Ir0.5Pt0.5O2阳极的电催化活性及氧化电解水制备
高洁,朱玉婵,任占冬,李文阳,全姗姗,刘晔,王又容,柴波
Ir0.5Pt0.5O2阳极的电催化活性及氧化电解水制备
高洁,朱玉婵,任占冬,李文阳,全姗姗,刘晔,王又容,柴波
(武汉轻工大学化学与环境工程学院,湖北武汉430023)
氧化电解水作为一种新型、高效、环保的杀菌剂,具有广阔的应用前景。但目前在氧化电解水制备过程中,其阳极电催化材料存在效率低和使用寿命短等问题。采用亚当斯融合法制备了Ir0.5Pt0.5O2复合氧化物电极。通过XRD表征,其晶型为典型的金红石型结构。SEM结果表明虽然颗粒之间存在团聚现象,但是可以明显观察到大量蜂窝状结构存在,提高了催化剂的比表面积和电化学面积。进一步的CV表征证明了这一点,同时在CV图中表现出明显的铂铱复合氧化物结构的特征。利用LSV技术分别考察了Ir0.5Pt0.5O2的析氯和析氧极化曲线,发现其单位表观面积上析氯活性明显提高,而析氧活性明显降低。计算表明Ir0.5Pt0.5O2的析氯反应Tafel斜率为56.3 mV·dec-1,反应机理为Volmer-Heyrovsky机理,速控步骤为电化学脱附步骤;其析氧反应Tafel斜率为126.6 mV·dec-1,控速步骤为催化剂表面氢氧化物的形成。进一步电化学阻抗实验表明在1 g·L-1NaCl溶液中, Ir0.5Pt0.5O2析氯电催化活性优于IrO2,这与前面研究结果一致。在此基础上,以Ir0.5Pt0.5O2/Ti为阳极制备氧化电解水,在相同条件下,其有效氯含量明显优于IrO2/Ti,同时电解效率也明显提高,强化试验寿命是IrO2/Ti的3.14倍,大大提高了电极性能,有利于其商品化使用。
Ir0.5Pt0.5O2复合材料;电解;析氯;析氧;反应动力学
引 言
氧化电解水作为一种新型无毒环保的杀菌剂,具有杀菌广谱、迅速、强力、持续等特点。近年来,随着对氧化电解水研究的不断深入,其应用范围也不断拓宽,其在医疗卫生[1-8]、食品安全、农作物生长[9-20]等多个领域均有应用研究。氧化电解水的制备是通过电解浓度极稀的氯化钠溶液得到的,与之相类似的体系有氯碱工业和海水电解,但是三者之间有着明显的区别,即氯化钠在各电解液中的浓度不同。在氯碱工业中经常使用的是饱和氯化钠溶液,而海水电解中氯化钠的质量分数也达到2%,但在氧化电解水制备中氯化钠的质量分数仅为0.05%。如此大的电解质浓度差异,造成了电催化反应类型和电解效率的不同。在氯碱工业中,主要的阳极反应为析氯反应;而在氧化电解水制备过程中,除了析氯反应外,还有大量的析氧反应发生,如何调配两者之间的反应选择性,提高电解效率,这些都取决于对电极材料的研究。如果仍采用氯碱工业中常用的Ru基金属氧化物电催化材料(析氯和析氧电位仅相差100 mV)[21-24],势必影响其电解效率和电极使用寿命,因为阳极析氧电流较大时会破坏电极表面金属氧化物涂层的缺氧固溶体结构,在涂层和钛基体界面产生不导电的TiO2钝化膜,从而导致了电解效率和电极寿命大大降低。与Ru基等金属氧化物电极相比,Ir基金属氧化物的析氯和析氧活性略低,但其电极的使用寿命会大幅度增加[25-28],但仍然很难满足这种特殊条件下实际使用的需要。而PtO2电极具有较高的析氧过电位[29-30],如果将PtO2引入IrO2中,这样可以进一步降低电极材料析氧活性,从而提高析氯选择性,提高电极使用寿命。本文旨在制备铂铱复合氧化物催化剂,考察其析氯、析氧反应电催化活性,并考察其制备氧化电解水的性能和电极使用寿命。
1 实验材料和方法
1.1 Ir0.5Pt0.5O2粉体催化剂的制备
本文采用改进亚当斯融合法[31-32]制备Ir0.5Pt0.5O2粉体催化剂,即在烧杯里加入10 ml等量的H2IrCl6·6H2O和H2PtCl6·6H2O溶液,控制溶液中金属离子总浓度为0.1 mol·L-1,加入过量30%的硝酸钠及10 ml异丙醇。将所得溶液在60℃下持续搅拌直到异丙醇挥发完全,然后再将混合物在 80℃烘干。将其冷却后,经充分研磨,并在管式炉中500℃下烧结30 min。将混合物冷却至室温后,用大量蒸馏水洗涤多次,以便除去所有的氯离子,并用AgNO3溶液检测洗涤液中无Cl-为止,并在 80℃下干燥完全,得到催化剂粉体。
1.2 Ir0.5Pt0.5O2/Ti的制备
本文通过热分解法制备钛基氧化物薄膜电极。即将预处理好的钛板浸入预先配制好的涂液(0.2 mol·L-1H2PtCl6·6H2O+H2IrCl6·6H2O乙醇异丙醇混合溶液)之中,然后以1 mm·s-1的速度将钛板平稳地从涂液中提拉出来,在黏度和重力作用下基板表面形成一层均匀的液膜,并于80~90℃烘干10 min至表面溶剂全部挥发,然后放入马弗炉在500℃条件下热氧化10 min,冷却至室温后再次涂膜,此过程重复15次,最后一次热氧化时间为1 h,并退火至室温。
1.3 催化剂表征及电化学测试
涂层表面形貌、组成和结构用扫描电镜(SEM),X射线衍射(XRD)技术分析。X射线衍射型号为Shimadzu XRD-600,Cu-Kα射线源,管电流30 mA,管电压40 kV,扫描范围10°~90°,扫描速率4(°)·min-1。扫描电镜型号为S-3000N,日本HITACHI公司。BET比表面积是在-196℃下的氮气氛中通过物理吸附测定,仪器型号为Micromeritics ASAP2020。电化学性能的测定在CHI700D电化学工作站上进行,电解池采用三电极体系,辅助电极为碳纸电极,参比电极为可逆氢参比电极或饱和甘汞电极,工作电极为载有Ir0.5Pt0.5O2/GC(表观面积为0.196 cm2)。在25℃,0.5 mol·L-1H2SO4溶液中测定电极的循环伏安曲线和析氧极化曲线,在饱和NaCl溶液(6 mol·L-1)中测定电极的析氯极化曲线,在1 g·L-1NaCl溶液中进行电化学阻抗测试,测试的频率范围为100 kHz~0.1 Hz, 扰动幅值10 mV,测试电势为1.3 V(SCE)。
1.4 氧化电解水的制备、性能测试及寿命实验
在自制离子膜电解槽中,阳极是Ir0.5Pt0.5O2/Ti电极(有效面积1 cm2),阴极为钛板。中间用阳离子交换膜将电解槽分成阳极区和阴极区,体积分别为100 ml。电解过程中。添加浓度为1g·L-1的 NaCl溶液作为电解质,电流密度为100 mA·cm-2,电极间距为4 cm,电解30 min,在阳极区得到EOW。氧化电解水物性测定,其中有效氯含量采用碘量法滴定;pH和ORP值采用pH酸度计和ORP仪直接测定,美国热电-奥立龙。氯离子含量测定采用电位滴定法,美国热电-奥立龙,工作电极为Ag电极,参比电极双盐桥饱和甘汞电极。强化实验寿命测试采用2 cm×2 cm Ti板作为阴极,1 cm×1 cm Ir0.5Pt0.5O2/Ti作为阳极,电解液是0.5 mol·L-1H2SO4,温度40℃,电流密度200 mA·cm-2。
2 实验结果与讨论
2.1 XRD谱图分析
由于IrO2和PtO2都是金红石型晶体,具有相同的对称性和相近的晶格常数,所以两者能在广泛配比范围内形成混晶。图1是Ir0.5Pt0.5O2和IrO2的XRD谱图,从图中可以看出IrO2是典型的金红石相晶体结构,对比JCPDS15-0870标准卡片,其在27.8°、34.7°、53.9°和66.6°的衍射峰分别是IrO2的(110)、(101)、(211)、(112)晶面的特征峰。而当IrO2中掺入PtO2,形成Ir0.5Pt0.5O2固溶体后,其在34.1°处是明显(101)晶面衍射峰,这是金红石型晶体典型特征。另外,在60.6°和71.8°处是其(002)、(301)晶面特征峰。通过Scherrer公式,对它们的(101)晶面进行拟合计算其粒径,其中IrO2为4.18 nm,而Ir0.5Pt0.5O2的粒径为3.89 nm,粒径明显变小,这将有助于提高电极表面的比表面积,从而提高电催化活性。
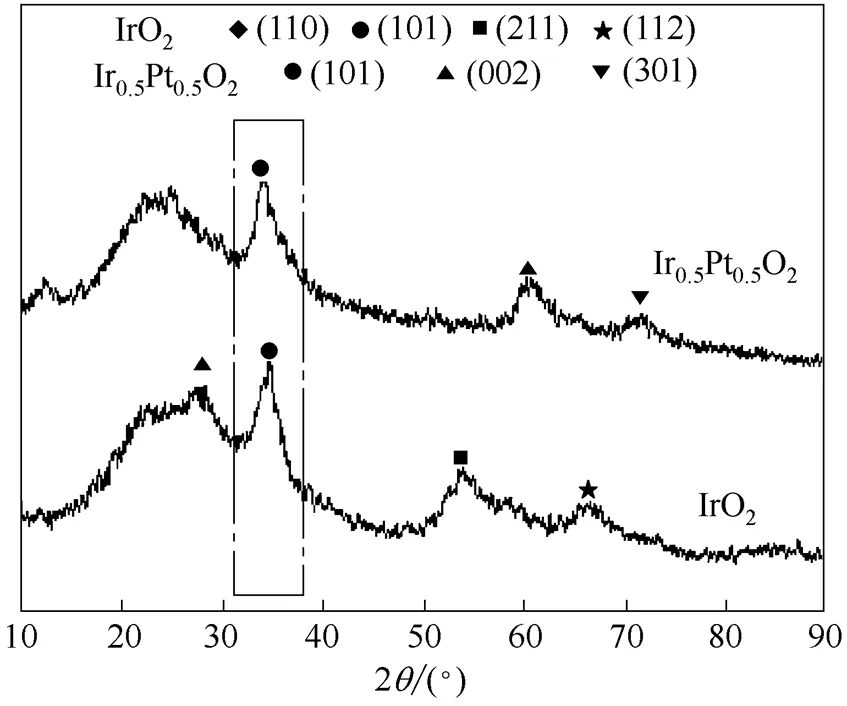
图1 Ir0.5Pt0.5O2和IrO2催化剂的XRD谱图
2.2 SEM图分析
图2是Ir0.5Pt0.5O2和IrO2催化剂的SEM图,从图中可以看出两种催化剂都出现不同程度的团聚现象,这可能是由于在制备过程中,需要高温退火氧化,所以出现大量颗粒聚集现象。在图2 (a)中可以看出,排除个别少数大的颗粒外,IrO2的粒径大小还是比较均匀,在100~200 nm之间。图2 (b)是Ir0.5Pt0.5O2的SEM图,仍然可以观察到粒径很大的颗粒,但在图中方框所标注区域内,可以观察到明显细小的网状结构,其晶粒尺寸远远小于IrO2粒径,这将大大有助于提高其比表面积,其BET比表面积达到255.4 m3·g-1,是IrO2的3.1倍。但比表面积是催化剂本身的物理性质,虽然其提高会有助于提高电催化活性,但其电化学面积才最能真正反映其电催化活性位点数目多少,所以以下将利用电化学循环伏安法来表征其电化学面积。
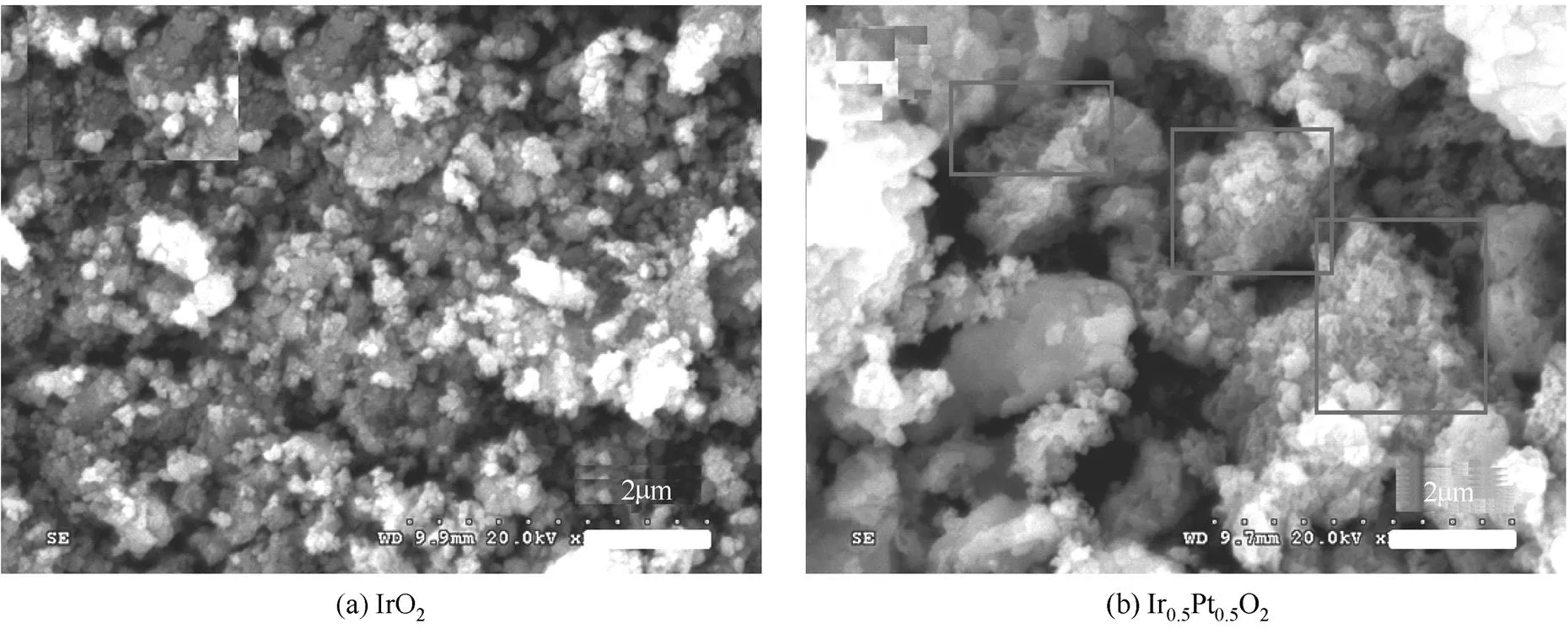
图2 Ir0.5Pt0.5O2和IrO2催化剂的SEM图
2.3 循环伏安(CV)表征
图3是Ir0.5Pt0.5O2和IrO2的CV曲线,其表面电量值能够代表活性位点的多少。图3 (a)是IrO2的CV曲线,这是典型IrO2循环伏安曲线,其中电势在0.9 V是Ir3+/Ir4+的氧化还原;电势在1.25 V是Ir4+/Ir5+的氧化还原,对其CV曲线进行积分,得到其氧化物表面电量为0.151 mC。当将PtO2掺入IrO2后,其CV特征发生明显的变化,结果如图3 (b)所示。从图中可以看出在1.0 V出现了明显氧化峰,其起始电势为0.75 V,这个氧化峰在纯IrO2的CV中没有观察到,这代表Pt表面氧化形成Pt-OH,而在0.85 V处是其还原峰。另外,在氢区(0.03~0.3 V),阴极电流明显增加,这是由于PtO2在电势较低情况下很容易被还原得到Pt,而Pt表面的HUPD电流会使阴极电流增加。对其表面电量进行积分,电量为0.4 mC,是纯IrO2的2.65倍,说明其表面电化学活性位点增多,这将有利于电催化反应活性的提高。此外,观察到Ir0.5Pt0.5O2的表面氧化起始电势比IrO2正移150 mV,说明其表面更难以发生氧化,这正是PtO2加入的结果,因为PtO2比IrO2析氧活性更差。

图3 Ir0.5Pt0.5O2和IrO2催化剂的CV曲线
2.4 氯析出(CER)电催化活性
氧化电解水中主要杀菌活性因子是HClO,而其产生是通过阳极析氯反应得到的,所以研究电极材料析氯反应活性是十分重要的,其析氯反应活性越高,产生的HClO含量越高,预示着杀菌活性越好[15]。图4是Ir0.5Pt0.5O2和IrO2在6 mol·L-1氯化钠溶液中的极化曲线。从图4 (a)中可以看出,Ir0.5Pt0.5O2和IrO2两者的析氯起始电势很接近,均在1.05 V(SCE)。但随着极化电势增加,它们在单位面积上氯析出反应活性有着明显不同,其中IrO2的析氯活性较差,析氯电流(@1.4 V)仅为40.3 mA·cm-2;但当形成铂铱复合氧化物后,其析氯活性明显增大,析氯电流(@1.4 V)达到了86.9 mA·cm-2,是IrO2的2.16倍。这一方面是因为当铂铱之间形成复合氧化物后,其电化学面积有所增加(见CV表征),从而带来了电催化活性的提高;另一方面则可能是由于PtO2本身析氧活性较差,所以其加入有利于抑制析氧反应的发生,从而提高析氯反应的选择性,提高析氯反应活性。为了明确电极材料组成对析氯反应电催化活性的影响,就必须排除电化学活性面积变化对催化反应活性的影响,所以将它们对其表面电量进行归一化。图4 (b)则是Ir0.5Pt0.5O2和IrO2对其表面电量进行归一化后的析氯活性,从图中可以看出,两者析氯反应活性比较接近,说明两者在每个表面活性位点上的析氯反应活性一样,并不会因为形成复合氧化物而发生改变。对比IrO2电极,Ir0.5Pt0.5O2电极在单位表观面积上析氯反应活性的提高很大程度上是因为其表面活性反应位点的增加。
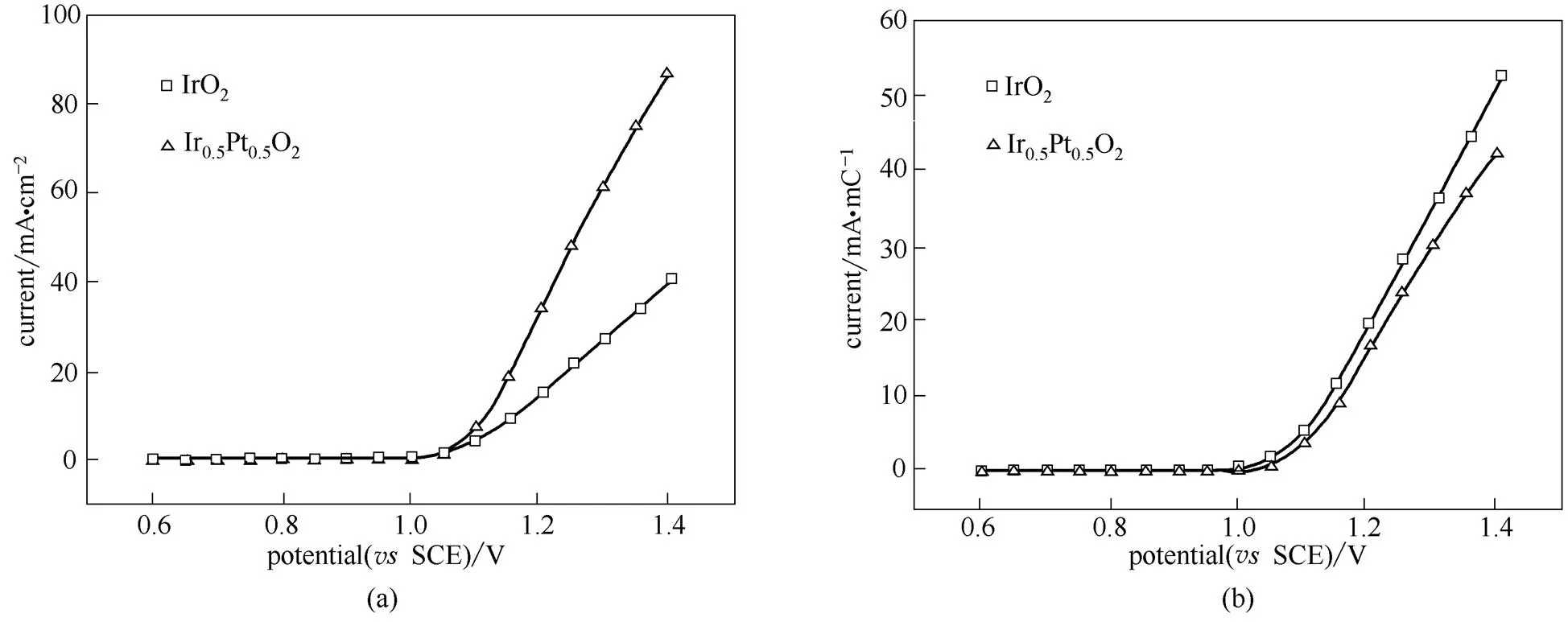
图4 Ir0.5Pt0.5O2和IrO2析氯反应的线性伏安曲线
从前面的研究得知Ir0.5Pt0.5O2具有较好的析氯反应活性,下面进一步分析其析氯反应机理。从图5中可以看出Ir0.5Pt0.5O2的Tafel斜率为56.3 mV·dec-1,其析氯反应机理应为Volmer-Heyrovsky机理[33-36],其反应过程分两步,其中第2步是其反
应的速控步骤,S代表活性位点。
所以,其析氯反应电流可以写成
将式(4)代入式(3)中,可以得到式(5),再经简单数学变换得到式(6)
2.5 氧析出(OER)电催化活性
前面考察了Ir0.5Pt0.5O2的氯析出反应活性,但在制备氧化电解水过程中,除了析氯反应之外,还会伴随着大量析氧反应的发生。析氧反应的存在一方面会降低析氯反应效率,另一方面会使电极表面金属氧化物涂层的缺氧固溶体结构发生破坏,涂层和钛基体界面产生不导电的TiO2钝化膜,从而大大降低电解效率和电极寿命,所以尽量避免析氧反应的发生。图7 (a)是单位表观面积上的析氧极化曲线,从图中可以看出,与析氯活性不同,Ir0.5Pt0.5O2的析氧活性与其本身的电化学面积并不呈正比。其中IrO2电化学面积小于Ir0.5Pt0.5O2,但其氧析出电流却大于后者。如再将它们的析氧活性对表面电量进行归一化,排除电化学面积的影响,如图7 (b)所示,它们析氧活性之间差别则进一步增大。以上研究结果说明析氧反应活性主要受电极组成的影响。由于PtO2本身的析氧活性较差,所以形成复合氧化物后,会使析氧活性减小,而析氧活性的减小正好可以提高析氯反应选择性以及电极寿命。

图7 Ir0.5Pt0.5O2和IrO2析氧反应的线性伏安曲线
为了进一步分析析氧反应活性变化的机理,首先做了两者的Tafel曲线,从图8中可以看出,IrO2和Ir0.5Pt0.5O2的析氧反应Tafel曲线斜率有很大不同,预示着其析氧反应机理的不同。在酸性体系中,文献中一般认为析氧反应过程如下[37-39]
为了更清晰地表达析氯和析氧选择性的变化,选择在电流密度为20 mA·cm-2下对比两者的析氯和析氧电势,结果表明IrO2分别为1.239 V和1.392 V,氯氧电势差为153 mV;而Ir0.5Pt0.5O2分别为1.154 V和1.432 V,氯氧电势差为278 mV。Ir0.5Pt0.5O2的氯氧电势差明显大于IrO2,意味着析氯反应选择性增加,电流效率提高。
2.6 电化学阻抗
由于氧化电解水制备时,氯化钠浓度为1 g·L-1,所以Ir0.5Pt0.5O2和IrO2的电化学阻抗测试在该浓度下进行,设定电极电势为1.3 V(SCE),其阻抗复平面图如图9所示。
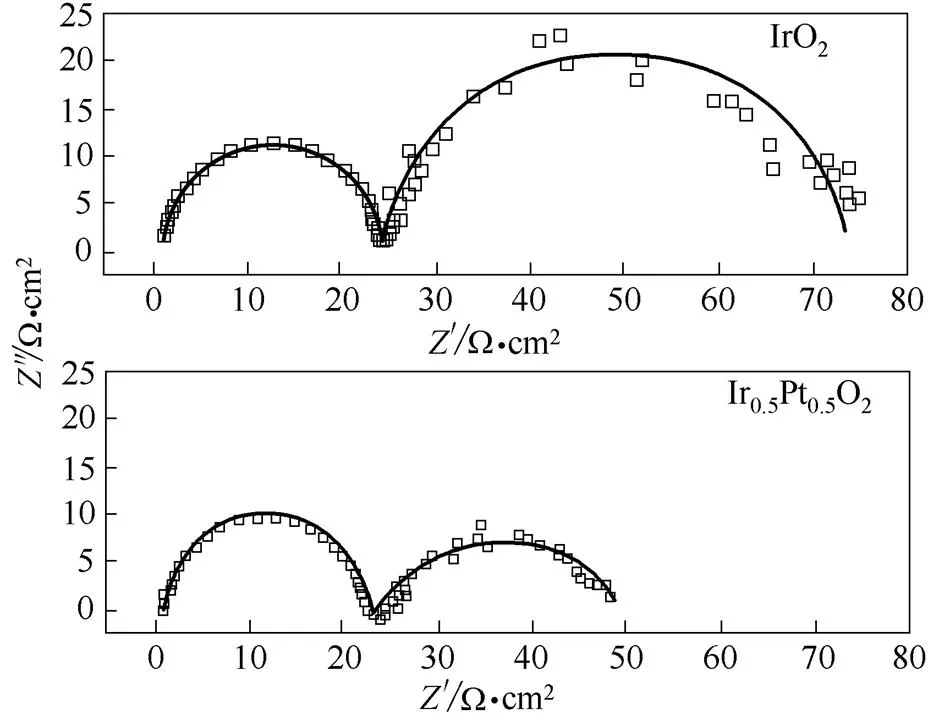
图9 Ir0.5Pt0.5O2和IrO2在1 g·L-1NaCl溶液中阻抗复平面图
电极电势为1.3 V(SCE)时,析氯反应已经明显发生,采用Zview软件进行拟合,其等效电路设计为s(ff)(ctdl),其中s代表溶液电阻,f代表电极材料膜电阻,ct代表析氯反应时电荷转移电阻;f代表电极材料膜电容,dl代表电极表面的双电层电容。从表1中可以看出在1.3 V时,Ir0.5Pt0.5O2的f小于IrO2,说明其导电性更好;而ct明显变小及dl明显变大,则说明其析氯反应电催化活性高,这与前面析氯极化曲线等研究结果一致。

表1 Ir0.5Pt0.5O2和IrO2在1g·L-1 NaCl溶液中等效电路拟合阻抗参数
2.7 氧化电解水制备和电极使用寿命
前面的研究表明形成铂铱复合氧化物后,其析氯和析氧活性发生明显变化。下面考察氧化电解水实际制备中Ir0.5Pt0.5O2电极的性能。表2是分别以Ir0.5Pt0.5O2/Ti、IrO2/Ti作为阳极材料,在自制离子膜电解槽中通过电解1 g·L-1NaCl溶液制备得到氧化电解水,并考察其pH、ORP和有效氯值等性能参数,以及电解后溶液中的氯离子含量及电解效率。如表2中所示,两种电极制备得到EOW的pH和ORP值相近,但有效氯含量和电解效率上相差较大,说明两者析氯反应活性有很大差别。其中Ir0.5Pt0.5O2电解得到的EOW中含有较多有效氯,而在前期研究中发现有效氯越高,氧化电解水的杀菌效率越高[15,40-41]。另外,从电解后溶液中氯离子含量以及电解效率来看,Ir0.5Pt0.5O2也要好于IrO2电极,这些说明Ir0.5Pt0.5O2更适合作为制备EOW的阳极电催化材料。
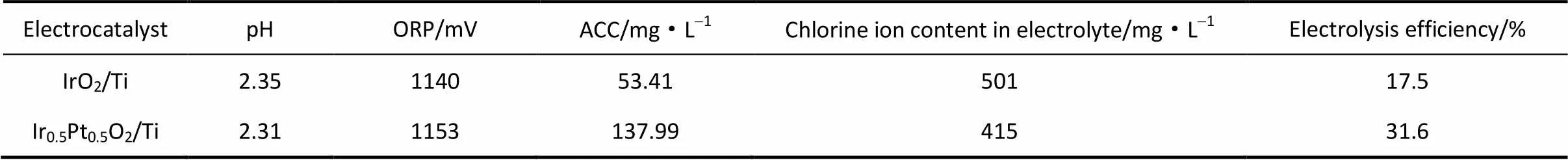
表2 Ir0.5Pt0.5O2/Ti和IrO2/Ti制备电解水的指标参数和电解效率
Note:Initial content of chloride ion in solution is 607 mg·L-1, volume of solution is 120 ml, current density is 100 mA·cm-2, electrode area is 1 cm2, electrolytic time is 0.5 h.
前面的研究中Ir0.5Pt0.5O2表现出很好的析氯反应活性和较高的电解效率,下面进一步考察其电极使用寿命,因为使用寿命对于电极实际应用是十分重要的。采用在0.5 mol·L-1H2SO4溶液中,电流密度200 mA·cm-2,温度40℃下进行强化寿命实验,结果如图10所示。从图10中可以看出,IrO2电极强化寿命为100 h,而Ir0.5Pt0.5O2电极强化寿命为341 h,提高了3.41倍,说明当铂铱形成复合氧化物后由于析氧活性的下降,减少了电极表面金属氧化物涂层的缺氧固溶体结构发生破坏,较好地防止不导电的TiO2钝化膜的形成,所以其使用寿命大幅度增加。

图10 Ir0.5Pt0.5O2/Ti和IrO2/Ti催化剂的强化寿命实验
3 结 论
针对目前氧化电解水制备过程中,其阳极电催化材料析氯反应选择性低和使用寿命短等问题,制备了铂铱复合氧化物电极。结果表明Ir0.5Pt0.5O2具有典型的金红石型结构,呈蜂窝状分布,具有较大比表面积和电化学面积,具有较好的析氯反应活性和较差的析氧反应活性。Ir0.5Pt0.5O2的析氯反应Tafel斜率为56.3 mV·dec-1,反应机理为Volmer-Heyrovsky机理;其析氧反应Tafel斜率为126.6 mV·dec-1,控速步骤为催化剂表面氢氧化物的形成。以上研究表明,Ir0.5Pt0.5O2电催化剂作为阳极材料来制备氧化电解水是十分适宜的。实验结果表明,以Ir0.5Pt0.5O2/Ti电极制备的氧化电解水中有效氯含量明显优于IrO2/Ti,同时电解效率也明显提高,强化实验寿命是IrO2/Ti的3.14倍,大大提高了电极性能,有利于其商品化使用。
References
[1] Thorn R M S, Lee S W H, Robinson G M, Greenman J, Reynolds D M. Electrochemically activated solutions: evidence for antimicrobial efficacy and applications in health care environments [J]......., 2012, 31 (5): 641-653
[2] Gulabivala K, Stock C J R, Lewsey J D, Ghori S, Ng Y L, Spratt D A. Effectiveness of electrochemically activated water as an irrigant in an infected tooth model [J]...., 2004, 37 (9): 624-631
[3] Chittoria R K, Yootla M, Sampatrao L M, Raman S V. The role of super oxidized solution in the management of diabetic foot ulcer: our experience [J]....., 2007, 9: 125-128
[4] Vorobjeva N V, Vorobjeva L I, Khodjaev E Y. The bactericidal effects of electrolyzed oxidizing water on bacterial strains involved in hospital infections [J]..., 2004, 28 (6): 590-592
[5] Fenner D C, Bürge B, Kayser H P, Wittenbrink M M. The anti-microbial activity of electrolysed oxidizing water against microorganisms relevant in veterinary medicine [J]......., 2006, 53 (3): 133-137
[6] Robinson G M, Lee S W H, Greenman J, Salisbury V C, Reynolds D M. Evaluation of the efficacy of electrochemically activated solutions against nosocomial pathogens and bacterial endospores [J]...., 2010, 50 (3): 289-294
[7] Morita C, Nishida T, Ito K. Biological toxicity of acid electrolyzed functional water: effect of oral administration on mouse digestive tractand changes in body weight [J]...., 2011, 56 (4): 359-366
[8] Park G W, Boston D M, Kase J A, Sampson M N, Sobsey M D. Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus [J]...., 2007, 73 (14): 4463-4468
[9] Keskinen L A, Burke A, Annous B A. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminateO157:H7 from lettuce leaves [J]...., 2009, 132 (2/3): 134-140
[10] Koide S, Shitanda D, Note M, Cao W. Effects of mildly heated, slightly acidic electrolyzed water on the disinfection and physicochemical properties of sliced carrot [J]., 2011, 22 (2/3): 452-456
[11] McCarthy S, Burkhardt III W. Efficacy of electrolyzed oxidizing water againstandon conveyor belt and raw fish surfaces [J]., 2012, 24 (1/2): 214-219
[12] Xie J, Sun X H, Pan Y J, Zhao Y. Combining basic electrolyzed water pretreatment and mild heat greatly enhanced the efficacy of acidic electrolyzed water againston shrimp [J]., 2012, 23 (2): 320-324
[13] Rahman S M E, Wang J, Oh D H. Synergistic effect of low concentration electrolyzed water and calcium lactate to ensure microbial safety, shelf life and sensory quality of fresh pork [J]., 2013, 30 (1): 176-183
[14] Ren Zhandong (任占冬), Zhu Yuchan (朱玉婵), Liu Ye (刘晔), Zhang Zhiyong (张智勇), Zhang Qi (张奇). Electrolyzed potential water sterilizing technics and mechanism on pork stuffing [J].(农业机械学报), 2009, 40 (12): 139-143
[15] Zhu Yuchan (朱玉婵), Ren Zhandong (任占冬), Liu Ye (刘晔), Zhang Zhiyong (张智勇). Sterilization characteristics of electrolyzed-oxidizing water and its sterilizing effect for meat [J].(化工学报), 2009, 60 (10): 2583-2589
[16] Cao W, Zhu Z W, Shi Z X, Wang C Y, Li B M. Efficiency of slightly acidic electrolyzed water for inactivation ofand its contaminated shell eggs [J]...., 2009, 130 (2): 88-93
[17] Graca A, Abadias M, Salazar M, Nunes C. The use of electrolyzed water as a disinfectant for minimally processed apples [J]..., 2011, 61 (2/3): 172-177
[18] Xiong K, Liu H J, Li L T. Product identification and safety evaluation of aflatoxin B1 decontaminated by lectrolyzed oxidizing water [J]...., 2012, 60 (38): 9770-9778
[19] Zhang Houcheng (张后成), Zhu Yuchan (朱玉婵), Ren Zhandong (任占冬), Pan Deng (潘登), Liu Ye (刘晔), Wang Yourong (王又容), Chai Bo (柴波). Sterilizing effect and mechanism of neutral electrolyzed oxidizing water on cabbage [J].(农业工程学报), 2013, 29 (22): 277-283
[20] Huang Y R, Hung Y C, Hsu S Y, Huang Y W, Hwang D F. Application of electrolyzed water in the food industry [J]., 2008, 19 (4): 329-345
[21] Trieu V, Schley B, Nattera H, Kintrup J, Bulan A, Hempelmann R. RuO2-based anodes with tailored surface morphology for improved chlorine electro-activity [J].., 2012, 78: 188-194
[22] Cao H Z, Lu D H, Lin J P, Ye Q, Wu J J, Zheng G Q. Novel Sb-doped ruthenium oxide electrode with ordered nanotube structure and its electrocatalytic activity toward chlorine evolution [J].., 2013, 91: 234-239
[23] Petrykin V, Macounová K, Okubea M, Mukerjeec S, Krtil P. Local structure of Co doped RuO2nano crystalline electrocatalytic materials for chlorine and oxygen evolution [J]., 2013, 202: 63-69
[24] Neodoa S, Rosestolato D, Ferro S, Battisti A D. On the electrolysis of dilute chloride solutions: influence of the electrode material on Faradaic efficiency for active chlorine, chlorate and perchlorate [J].., 2012, 78: 282-291
[25] Hu W, Chen S L, Xia Q H. IrO2/Nb-TiO2electrocatalyst for oxygen evolution reaction in acidic medium [J]..., 2014, 39 (13): 6967-6976
[26] Xu J Y, Liu G Y, Li J L, Wang X D. The electrocatalytic properties of an IrO2/SnO2catalyst using SnO2as a support and an assisting reagent for the oxygen evolution reaction [J].., 2012, 59: 105-112
[27] HuW, Wang Y Q, Hu X H, Zhou Y Q, Chen S L. Three-dimensional ordered macroporous IrO2as electrocatalyst for oxygen evolution reaction in acidic medium [J]...., 2012, 22: 6010-6016
[28] Ye Z G, Meng H M, Sun D B. New degradation mechanism of Ti/IrO2+MnO2anode for oxygen evolution in 0.5M H2SO4solution [J].., 2008, 53: 5639-5643
[29] Stoyanova A, Borisov G, Lefterova E, Slavcheva E. Oxygen evolution on Ebonex-supported Pt-based binary compounds in PEM water electrolysis [J]..., 2012, 37 (21): 16515-16521
[30] Reier T, Oezaslan M, Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials [J].., 2012, 2 (8): 1765-1772
[31] Adams R, Shriner R L. Platinum oxide as a catalyst in the reduction of organic compoundsⅢpreparation and properties of the oxide of platinum obtained by the fusion of chloroplatinic acid with sodium nitrate [J]....., 1923, 45: 2171-2179
[32] Song S D, Zhang H M, Ma X P, Shao Z G, Zhang Y N, Yi B L. Bifunctional oxygen electrode with corrosion-resistive gas diffusion layer for unitized regenerative fuel cell [J]..., 2006, 8: 399-405
[33] Santana M H P, Faria A D L. Oxygen and chlorine evolution on RuO2+TiO2+CeO2+Nb2O5mixed oxide electrodes [J].., 2006, 51: 3578-3585
[34] Hansen H A, Man I C, Studt F, Abild-Pedersen F, Bligaard T, Rossmeisl J. Electrochemical chlorine evolution at rutile oxide (110) surfaces [J]....., 2010, 12: 283-290
[35] Guerrini E, Consonni V, Trasatti S. Surface and electrocatalytic properties of well-defined and vicinal RuO2single crystal faces [J]..., 2005, 9: 320-329
[36] Ferro S, Battisti A D. Electrocatalysis and chlorine evolution reaction at ruthenium dioxide deposited on conductive diamond [J]...., 2002, 106: 2249-2254
[37] Ye Z G, Meng H M, Chen D, Yu H Y, Huan Z S, Wang X D, Sun D B. Structure and characteristics of Ti/IrO2()+MnO2(1-) anode for oxygen evolution [J]., 2008, 10: 346-354
[38] Macounova K, Makarova M, Krtil P. Oxygen evolution on nanocrystalline RuO2and Ru0.9Ni0.1O2-δelectrodes-DEMS approach to reaction mechanism determination [J]..., 2009, 11: 1865-1868
[39] Tsuji E, Imanishi A, Fukui K, Nakato Y. Electrocatalytic activity of amorphous RuO2electrode for oxygen evolution in an aqueous solution [J].., 2011, 56: 2009-2016
[40] Zhu Yuchan (朱玉婵), Ren Zhandong (任占冬), Liu Ye (刘晔), Chen Hongmei (陈红梅). Sterilizing effect and neutral electrolyzed oxidizing water [J]....(中国公共卫生), 2011, 27 (6): 805-806
[41] Ren Zhandong (任占冬), Zhu Yuchan (朱玉婵), Liu Ye (刘晔), Zhou Xiaorong (周晓荣), Zhang Zhiyong (张智勇). Sterilizing effect and mechanism of electrolyzed water [J]..... (中华预防医学), 2008, 8: 578-581
Electrocatalytic performance of Ir0.5Pt0.5O2anode and preparation of electrolyzed oxidizing water
GAO Jie, ZHU Yuchan, REN Zhandong, LI Wenyang, QUAN Shanshan, LIU Ye, WANG Yourong, CHAI Bo
School of Chemical and Environmental EngineeringWuhan Polytechnic UniversityWuhanHubeiChina
Electrolyzed oxidizing water (EOW), as an innovative disinfectant characterized by its high efficiency, broad antimicrobial spectrum, and non-toxic residues, has been broadly used in health care industry, medicines, agriculture, and food processing. EOW is usually generated by electrolysis of a dilute NaCl solution in a chamber with two cells separated by membrane, and is obtained from the anode side. But low current efficiency and short service life of the anode in EOW generators restrict the application of EOW. Ir0.5Pt0.5O2anode was prepared by the improved Adams fusion method. The properties of Ir0.5Pt0.5O2anode was investigated with X-ray diffraction (XRD), scanning electron microscope (SEM) and electrochemistry cyclic voltammetry (CV). The crystal type is rutile with (101), (002) and (301) crystal planes. A large number of cellular structures were observed on the surface of the anode, which greatly increased specific surface area of the anode. With increasing specific surface area, electric charge was enhanced to 0.4 mC, which was 2.65 times of pure IrO2. Electrochemical characteristics of the anode surface, such as oxidation peaks at 1.0 V(Pt-OH) and 0.9 V(Ir3+/Ir4+) proved the formation of platinum iridium oxide. The activities of chlorine evolution and oxygen evolution were also studied through linear sweep voltammetry (LSV). Compared with IrO2, chlorine evolution activity in unit apparent surface area increased significantly, but oxygen evolution activity decreased obviously. The slope of Tafel was 56.3 mV·dec-1for chlorine evolution reaction (CER), and the mechanism was Volmer-Heyrovsky in which the rate controlling step was electrochemical desorption. The slope of Tafel was 126.6 mV·dec-1for oxygen evolution reaction (OER), and the rate controlling step was formation of surface hydroxide on the catalyst surface. Electrochemical surface structure and electrochemical performance of Ir0.5Pt0.5O2oxide coatings in 1 g·L-1NaCl solution were investigated with electrochemical impedance spectroscopy (EIS). CER activity of Ir0.5Pt0.5O2was better than IrO2, which was in agreement with previous research. In the actual EOW preparation, electrolysis efficiency and available chlorine content (ACC) of EOW on the Ir0.5Pt0.5O2anode were much greater than IrO2anode under the same condition. The accelerated life of Ir0.5Pt0.5O2anode was 3.14 times of the IrO2anode and the performance of the anode was greatly improved, which favored its commercial use.
Ir0.5Pt0.5O2composites; electrolysis; chlorine evolution; oxygen evolution; reaction kinetics
2014-08-05.
Prof. ZHU Yuchan, zhuyuchan@163.com; Prof. REN Zhandong, renzhandong@163.com
10.11949/j.issn.0438-1157.20141176
TQ 151.2
A
0438—1157(2015)03—0992—09
国家自然科学基金项目(31101370);湖北省自然科学基金项目(2012FFB04803);武汉轻工大学校立科研计划项目(2015d8)。
2014-08-05收到初稿,2014-12-08收到修改稿。
联系人:朱玉婵,任占冬。第一作者:高洁(1990—),女,硕士研究生。
supported by the National Natural Science Foundation of China (31101370) and the Natural Science Foundation of Hubei Province (2012FFB04803).

