, Chen J H. Biosynthesis and metabolism of ascorbic acid and regulation of related genes in plants[J]. Plant Physiology Journal, 2009, 45(9):925-930.
[21]Barth C, Conklin P L. The role of ascorbic acid in the control of flowering time and the onset of senescence[J]. Journal of Experimental Botany, 2006, 57(8):1657-1665.
[22]Kotchoni S O, Larrimore K E, Mukherjee M, et al. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis[J]. Plant Physiology, 2009, 149(2):803-815.
[23]黄文敏,邢伟,李敦海,等. 外源抗坏血酸对烟草细胞生长及衰老的影响[J]. 农业环境科学学报, 2006, 25(5):1157-1161.
Huang W M, Xing W, Li D H, et al. Effects of exogenous ascorbic acid on the growth and senescence of tobacco BY-2 suspension cells[J]. Journal of Agro-Environment Science, 2006, 25(5):1157-1161.
[24]Gillespie K M, Ainsworth E A. Measurement of reduced, oxidized and total ascorbate content in plants[J]. Nature Protocols, 2007, 2(4):871-874.
[25]柯展鸿,曾庆婷,何月雯,等. 模拟SO2污染对广东8种常见果树的胁迫效应[J]. 广东农业科学, 2013, 40(2):32-36.
Ke Z H, Zeng Q T, He Y W, et al. Effects of simulated SO2pollution on eight common fruit trees of Guangdong province[J]. Guangdong Agricultural Sciences, 2013, 40(2):32-36.
[26]White A J, Critchley C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus[J]. Photosynthesis Research, 1999, 59(1):63-72.
[27]Ralph P J, Gademann R. Rapid light curves: A powerful tool to assess photosynthetic activity[J]. Aquatic Botany, 2005, 82(3):222-237.
[28]张守仁. 叶绿素荧光动力学参数的意义及讨论[J]. 植物学通报, 1999, 16(4):444-448.
Zhang S R. A discussion on chlorophyll fluorescence kinetics parameters and their significance[J]. Chinese Bulletin of Botany, 1999, 16(4):444-448.
[29]Liu Y, Yu L, Wang R. Level of ascorbic acid in transgenic rice for l-galactono-1,4-lactone dehydrogenase overexpressing or suppressed is associated with plant growth and seed set[J]. Acta Physiologiae Plantarum, 2011, 33(4): 1353-1363.
[30]Urzica E I, Adler L N, Page M D, et al. Impact of oxidative stress on ascorbate biosynthesis in Chlamydomonas via regulation of the VTC2 gene encoding a GDP-L-galactose phosphorylase[J]. Journal of Biological Chemistry, 2012, 287(17):14234-14245.
[31]Tamaoki M, Mukai F, Asai N, et al. Light-controlled expression of a gene encoding L-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana[J]. Plant Science, 2003, 164(3):1111-1117.
[32]Maxwell K, Jonhnsin G N. Chlorophyll fluorescence-a practical guide[J]. Journal of Experimental Botany, 2000, 51(345):659-668.
[33]梁芳,郑成淑,孙宪芝,等. 低温弱光胁迫及恢复对切花菊光合作用和叶绿素荧光参数的影响[J]. 应用生态学报, 2010,21(1):29-35.
Liang F, Zheng C S, Sun X Z, et al. Effects of low temperature and weak light stress and its recovery on the photosynthesis and chlorophyll fluorescence parameters of cue flower chrysanthemum[J]. Chinese Journal of Applied Ecology, 2010,21(1):29-35.
[34]Zimmermann P, Zentgraf U. The correlation between oxidative stress and leaf senescence during plant development[J]. Cellular & Molecular Biology Letters, 2005, 10(3): 515-534.
[35]Ivanov B N. Role of ascorbic acid in photosynthesis[J]. Biochemistry, 2014, 79(3):282-289.
[36]Gallie D R. Increasing vitamin C content in plant foods to improve their nutritional value:Successes and challenges[J]. Nutrients, 2013, 5(9):3424-3446.
【中文责编:成文英文责编:李海航】
Effects of Ascorbate on Darkness Induced Aging in Arabidopsis
Li Mei, Chen Xiaofeng, Peng Changlian*
(School of Life Sciences, South China Normal University, Guangzhou 510631, China)
Arabidopsisthalianahad been selected as the research material in this study, to test the darkness stress on the eco-physiological characteristics (including phenotype, total ascorbate content and the chlorophyll fluorescence) of three genotype plants with different endogenous ascorbate contents (GLDHgene over-expression named asgldh236OE, wide typeColand the AsA deficient mutantsvtc2-1). The results indicated that the contents of total ascorbic acid of the three genotype plants were trended down under the darkness. Compared with the wide type (Col), total ascorbate content ofvtc2-1 was significantly reduced (P<0.05). The leaf etiolation speed ofvtc2-1 was faster thanCol, butgldh236OEwas slower. The darkness induced the decrease ofFv/Fm and Yield, which hindered photosynthetic efficiency of PSII. It also reduced the NPQ ofvtc2-1, and exogenous applied ascorbic acid markedly enhanced its capability of the heat dissipation (P<0.05). The present results indicated that the deficient of endogenous ascorbate reduced plants’ resistance to dark stress and exogenous ascorbate relieved the stress of the dark in part.
Arabidopsisthaliana; ascorbate; darkness; the chlorophyll fluorescence parameters; stress resistance
2015-04-07《华南师范大学学报(自然科学版)》网址:http://journal.scnu.edu.cn/n
国家自然科学基金项目(31270287)
彭长连,研究员,Email:pengchl@scib.ac.cn.
Q945.3
A
1000-5463(2015)05-0071-07

