环境中抗生素及其生态毒性效应研究进展
徐永刚, 宇万太, 马 强, 周 桦, 姜春明
中国科学院沈阳应用生态研究所,沈阳 110016
环境中抗生素及其生态毒性效应研究进展
徐永刚, 宇万太*, 马 强, 周 桦, 姜春明
中国科学院沈阳应用生态研究所,沈阳 110016
近年来,越来越多的抗生素类药物用于在医疗、畜禽和水产养殖业。由于其机体代谢率低,大部分以原药或代谢物的形式经由尿液和粪便排出体外进入环境中,造成抗生素在水体和土壤等环境介质中的残留。这些残留的抗生素会导致潜在的环境风险,其中最严重的是会诱发和传播各类抗生素抗性基因(antibiotic resistance genes, ARGs),进而对人类健康产生威胁。本文介绍了环境中抗生素的来源,归趋和残留状况,并且对其所引起的生态毒性效应以及ARGs进行总结,最后指出了目前研究中存在的问题,并对未来研究进行了展望。
抗生素;生态毒性效应;抗性基因
抗生素类药物(以下简称抗生素)是由微生物(包括细菌、真菌、放线菌属)或高等动植物在生活过程中所产生的具有抗病原体或其它活性的一类次级代谢产物,能干扰其他生活细胞发育功能的化学物质。现常用的抗生素除了包括β-内酰胺类,氨基糖苷类,氯霉素类,大环内脂类和四环素类抗生素等微生物培养液中提取物的抗生素,还包括磺胺类和喹诺酮类等用化学方法合成或半合成的药物。抗生素除了用于人类和动物感染性疾病的治疗,也被作为促长剂添加于饲料中在养殖业中大量应用。据统计,美国1999年抗生素的使用量为2.68万t,其中60%用于人类的疾病治疗和预防,32%用作养殖业生长促进剂,另8%用作治疗剂[1]。1999年,欧盟和瑞士共消耗抗生素1.33万t,其中65%是医用,29%是动物养殖兽药,6%是动物生长促进剂[2]。中国是抗生素使用大国,也是抗生素生产大国。根据化学工业制药工业协会2005年统计数据,我国每年抗生素原料生产量约为21万t,其中有9.7万t(占年总产量的46.1%)的抗生素用于养殖业。
大部分抗生素具有水溶性,人类和动物服用的抗生素有30%~90%将随粪尿排出体外[3]。大量未被代谢的抗生素经不同途径进入环境,虽然抗生素的半衰期不长,但由于频繁使用并进入环境,导致水体、沉积物和土壤等环境介质抗生素及其代谢活性产物浓度逐渐提高[3]。环境残留的抗生素不仅可抑制微生物的生长和活性,也可对动植物产生毒性效应,进而干扰其生态功能,对维持生态系统的稳定构成潜在风险[4]。另外,抗生素还可诱导微生物产生抗性基因,其可在水、土壤和空气等环境介质中以及动植物体内传播扩散,从而对人类健康构成巨大威胁[5,6]。
1 环境抗生素的来源和和归趋
1.1 环境中抗生素的来源
自然界存在于土壤中的一些细菌种类本身可以合成抗生素,如链霉菌等放线菌类,但是抗生素的环境本底值总体是非常微量的。目前,环境中抗生素主要来源于抗生素工业废水、医用抗生素和农用抗生素。图1列举了环境中抗生素的来源及其迁移途径。
医用抗生素的使用主要在医院和家庭。此类抗生素不能被人体完全吸收或代谢,未吸收的抗生素和代谢产物通过粪尿排出体外进入废水系统。医院里抗生素使用较为频繁且集中,因此医院废水中抗生素种类较少,以喹诺酮类和磺胺类药物为主,但其浓度较高,含量可达μg·L-1级别[8-9]。家庭使用的抗生素一部分通过人体排泄进入环境水体,另一部分作为固体垃圾被丢弃,并极有可能因为浸泡、腐蚀而渗入水体环境。
农用抗生素主要在畜禽养殖和水产养殖业中使用。它们除了被用来预防和治疗动物疾病,还以亚治疗剂量长期添加于饲料中作为促生长剂使用。在畜禽养殖业中,大部分的抗生素都不能被动物体吸收或代谢通过粪尿排泄出体外。目前,高浓度抗生素频繁发现于集约化畜禽养殖场废水和畜禽粪便中,其检出浓度多在μg·L-1或mg·kg-1级别,且主要以四环素、喹诺酮和磺胺类等兽药抗生素为主[10-13]。这些废弃物无论用作肥料施用于农田,还是直接排放,都可能随着地表径流汇入江河或淋溶至地下水,并很有可能通过饮用水源和植物吸收积累进入食物链,威胁人类健康。
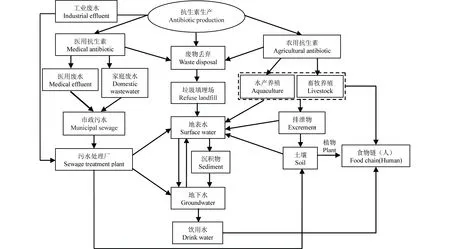
图1 环境中抗生素的来源与迁移途径Fig. 1 The sources and migration paths of antibiotics in the environment[7]
水产养殖中抗生素的使用主要以直接投放为主,因此大量未被水产养殖生物吸收及随粪便排泄的抗生素残留于水体,或沉降富集于底泥,成为水环境抗生素的一个重要污染源。尽管各国对水产养殖用药规定不同,但磺胺类和喹诺酮类药物均被频繁检出,其浓度高达几十mg·L-1或mg·kg-1[14-16]。
医药生产企业污水废料也是环境抗生素的重要来源,但对其抗生素类型和含量的报道不多。仅有的几篇文献资料显示,制药厂废水中抗生素种类与其所生产的药品种类有关,其含量很高,浓度在mg·L-1级别[17,18]。
医院、养殖和制药废水通过市政污水收集系统进入污水处理厂,经过稀释作用使其抗生素含量降低,浓度多在几百到几千ng·L-1范围。然而现有的常规水处理技术很难将其去除,污水处理厂出水中抗生素浓度仍在ng·L-1级别。在污水厂进水和出水中,最常被检出的是大环内酯类、磺胺类和喹诺酮类抗生素(表1)。另外,在污水厂处理流程中抗生素会不断地吸附在活性污泥上而不容易降解,对抗生素具有一定的蓄积作用。四环素类、喹诺酮类和磺胺类抗生素已在污泥中被频繁的检出,且检测浓度多在μg·kg-1级别(表1)。污水处理厂尾水直接排放入河流或作为农田灌溉用水以及污泥施用于农田都成为环境抗生素污染的一个主要来源。
1.2 抗生素在环境中的归趋
和其它有机污染物一样,进入土壤、水和沉积物等环境介质的抗生素通过吸附、迁移和降解等一系列生化过程在环境介质间发生再分配,这将直接影响抗生素在环境中的残留浓度及其生物学效应。
1.2.1 吸附和迁移
吸附行为是抗生素在环境介质间的重要物理化学过程,其直接决定了迁移与降解过程。抗生素通过范德华力、色散力、诱导力和氢键等分子间作用力与环境介质的吸附位点相吸附,或者抗生素的分子功能基团(如羧酸、醛、胺类)与环境介质表面发生化学反应形成络合物或螯合物。因此,抗生素的性质(如亲水性、空间构型和官能团)决定了其在环境介质上的吸附能力[33]。例如,四环素类和喹诺酮类抗生素具有较多的羧基、羰基和酰胺基等极性官能团使其与环境介质有很强的亲和力;尽管大环内酯类抗生素含有的极性官能团不少,但因分子较大阻碍环境介质对其的吸附;氨基糖苷类和β-内酰胺类抗生素极性较强,因此固相介质对其吸附能力较弱;磺胺类抗生素仅含有苯胺基和酰胺基两个离子型官能团,与环境介质的吸附作用很弱。其它种类的抗生素与环境介质的吸附研究较少。综合资料表明[34-36],抗生素吸附作用强弱顺序依次为四环素类>氟喹诺酮类>大环内酯类>氨基糖苷类>β-内酰胺类>磺胺类。
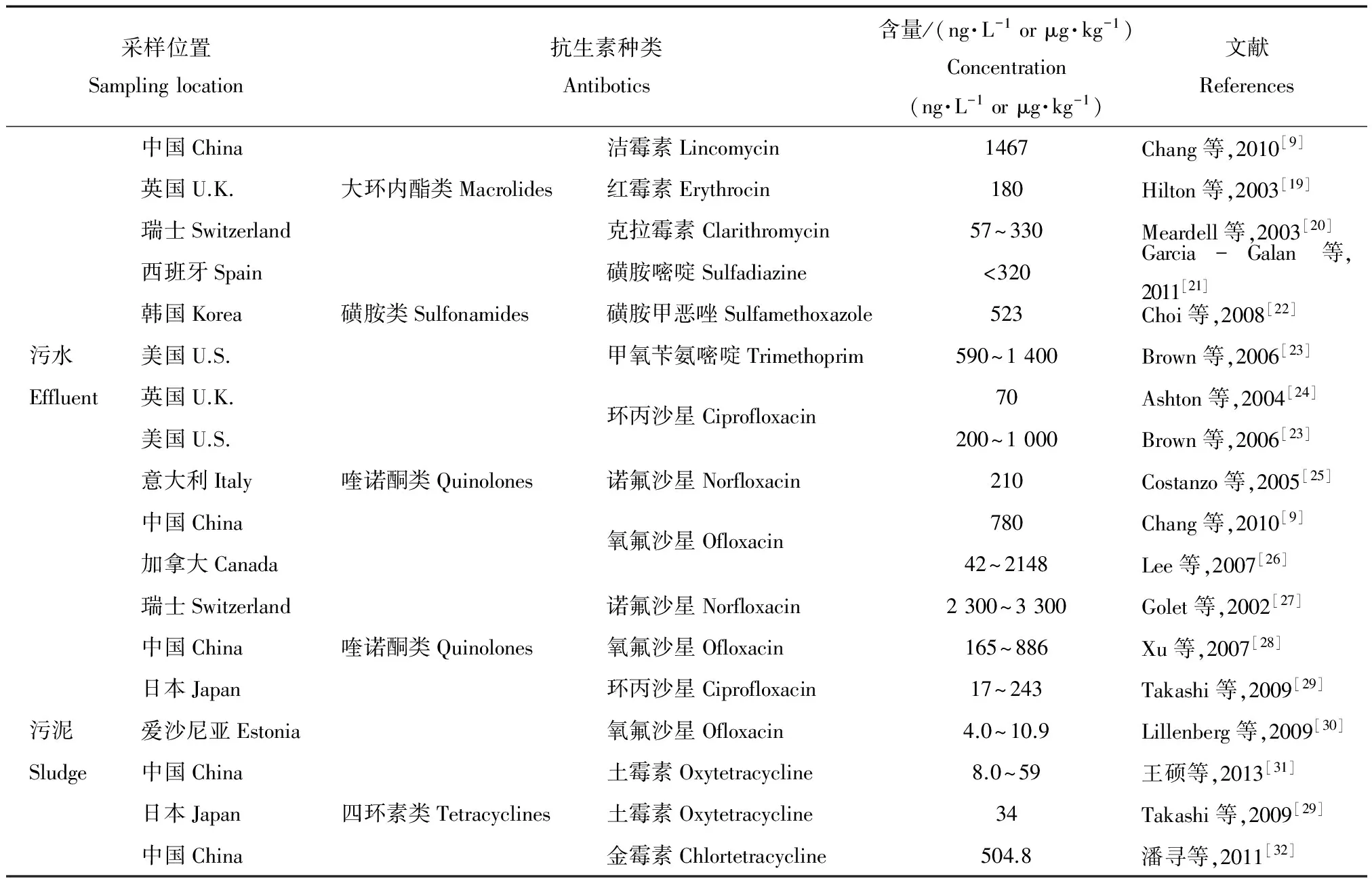
表1 污水处理厂污水和污泥中抗生素含量
环境介质对抗生素的吸附行为也受环境因子的调控。研究最多的是pH的影响,它通过改变抗生素和吸附介质的电荷状态对吸附产生显著影响。多数研究显示抗生素的吸附能力随土壤或沉积物pH的增加而降低[37-39]。此外,不同形态的金属离子对抗生素吸附的影响不同。比如,多价态金属离子通过共价键连接抗生素带负电部分与固体表面的负吸附位,形成抗生素-金属离子-吸附介质三相络合物,进而促进吸附[40]。然而,Na+、K+等一价金属离子通过与阳离子态/0价态的抗生素竞争吸附位进而减少吸附[41]。粘粒矿物和有机质组分是抗生素在土壤和沉积物中的主要吸附位点,因此多数研究发现抗生素的Kd值与粘土矿物和有机质含量成正相关[42,43]。
抗生素在土壤或沉积物剖面内的迁移与吸附作用相关联。吸附性能越强的物质,与土壤等介质的结合能力越强,则迁移能力越差,因此,多数研究者通过土柱淋溶实验发现磺胺类药物能淋溶较深土层或地下水[44,45]。
1.2.2 降解
抗生素在环境中可能发生水解、光解(统称非生物降解)和微生物降解等一系列降解反应。视环境条件的不同,抗生素会发生一种或多种降解反应,比如,粪便、土壤和沉积物中主要是微生物降解,而水体中主要以非生物降解方式为主,特别是厌氧条件下[46]。应该注意的是,一般降解过程会降低抗生素的药效,但有些抗生素的代谢物有着抗生素本身的毒性甚至更毒,且可能转化回抗生素原药[47]。
水解是水体中抗生素降解的重要方式。β-内酰胺类、大环内酯类和四环素类抗生素易溶于水发生水解。pH是影响抗生素水解的重要环境因子。例如,大环内酯类抗生素在中性pH条件下水解慢,且活性较低[48];β-内酰胺类在弱酸至强碱条件下水解速度都很快[49]。此外,部分抗生素的水解还受到温度和离子强度的影响[50,51]。

微生物降解是大部分抗生素在固相环境中降解的重要途径,其速率主要受抗生素种类的影响[55,56]。同样,环境抗生素微生物降解速率也受众多环境因素的影响,尤其是影响降解微生物生存和活性的环境因子,比如温度、营养物质、供氧状况、生物量以及环境中抗生素浓度水平等[57,58]。
2 环境中抗生素的残留状况
2.1 自然水体
环境中的绝大部分抗生素最终都会进入水环境,因此其对水环境的影响最为严重,也是最受关注的环境污染问题。目前的相关研究表明,世界各国和地区的地表水、地下水和饮用水中抗生素残留现象是比较普遍[59]。与污废水相比,地表水中抗生素存在的种类较多,但含量较低,其含量在几十到几百ng·L-1范围(表2)。地表水中的抗生素也有很大部分被吸附到沉积物中,但其含量较污泥和养殖场底泥低很多,其抗生素含量多在几十到几百μ g·kg-1范围(表1)。沉积物中以喹诺酮类和四环素类抗生素为主,这主要是由于喹诺酮类和四环素类抗生素在固体颗粒上吸附能力较强。抗生素在水和沉积物间的迁移转化是一个动态平衡的过程,即沉积物是抗生素的储存库,同时也是水中抗生素潜在的污染源。
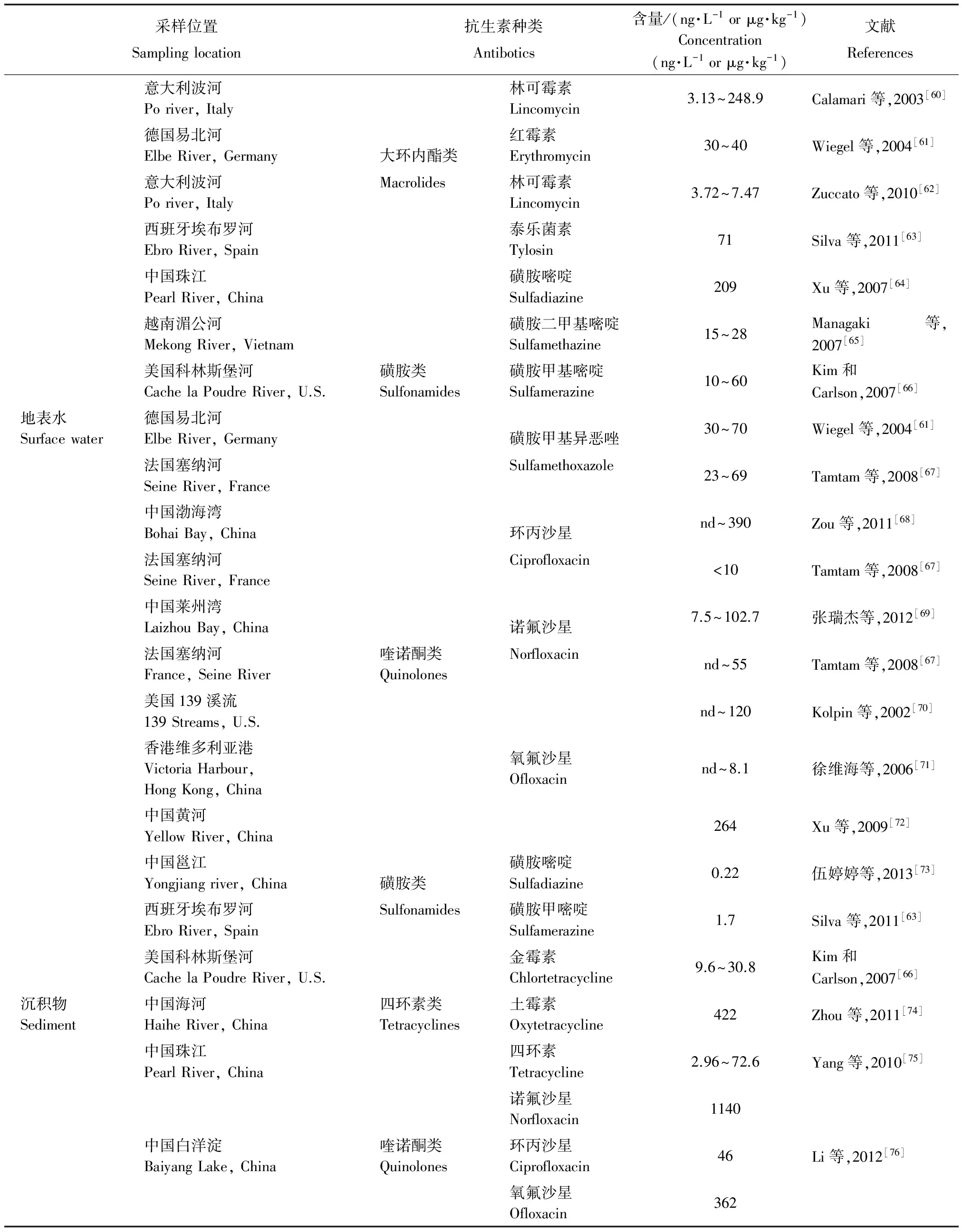
表2 地表水和沉积物中抗生素的污染水平
注:nd代表未检出.
Note: nd=not detected
国内外许多文献报道了地下水中抗生素的存在,但由于土壤层的天然净化作用,地下水受抗生素污染程度较低,一般检测到的含量在几十ng·L-1,且主要以迁移能力强的磺胺类药物为主(表3)。应该引起重视的是世界多地饮用水中也检测到抗生素的残留(表3)。尽管饮用水中抗生素含量不高(一般在几个ng·L-1范围),但其中的抗生素残留仍然对人类健康有着不容忽视的影响。
2.2 土壤中抗生素的残留状况
抗生素随污水灌溉、畜禽粪便和污泥施肥等途径进入土壤环境。不同国家和地区土壤中抗生素残留水平有所差异,但总体看来土壤中抗生素含量一般在μg·kg-1级,如表4。医用和农用抗生素在土壤中可被大量检出,包括四环素、喹诺酮类、磺胺类和大环内脂类抗生素等。综合资料表明[94],人为干扰的土壤中抗生素总量容易超过欧盟医药产品排放基准值(100 μg·kg-1)。
以上研究表面,目前有关抗生素在各种环境中的存在和污染水平的报道日渐增多,但是仍然缺乏合适的且具有可比性的分析测定方法。世界各国或地区报道的抗生素污染水平的差异部分源于此。
3 环境中抗生素的生态毒性效应
抗生素在药物设计时主要是针对人体和动物体内的病原性致病菌,因此,环境中残留的抗生素也会对环境中其他有机体产生不同程度的潜在的生态毒性效应。
3.1 对土壤生物的生态毒性效应
3.1.1 微生物
由于抗生素本身设计为抗菌药物,能直接杀死或抑制土壤中相关微生物的生长,从而影响微生物活性或功能。与其它污染物相似,抗生素的微生物毒性作用呈现剂量效应[95-97]。多数研究显示低浓度的抗生素无显著毒性效应,这可能与土壤吸附有很大关系[98]。有意思的是,部分研究显示添加低剂量的抗生素甚至可以对土壤微生物产生刺激作用,其原因可能在于这些抗生素可以作为某些微生物的碳源促进其生长[99]。但也有学着研究发现,在低浓度抗生素条件下微生物活性也会受到显著影响。例如,Toth等[100]研究发现磺胺二甲嘧啶和莫能菌素在环境浓度条件下(≤200 μg·kg-1)对土壤铁还原和硝化作用产生显著影响。

表3 地下水和饮用水中抗生素的污染水平

表4 土壤中抗生素残留状况
抗生素类药物有其靶标的微生物,因此,靶标微生物受到抑制后,环境中的其它微生物可以获得大量的资源,从而刺激其快速生长繁殖,对整个环境微生物群落结构产生影响。多位研究者采用分子指纹技术(如DGGE、T-RFLP等)研究了抗生素对土壤微生物群落结构的影响[101-103],结果显示,抗生素处理土壤后,代表不同微生物种类的条带会出现或消失,这说明抗生素对土壤微生物群落结构产生了显著影响。多数抗生素为抑细菌或灭细菌药物,因此此类抗生素的添加能降低土壤细菌数量,进而增加土壤真菌/细菌比例[104,105]。部分抗生素对革兰氏阳性菌(G+)和革兰氏阴性菌(G-)也具有选择性,因此G+/G-比例也会受到影响[106,107]。应该注意的是,抗生素也会直接作用于非靶标微生物。例如,Mohamed等[108]研究显示,游霉素(一种真菌抑制剂)在较高浓度下也会对细菌数量和群落结构产生影响。
许多研究者发现抗生素对土壤微生物群落功能多样性的影响[109,110]。但是直到现在,抗生素对参与生态系统过程的功能微生物群落的研究仍然很少。仅有Schauss等[111]和Kleineidam等[112]分别研究了猪粪配施磺胺嘧啶对氨氧化和反硝化微生物群落的影响。
3.1.2 动物
土壤动物(如蚯蚓,线虫,变形虫和轮虫等)不但对土壤起着天然的“过滤”和“净化”作用,而且在环境监测和生态毒性诊断研究中等方面也起着重要的作用。现有研究显示,只有在极高浓度条件下(效应浓度已远超过实际环境浓度),抗生素才会对土壤动物产生毒副作用[113,114]。Baguer等[115]研究了不同浓度土霉素和泰乐菌素对土壤无脊椎动物蚯蚓、跳虫和线蚓的影响,结果发现这两种药物对所测定的三种土壤动物的毒性较低,其ECl0达到150 mg·kg-1。陈海刚等[116]也发现喹乙醇、阿散酸和土霉素只有在高浓度条件下(500、500和125 mg·L-1)才会显著影响赤子爱胜蚓(Eisenia foetida )体中的纤维素酶和超氧化物歧化酶(SOD酶)活力。尽管抗生素对土壤动物的急性毒性低,但并不能就此忽略。因为它们及代谢物在环境中可发生迁移,并可在环境生物中蓄积并造成蓄积毒性。因而有必要进一步进行抗生素及其降解产物对土壤动物的慢性毒性或蓄积毒性试验。
3.1.3 植物
抗生素对植物生长发育的影响除与其自身的性质和使用剂量,还与培养介质和植物品种等有关。Boxall等[117]研究发现,土培条件下1 mg·kg-1浓度土霉素、保泰松和恩诺沙星能显著抑制胡萝卜和莴苣生长,而相同浓度的阿莫西林、磺胺嘧啶、泰乐素、甲氧苄啶和氟苯尼考等对这两种蔬菜生长没有影响。与其它污染物相似,抗生素在低浓度下可促进植物生长,高浓度则抑制植物生长[118,119]。同种抗生素对不同植物的生态毒性差异非常大。Eggen等[120]研究表明,当环丙沙星添加浓度为10 mg·kg-1时对大麦和胡萝卜的生长产生抑制作用,其中胡萝卜对环丙沙星的毒性效应更为敏感。
3.2 对水生生物的生态毒性效应
目前开展的相关研究较多针对水体的微生物、藻类、枝角类(或称“溞类”)及鱼类等,且多集中在急性毒害研究。一般来讲,抗生素对微生物和藻类产生毒性效应的浓度与高营养级生物相比要低2-3个数量级。研究表明,抗生素对低等水生生物(藻类和微生物)的半数效应浓度(EC50)多在μg·L-1级别[121,122]。例如,Robinson等[123]研究七种喹诺酮类药物对五种水生生物的影响发现,其中对蓝细菌(Microcystis aeruginosa )、浮萍(Lemna minor )和月牙藻(Pseudokirchneriella subcapitata )的EC50分别为49、106和7400 μg·L-1,而在10 mg·L-1范围内对大型溞(Daphnia magna )和黑头呆鱼(Pimephales promelas )无显著影响。其它研究也显示,环境相关浓度的抗生素对高等水生生物无明显影响[124,125]。但是也有研究显示,鱼类对大环内酯类药物比较敏感,其EC50都在100 μg·L-1以下[126,127]。显然,不同种类的抗生素对同种生物的毒性效应差异很大。
尽管抗生素在沉积物和活性污泥中广泛存在,但是相对于土壤环境,抗生素对这些环境中微生物的影响研究较少。由于沉积物和污泥对抗生素有强烈的吸附作用,抗生素可能降低甚至失去其抗菌活性。因此,多数研究显示抗生素对沉积物和活性污泥中微生物数量、活性及群落结构的效应浓度较高,都在mg·kg-1或mg·L-1级别[128-131]。
4 环境中的抗生素抗性基因问题
进入环境中的抗生素除了会造成化学污染外,还可能会诱导抗性微生物和抗性基因(antibiotics resistance genes,ARGs)的产生,并加速抗生素抗性的传播和扩散。这些抗性微生物可能会通过直接或者间接接触(如食物链)等途径进入人体,增加人体的耐药性,从而给人类公共健康带来威胁。自Piuden等[132]首次提出将ARGs作为一种环境污染物后,对其来源、分布和传播的研究开始受到人们的重视。
4.1 环境中ARGs的来源
环境中ARGs的来源主要有以下两种:
(1)内在抗性 内在抗性是指存在于细菌的基因组上的抗性基因的原型、准抗性基因或平时没有表达的抗性基因[133]。土壤中一些土著微生物本身就能够产生低浓度的抗生素,这些低浓度抗生素均可作为微生物种群间或种群内的信号分子,使微生物可通过随机突变或表达潜在抗性基因而获得抗性,因此土壤中必然存在着相应的ARGs。人类多次从千年冻土筛选到多种ARGs[134-136],足以证明ARGs早就存在于自然界中,而并非是由于现代临床治疗过程中抗生素的使用而造成。另外,环境中残留的抗生素构成筛选抗性细菌的环境选择压力,从而促进环境中ARGs的产生,加速内在ARGs突变和水平转移。目前许多研究发现环境中的抗生素残留与抗性基因之间有很好的相关性[137-138]。
(2) 外源输入 抗生素主要用于人类医疗业和养殖业,在其肠道内诱导出抗药菌株。从基因水平上看,抗药菌株都是由于其体内基因发生变异产生ARGs而表现出抗药性,这些抗性菌株随粪便排泄进入环境,使其成为环境中ARGs的重要来源。
医用抗生素首先在人体内诱导出ARGs,这些ARGs随粪便菌群排出体外,进入医疗废水。因此,医疗废水中ARGs的检出率较高,且以人用抗生素对应的ARGs种类为主,例如,β-内酰胺类[139]、氨基糖苷类[140]和大环内酯类[141]等。
在养殖业中,抗生素的使用极大地刺激了动物肠道内ARGs的发展。一些研究人员从畜禽粪便中分离出抗性菌株(如粪肠球菌、大肠杆菌等),并检测到多种ARGs[142-144]。畜禽粪便施肥是ARGs进入土壤环境的主要途径。另外,多位研究人员对美国不同地区养殖场废水的研究发现,四环素和磺胺类抗性基因等广泛存在于养殖废水中[138,145,146]。最近,Li等[147]在北京3个地区的养猪场废水中检测出五种喹诺酮类抗性基因(qnrD、qnrS、qepA、oqxA和oqxB),其绝对浓度在1.66×107~4.06×108copies·mL-1之间。以上研究也发现,养殖废水的排放已经对周边纳污水体构成污染。水产养殖业中,养殖生物粪便直接排放到水环境中,使得全球不同地区水产养殖区域水体和底泥中均检测到多种ARGs,且以磺胺类和四环素类ARGs为主[148-150]。
ARGs可以随医疗废水和动物养殖场污水等进入市政污水处理系统,使其成为ARGs聚集的“汇”。然而由于现有的水处理技术对ARGs没有明显的去除效果,国内外研究发现污水出水中仍有相当浓度的ARGs存在(表5)。由于处理厂污水与活性污泥间接触紧密,造成活性污泥中也可检出多种ARGs(表5)。市政污水处理系统中ARGs通过出水排放及污泥施肥进入到水体和土壤环境,造成受纳环境ARGs污染。
4.2 环境中AGRs的污染状况
各类污废水可通过不同途径进入地表水(海洋、湖泊、河流等),故在地表水和沉积物已普遍检测到ARGs。Stoll等[157]研究了24种抗性基因在德国和澳大利亚河流表层水中的分布,他们发现磺胺类和甲氧苄氨嘧啶抗性基因是最普遍的。Luo等[158]在海河(中国天津)河水和沉积物中检测出4种磺胺抗性基因(sul1、sul2、sul3、sulA)和7种四环素抗性基因(tetB、tetM、tetO、tetQ、tetS、tetT、tetW),沉积物中的抗性基因是水样中的120~2,000倍。Pei等[159]美国科罗拉多州北部沉积物多处点位检测到2种抗四环素基因(tetW,tetO)和两种磺胺抗性基因(sul1,sul2)。这些研究表明,地表水和沉积物已成为ARGs的库,并可能加速了ARGs的传播。
ARGs能够通过渗透、泄漏等途径从农田或沉积物进入地下水。目前,多人从养猪场附近的地下水中检测到多种四环素抗性基因[160,161]。如果地下水作为饮用水并很可能将ARGs通过饮水与食物带入食物链,在各个环境介质中传播转化,可能导致ARGs的全球性污染。
相对于水环境而言,目前有关ARGs在土壤环境中的研究还不系统,但已有研究表明ARGs可通过污水灌溉、施用畜禽粪便或污泥传播给土壤土著微生物。例如,Dalkmann等[162]墨西哥中部污水灌溉区域的土壤中ARGs的积累,仅在污水灌溉土壤中发现qnrS和qnrB,而非灌溉土壤没有发现;Schmitt等[163]检测了猪粪中,以及施用猪粪前后的土壤中的四环素类抗性基因,通过对比发现有一部分抗性基因是施肥前的土壤中所没有的,而是猪粪中特有的,因此可以推断这部分抗性基因是由猪粪带入土壤中的。此外,多项研究发现畜禽养殖场周边的土壤存在多种ARGs[129,164],这些研究表明畜禽粪便中的ARGs作为污染源能够迁移到土壤土著微生物。
目前在医院室内和养殖场附近的空气环境中已分离到多种携带ARGs的细菌。例如,Gilbert等[165]在加拿大一家医院的空气中分离的细菌中检测出多种ARGs,其中erm(X)、tet(G)和erm(F)具有较高的检出率。Sapkota等[166]在大型养猪场室内发现空气中气生革兰氏阳性细菌具有高水平的多药物抗性,所有受试菌(肠球菌和链球菌)均含有多重对大环内脂类、林可胺类、红霉素及四环素的抗性基因,50%的肠球菌和44%的链状球菌含有多重四环素抗性基因。以上研究表明,空气中的抗性菌可能是ARGs的储存库,吸入这些环境的空气是ARGs向人体传播的一个暴露途径。

表5 污水处理厂污水和污泥中抗生素抗性基因含量
注:-代表未检测
Note: -non-detection
5 研究展望
综上所述,虽然抗生素在环境中的浓度较低,但其对生态环境以及人类健康的潜在危害不容忽视。在过去10年间,随着各种环境中ARGs的不断发现,ARGs可以在各个环境介质中迁移、转化,并很可能将ARGs带入食物链传播,最终使ARGs污染具全球性。尽管人们对环境中的抗生素残留及其生态毒性效应和ARGs污染情况进行了大量的研究,但是仍然存在很多问题需要进一步研究:
环境中抗生素常与其它多种抗菌药物及有机无机污染物(如重金属、杀虫剂和多环芳烃等)共存,但缺乏对以上两者或三者在环境介质(主要是土壤)中的吸附-解吸、迁移和降解等行为或过程进行研究以及其复合污染的环境效应研究。
尽管现有研究已明确环境中的抗生素残留能产生一定的生态毒性效应,但是对它们的效应评价缺乏必要的研究,特别是长时间暴露在低剂量的生态毒性效应研究;另外尚缺乏环境因素对其生态毒性效应影响及其调控机理方面的研究。
在不同的环境介质中均发现多种ARGs,但是应该存在更多的ARGs,应加强对ARGs检测方法的研究,并对其ARGs的起源、转化和传播等研究领域进行积极探索。
ARGs作为一种环境中的新型污染物,对环境和人类健康的危害日趋明显,因此我们应该积极地寻找措施以降低其危害,例如,在养殖业中合理使用抗生素,避免经验用药;研究消除环境中抗生素及其抗性基因的方法与技术。
[1] Shea K M. Antibiotic resistance: What is the impact of agricultural uses of antibiotics on children's health [J]. Pediatrics, 2003, 112(1): 253-258
[2] European Federation of Animal Health (FEDESA). Antibiotics Use in Farm Animals does not threaten Human Health [R]. FEDESA/FEFANA Press release, 2001: 167-169
[3] Halling-Sørensen B, Nors N S, Lanzky P F, et al. Occurrence, fate and effects of pharmaceutical substances in the environment-a review [J]. Chemosphere, 1998, 36(2): 357-393
[4] Li W C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil [J]. Environmental Pollution, 2014, 187: 193-201
[5] Martínez J L. Antibiotics and antibiotic resistance genes in natural environments [J]. Science, 2008, 321(5887): 365-367
[6] 周启星, 罗义, 王美娥. 抗生素的环境残留、生态毒性及抗性基因污染[J]. 生态毒理学报, 2007, 2(3): 243-251
Zhou Q X, Luo Y, Wang M E. Environmental residues and ecotoxicity of antibiotics and their resistance gene pollution: A Review [J]. Asian Journal of Ecotoxicology, 2007, 2(3): 243-251 (in Chinese)
[7] Kümmerer K. Significance of antibiotics in the environment [J]. Journal of Antimicrobial Chemotherapy, 2003, 52(1): 5-7
[8] Duong H A, Pham N H, Nguyen H T, et al. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam [J]. Chemosphere, 2008, 72(6): 968-973
[9] Chang X, Meyer M T, Liu X, et al. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China [J]. Environmental Pollution, 2010, 158(5): 1444-1450
[10] Hamscher G, Sczesny S, H?per H, et al. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high performance liquid chromatography with electrospray ionization tandem mass spectrometry [J]. Analytical Chemistry, 2002, 74(7): 1509-1518
[11] Martínez-Carballo E, González-Barreiro C, Scharf S, et al. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria [J]. Environmental Pollution, 2007, 148(2): 570-579
[12] Zhao L, Dong Y H, Wang H, et al. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China [J]. Science of the Total Environment, 2010, 408(5): 1069-1075
[13] 邰义萍, 罗晓栋, 莫测辉, 等. 广东省畜牧粪便中喹诺酮类和磺胺类抗生素的含量与分布特征研究[J]. 环境科学, 2011, 32(4): 1188-1193
Tai Y P, Luo X D, Mo C H, et al. Occurrence of quinolone and sulfonamide antibiotics in swine and cattle manures from large-scale feeding operations of guangdong province [J]. Chinese Journal of Environmental Science, 2011, 32(4): 1188-1193 (in Chinese)
[14] 阮悦斐, 陈继淼, 郭昌胜, 等. 天津近郊地区淡水养殖水体的表层水及沉积物中典型抗生素的残留分析[J]. 农业环境科学学报, 2011, 30(12): 2586-2593 Ruan Y F, Chen J M, Guo C S, et al. Distribution characteristics of typical antibiotics in surface water and sediments from freshwater aquaculture water in Tianjin suburban areas, China [J]. Journal of Agro-Environment Science, 2011, 30(12): 2586-2593 (in Chinese)
[15] Le T X, Munekage Y. Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam [J]. Marine Pollution Bulletin, 2004, 49(11-12): 922-929
[16] Zou S, Xu W, Zhang R, et al. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities [J]. Environmental Pollution, 2011, 159(10): 2913-2920
[17] Sim W J, Lee J W, Lee E S, et al. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures [J]. Chemosphere, 2011, 82(2): 179-186
[18] Liu M M, Zhang Y, Yang M, et al. Abundance and distribution of tetracycline resistance genes and mobile elements in an oxytetracycline production wastewater treatment system [J]. Environmental Science & Technology, 2012, 46(14): 7551-7557
[19] Hilton M J, Thomas K V. Determination of selected human pharmaceutical compounds in effluent and surface water samples by high-performance liquid chromatography-electrospray tandem mass spectrometry [J]. Journal of Chromatography A, 2003, 1015(1): 129-141
[20] Meardell C S. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in Glatt Valley watershed, Switzerland [J]. Environmental Science & Technology, 2003, 24(5): 5479-5487
[21] Garcia-Galan M J, Diaz-Cruz M S, Barcelo D. Occurrence of sulfonamide residues along the Ebro river basin: Removal in wastewater treatment plants and environmental impact assessment [J]. Environment International, 2011, 37(2): 462-473
[22] Choi K, Kim Y, Park J, et al. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea [J]. Science of the Total Environment, 2008, 405(1-3): 120-128
[23] Brown K D, Kulis J, Thomson B, et al. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico [J]. Science of the Total Environment, 2006, 366(2-3): 772-783
[24] Ashton D, Hilton M, Thomas K V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom [J]. Science of the Total Environment, 2004, 333(1-3): 167-184
[25] Costanzo S D, Murby J, Bates J. Ecosystem response to antibiotics entering the aquatic environment [J]. Marine Pollution Bulletin, 2005, 51(1-4): 218-223
[26] Lee H B, Peart T E, Svoboda M L. Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography A, 2007, 1139(1): 45-52
[27] Golet E M, Strehler A, Alder A C, et al. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction [J]. Analytical Chemistry, 2002, 74(21): 5455-5462
[28] Xu W H, Zhang G, Li X D, et al. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China [J]. Water Research, 2007, 41(19): 4526-4534
[29] Takashi O, Naoyuki Y, Hiroaki T, et al. Development of extraction method of pharmaceuticals and their occurrences found in Japanese wastewater treatment plants [J]. Environment International, 2009, 35(5): 815-820
[30] Lillenberg M, Yurchenko S, Kipper K, et al. Presence of fluoroquinolones and sulfonamides in urban sewage sludge and their degradation as a result of composting [J]. Journal of Chromatography A, 2009, 1216(32): 5949-5954
[31] 王硕, 张晶, 邵兵. 超高效液相色谱-串联质谱测定污泥中氯霉素、磺胺类、喹诺酮类、四环素类与大环内酯类抗生素[J]. 分析测试学报, 2013, 32(2): 179-185
Wang S, Zhang J, Shao B, et al. Analysis of chloramphenicol, sulfonamides, fluoroquinolones, tetracyclines and macrolides in sewage sludge by ultra performance liquid chromatography-tandem mass spectrometry [J]. Journal of Instrumental Analysis, 2013, 32(2): 179-185 (in Chinese)
[32] 潘寻, 贲伟伟, 强志民. 高效液相色谱-质谱联用法同步测定城市污水处理厂活性污泥中的多类抗生素残留 [J]. 分析测试学报, 2011, 30(4): 448-452
Pan X, Ben W W, Qiang Z M. Simultaneous determination of several classes of antibiotics in the activated sludge of municipal sewage treatment plants by high performance liquid chromatography-mass spectrometry [J]. Journal of Instrumental Analysis, 2011, 30(4): 448-452 (in Chinese)
[33] Tolls J. Sorption of veterinary pharmaceuticals in soils: A Review [J]. Environmental Science & Technology, 2001, 35(17): 3397-3406
[34] Hailing-Søensen B, Nielsen S N, Lansky P F, et a1. Occurrence, fate, and effects of pharmaceuticals in the environment-A review [J]. Chemosphere, 1998, 36(2): 357-365
[35] Pan B, Ning P, Xing B S. Sorption of pharmaceuticals and personal care products [J]. Environmental Science and Pollution Research, 2009, 16(1): 106-116
[36] Wegst-Uhrich S R, Navarro D A G, Zimmerman L, et al. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides [J]. Chemistry Central Journal, 2014, 8(1): 5 doi: 10.1186/1752-153X-8-5
[37] ter Laak T L, Gebbink W A, Tolls J. The effect of pH and ionic strength on the sorption of sulfachloropyridazine, tylosin, and oxytetracycline to soil [J]. Environmental Toxicology and Chemistry, 2006, 25(4): 904-911
[38] Sassman S A, Lee L S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange [J]. Environmental Science & Technology, 2005, 39(19): 7452-7459
[39] Guo X T, Chen Y, Wu Y A. The influences of pH and ionic strength on the sorption of tylosin on goethite [J]. Environmental Science and Pollution Research, 2014, 21(4): 2572-2580
[40] Mackay A A, Canterbury B. Oxytetracycline sorption to organic matter by metal-bridging [J]. Journal of Environmental Quality, 2005, 34(6): 1964-1971
[41] Gu C, Karthikeyan K G., Sibley S D, et al. Complexation of the antibiotic tetracycline with humic acid [J]. Chemosphere, 2007, 66(8): 1494-1501
[42] Zhang M K, Wang L P, Zheng S A. Adsorption and transport characteristics of two exterior-source antibiotics in some agricultural soils [J]. Acta Ecologica Sinica, 2008, 28(2): 761-766
[43] Leal P, Marques R, Allenoi F, et al. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils [J]. Chemosphere, 2013, 92(8): 979-985
[44] Boxall A, Blackwell P, Cavallo R, et al. The sorption and transport of a sulphonamide antibiotic in soil systems [J]. Toxicology Letters, 2002, 131(1-2): 19-28
[45] Roy K, Cho Milt M, Myriam U, et al. Transformation and sorption of the veterinary antibiotic sulfadiazine in two soils: A short-term batch study [J]. Environmental Science & Technology, 2010, 44(12): 4651-4657
[46] Sarmah A K, Meyer M T, Boxall B A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment [J]. Chemosphere, 2006, 65(5): 725-759
[47] Jiao S, Zheng S, Yin D, et al. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria [J]. Chemosphere, 2008, 73(3): 377-382
[48] 张卫, 虞云龙, 谭成侠, 等. 阿维菌素水解动力学的研究[J]. 农业环境科学学报, 2004, 23(1): 174-176
Zhang W, Yu Y L, Tan C X, et al. Hydrolysis kinetics and mechanism of abamectin [J]. Journal of Agro-Environment, Science, 2004, 23(1): 174-176 (in Chinese)
[49] Längin A, Alexy R, König A, et al. Deactivation and transformation products in biodegradability testing of β-lactams piperacillin and amoxicillin [J]. Chemosphere, 2009, 75(3): 347-354
[50] 祖亭, 颜承农, 王润涛. 金属离子催化β-内酰胺类抗生素水解的荧光光谱研究[J]. 分析试验室, 2001, 20(4): 1-4
Zu T, Yan C N, Wang R T. Study on catalyzed hydrolysis of β-lactams antibiotics by metal ions with spectrofluorimetry [J]. Chinese Journal of Analysis Laboratory, 2001, 20(4): 1-4 (in Chinese)
[51] Keith A L, Craig D A, Michael T M, et a1. Effects of ionic strength, temperature, and pH on degradation of selected antibiotic [J]. Journal of Environmental Quality, 2008, 37(2): 378-386
[52] Torniainen K, Tammilehto S, Ulvi V. The effect of pH, buffer type and drug concentration on the photodegradation of ciprofloxacin [J]. International journal of pharmaceutics, 1996, 132(1-2): 53-61
[53] Fisher J M, Reese J G, Pellechia P J, et al. Role of Fe(III), phosphate, dissolved organic matter, and nitrate during the photodegradation of domoic acid in the marine environment [J]. Environmental Science & Technology, 2006, 40(7): 2200-2205
[54] 刘伟, 王慧, 陈小军, 等. 抗生素在环境中降解的研究进展[J]. 动物医学进展, 2009, 30(3): 89-94
Liu W, Wang H, Chen X J, et al. Progress on degradation of antibiotics in environment [J]. Progress in Veterinary Medicine, 2009, 30(3): 89-94 (in Chinese)
[55] Schlüsener M P, Bester K. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil [J]. Environmental Pollution, 2006, 143(3): 565-571
[56] Holly D, Satish G, Sally N. Antibiotic degradation during manure composting [J]. Journal of Environmental Quality, 2008, 37(3): 1245-1253
[57] Ingerslev F, Halling-Sφensen B. Biodegradability of metronidazole, olaquindox, and tylosin and formation of tylosin degradation products in aerobic soil-manure slurries [J]. Ecotoxicology and Environmental Safety, 2001, 48(3): 311-320
[58] Cetecioglu Z, Ince B, Azman S, et al. Biodegradation of tetracycline under various conditions and effects on microbial community [J]. Applied Biochemistry and Biotechnology, 2014, 172(2): 631-640
[59] Kümmerer K. Antibiotics in the aquatic environment-A review-Part I [J]. Chemosphere, 2009, 75(4): 417-434
[60] Calamari D, Zuccato E, Castiglioni S, et al. Strategic survey of therapeutic drugs in the rivers Po and Lambro in Northern Italy [J]. Environmental Science & Technology, 2003, 37(7): 1241-1248
[61] Wiegel S, Aulinger A, Brockmeyer R, et al. Pharmaceuticals in the river Elbe and its tributaries [J]. Chemosphere, 2004, 57(2): 107-126
[62] Zuccato E, Castiglioni S, Bagnati R, et al. Source, occurrence and fate of antibiotics in the Italian aquatic environment [J]. Journal of Hazardous Materials, 2010, 179(1-3): 1042-1048
[63] Silva B F, Jelic A, López-Serna R, et al. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain [J]. Chemosphere, 2011, 85(8): 1331-1339
[64] Xu W H, Zhang G, Zou S C, et al. Determination of selected antibiotics in the Victoria Harbour and the Pearl river, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry [J]. Environmental Pollution, 2007, 145(3): 672-679
[65] Managaki S, Murata A, Takada H, et al. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta [J]. Environmental Science & Technology, 2007, 41(23): 8004-8010
[66] Kim S C, Carlson K. Quantification of human and veterinary antibiotics in water and sediment using SPE/LC/MS/MS [J]. Analytical and Bioanalytical Chemistry, 2007, 387(4): 1301-1315
[67] Tamtam F, Mercier F, Le Bot B, et al. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions [J]. Science of The Total Environment, 2008, 393(1): 84-95
[68] Zou S, Xu W, Zhang R, et al. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities [J]. Environmental Pollution, 2011, 159(10): 2913-2920
[69] 张瑞杰, 张干, 郑芊, 等. 喹诺酮类抗生素在莱州湾及主要入海河流中的含量和分布特征[J]. 海洋环境科学, 2012, 31(1): 53-61
Zhang R J, Zhang G, Zhen Q, et al. Concentrations and spatial distributions of selected quinolones antibiotics in Laizhou Bay and main rivers flowing into the bay [J]. Marine Environmental Science, 2012, 31(1): 53-61 (in Chinese)
[70] Kolpin D W, Furlong E T, Meyer M T, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: A national reconnaissance [J]. Environmental Science & Technology, 2002, 36(6): 1202-1211
[71] 徐维海, 张干, 邹世春, 等. 香港维多利亚港和珠江广州河段水体中抗生素的含量特征及其季节变化 [J]. 环境科学, 2006, 27(12): 2458-2462
Xu W H, Zhang G, Zou S C, et al. Occurrence and seasonal changes of antibiotics in the Victoria Harbour and the Pearl River, South China [J]. Chinese Journal of Environmental Science, 2006, 27(12): 2458-2462 (in Chinese)
[72] Xu W H, Zhang G, Zou S C, et al. A preliminary investigation on the occurrence and distribution of antibiotics in the Yellow River and its tributaries, China [J]. Water Environment Research, 2009, 81(3): 248-254
[73] 伍婷婷, 张瑞杰, 王英辉,等. 邕江南宁市区段表层沉积物典型抗生素污染特征[J]. 中国环境科学, 2013, 33(2): 336-344
Wu T T, Zhang R J, Wang Y H, et al. Investigation of the typical antibiotics in the sediments of the Yongjiang River, Nanning City, South China [J]. China Environmental Science, 2013, 33(2): 336-344 (in Chinese)
[74] Zhou L J, Ying G G, Zhao J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China [J]. Environmental Pollution, 2011, 159(7): 1877-1885
[75] Yang J F, Ying G G, Zhao J L, et al. Simultaneous determination of four classes of antibiotics in sediments of the Pearl Rivers using RRLC-MS/MS [J]. Science of the Total Environment, 2010, 408(16): 3424-3432
[76] Li W H, Shi Y L, Gao L H, et al. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China [J]. Chemosphere, 2012, 89(11): 1307-1315
[77] Hu X G, Zhou Q X, Luo Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northem China [J]. Environmental Pollution, 2010, 158(9): 2992-2998
[78] Lindsey M E, Meyer M, Thurman E M. Analysis of trace levels of sulfonamide and tetracycline antimicrobials, in groundwater and surface water using solid-phase extraction and liquid chromatography mass spectrometry [J]. Analytical Chemistry, 2001, 73(19): 4640-4646
[79] Hirsch R, Ternes T, Haberer K, et al. Occurrence of antibiotics in the aquatic environment [J]. Science of the Total Environment, 1999, 225(1-2): 109-118
[80] Diaz-Cruz M S, Garcia-Galan M J, Barcelo D. Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry [J]. Journal of Chromatography A, 2008, 1193(1-2): 50-59
[81] Fram M S, Belitz K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking water supply in California [J]. Science of the Total Environment, 2011, 409(18): 3409-3417
[82] Zuccato E, Calamari D, Natangelo M, et al. Presence of therapeutic drugs in the environment [J]. Lancet, 2000, 355(9217): 1789-1790
[83] Vieno N M, Hrkki H, Tuhkanen T, et al. Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant [J]. Environmental Science & Technology, 2007, 41(14): 5077-5084
[84] Benotti M J, Trenholm R A, Vanderford B J, et al. Pharmaceuticals and endocrine disrupting compounds in US drinking water [J]. Environmental Science & Technology, 2009, 43(3): 597-603
[85] Ye Z Q, Weinberg H S, Meyer M T. Trace analysis of trimethoprim and sulfonamide, macrolide, quinolone, and tetracycline antibiotics in chlorinated drinking water using liquid chromatography electrospray tandem mass spectrometry [J]. Analytical Chemistry, 2007, 79(3):1135-1144
[86] Jacobsen A M, Halling-Sørensen B, Ingerslev F, et al. Simultaneous extraction of tetracycline, macrolide and sulfonamide antibiotics from agricultural soils using pressurised liquid extraction, followed by solid-phase extraction and liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography A, 2004, 1238(1-2): 157-170
[87] Hou J, Wan W N, Mao D Q, et al. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China [J]. Environmental Science and Pollution Research, 2015, 22(6): 4545-4554
[88] Boxall A B A, Fogg L A, Baird D J, et al. Targeted Monitoring Study for Veterinary Medicines in the Environment [R]. Science Report SC030183/SR. UK Environment Agency, Bristol. 2005
[89] Hamscher G, Sczesny S, Höper H, et al. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high performance liquid chromatography with electrospray ionization tandem mass spectrometry [J]. Analytical Chemistry, 2002, 74(7): 1509-1518
[90] Brambilla G, Patrizii M, De Filippis S P, et al. Oxytetracycline as environmental contaminant in arable lands [J]. Analytica Chimica Acta, 2007, 586(1-2): 326-329
[92] Xie Y F, Li X W, Wang J F, et al. Spatial estimation of antibiotic residues in surface soils in a typical intensive vegetable cultivation area in China [J]. Science of The Total Environment, 2012, 430: 126-131
[93] 李彦文, 莫测辉, 赵娜, 等. 菜地土壤中磺胺类和四环素类抗生素污染特征研究 [J]. 环境科学, 2009, 6: 1762-1766
Li Y W, Mo C H, Zhao N, et al. Investigation of sulfonamides and tetracyclines antibiotics in soils from various vegetable fields [J]. Chinese Journal of Environment Science, 2009, 30(6): 1762-1766 (in Chinese)
[94] Tamtam F, van Oort F, Le Bot B, et al. Assessing the fate of antibiotic contaminants in metal contaminated soils four years after cessation of long-term waste water irrigation [J]. Science of The Total Environment, 2011, 409(3): 540-547
[95] Thiele-Bruhn S. Microbial inhibition by pharmaceutical antibiotics in different soils-dose-response relations determined with the iron(III) reduction test [J]. Environmental Toxicology and Chemistry, 2005, 24(4): 869-876
[96] Kong W D, Zhu Y G, Fu B J, et al. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community [J]. Environmental Pollution, 2006, 143(1): 129-137
[97] Kotzerke A, Hammesfahr U, Kleineidam K, et al. Influence of difloxacin-contaminated manure on microbial community structure and function in soils [J]. Biology and Fertility of Soils, 2011, 47(2): 177-186
[98] Thiele-Bruhn S, Beck I C. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass [J]. Chemosphere, 2005, 59(4): 457-465
[99] Dantas G, Sommer M O A, Oluwasegun R D, et al. Bacteria subsisting on antibiotics [J]. Science, 2008, 320(5872): 100-103
[100] Toth J D, Feng Y, Dou Z. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification [J]. Soil Biology and Biochemistry, 2011, 43(12): 2470-2472
[101] Westergaard K, Müller A K, Christensen S, et al. Effects of tylosin as a disturbance on the soil microbial community [J]. Soil Biology and Biochemistry, 2001, 33(15): 2061-2071
[102] Zielezny Y, Groeneweg J, Vereecken H, et al. Impact of sulfadiazine and chlorotetracycline on soil bacterial community structure and respiratory activity [J]. Soil Biology and Biochemistry, 2006, 38(8): 2372-2380
[103] Demoling L A, Bååth E, Greve G, et al. Effects of sulfamethoxazole on soil microbial communities after adding substrate [J]. Soil Biology and Biochemistry, 2009, 41(4): 840-848
[104] Girardi C, Greve J, Lamshöft M, et al. Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities [J]. Journal of Hazardous Materials, 2011, 198: 22-30
[105] Hammesfahr U, Heuer H, Manzke B, et al. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils [J]. Soil Biology and Biochemistry, 2008, 40(7): 1583-1591
[106] Hund-Rinke K, Simon M, Lukow T. Effects of tetracycline on the soil microflora: Function, diversity, resistance [J]. Journal of Soils and Sediments, 2004, 4(1): 11-16
[107] Schnabel E L, Jones A L. Distribution of tetracycline resistance genes and transposons among phylloplane bacteria in Michigan apple orchards [J]. Applied and Environmental Microbiology, 1999, 65(11): 4898-4907
[108] Mohamed M A N, Ranjard L, Catroux C, et al. Effect of natamycin on the enumeration, genetic structure and composition of bacterial community isolated from soils and soybean rhizosphere [J]. Journal of Microbiological Methods, 2005, 60(1): 31-40
[109] Liu F, Wu J, Ying G G, et al. Changes in functional diversity of soil microbial community with addition of antibiotics sulfamethoxazole and chlortetracycline [J]. Applied Microbiology and Biotechnology, 2012, 95(6): 1615-1623
[110] 王加龙, 刘坚真, 陈杖榴, 等. 恩诺沙星残留对土壤微生物数量及群落功能多样性的影响[J]. 应用与环境生物学报, 2005, 11(1): 86-89
Wang J L, Liu J Z, Chen Z L, et al. Effect of enrofioxacin residue on number and community function diversity of soil microbes [J]. Chinese Journal of Applied & Enviromental Biology, 2005, 11(1): 86-89 (in Chinese)
[111] Schauss K, Focks A, Leininger S, et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils [J]. Environmental Microbiology, 2009, 11(2): 446-456
[112] Kleineidam K, Sharma S, Kotzerke A, et al. Effect of sulfadiazine on abundance and diversity of denitrifying bacteria by determining nirK and nirS genes in two arable soils [J]. Microbial Ecology, 2010, 60(4): 703-707
[113] 李银生, 曾振灵, 陈杖榴, 等. 三种兽药对蚯蚓的急性毒性试验 [J]. 农业环境科学学报, 2004, 23(6): 1065-1069
Li Y S, Zeng Z L, Chen Z L, et al. LC50the acute toxicity of three veterinary pharmaceuticals to earthworms [J]. Journal of Agro-Environment Science, 2004, 23(6): 1065-1069 (in Chinese)
[114] Gao Y H, Sun Z J, Sun X S, et al. Toxic effect of olaquindox antibiotic on Eisenia fetida [J]. European Journal of Soil Biology, 2007, 43(S1): S252-S255
[115] Baguer A J, Jensen J, Krogh P H. Effects of the antibiotics oxytetracycline and tylosin on soil fauna [J]. Chemosphere, 2000, 40(7): 751-757
[116] 陈海刚, 李兆利, 徐摇韵, 等. 三种兽药添加剂对赤子爱胜蚓体内纤维素酶和SOD酶的活性影响 [J]. 南京大学学报(自然科学), 2006, 42(4): 435-439
Chen H G, Li Z L, Xu Y Y, et al. Cellulase and superoxide dismutase activities in the earthworm Eisenia foetida exposed to three veterinary drugs and additives [J]. Journal of Nanjing University (Natural Sciences), 2006, 42(4): 435-439 (in Chinese)
[117] Boxall A B, Johnson P, Smith E J, et al. Uptake of veterinary medicines from soils into plants [J]. Journal of Agricultural and Food Chemistry, 2006, 54(6): 2288-2297
[118] Batchelder A R. Chlortetracycline and oxytetracycline effects on plant growth and development in soil systems [J]. Journal of Environmental Quality, 1982, 11(4): 675-678
[119] Migliore L, Cozzolino S, Fiori M. Phytotoxicity to and uptake of enrofloxacin in crop plants [J]. Chemosphere, 2003, 52(7): 1233-1244
[120] Eggen T, Asp T N, Grave K, et al. Uptake and translocation of metformin, ciprofloxacin and narasin in forage and crop plants [J]. Chemosphere, 2011, 85(1): 26-33
[121] Chen J Q, Guo R X. Access the toxic effect of the antibiotic cefradine and its UV light degradation products on two freshwater algae [J]. Journal of Hazardous Materials, 2012, 209-210: 520-523
[122] Isidori M, Lavorgna M, Nardelli A, et al. Toxic and genotoxic evaluation of six antibiotics on non-target organisms [J]. Science of The Total Environment, 2005, 346(1-3): 87-98
[123] Robinson A A, Belden J B, Lydy M J. Toxicity of fluoroquinolone antibiotics to aquatic organisms [J]. Environmental Toxicology and Chemistry, 2005, 24(2): 423-430
[124] 魏瑞成, 包红朵, 郑摇勤, 等. 粪源抗生素金霉素和喹乙醇在养殖水体中的残留及对锦鲤的生态毒理效应研究 [J]. 农业环境科学学报, 2009, 28(9): 1800-1805
Wei R C, Bao H D, Zheng Y Q, et al. Chlortetracycline and olaquindox residues of manure-derived antibiotics in the aquatic water and their ecotoxicological effects on Koi Carp [J]. Journal of Agro-Environment Science, 2009, 28(9): 1800-1805 (in Chinese)
[125] De Liguoro M, Fioretto B, Poltroneri C, et al. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim [J]. Chemosphere, 2009, 75(11): 1519-1524
[126] 赵于丁, 王冬兰, 来有鹏, 等. 一些常用农药对斑马鱼的毒性与安全评价 [J]. 农药科学与管理, 2008, 29: 25-29
Zhao Y D, Wang D L, Lai Y P, et al. Toxicity of some pesticides to Brachydanio rerio and safety valuation [J]. Pesticide Science and Administration, 2008, 29(8): 25-29 (in Chinese)
[127] 李世凯, 张健龙, 江敏, 等. 伊维菌素对斑马鱼(Danio rerio)生理生化特性的影响 [J]. 安全与环境学报, 2014, 14(1): 300-305
Li S K, Zhang J L, Jiang M, et al. Effects of ivermectin on the physiological and biochemical characteristic features of Danio rerio [J]. Journal of Safety and Environmen, 2014, 14(1): 300-305 (in Chinese)
[128] Halling-Sφrensen B. Inhibition of aerobic growth and nitrification of bacteria in sewage sludge by antibacterial agents [J]. Archives of Environmental Contamination and Toxicology, 2001, 40(4): 451-460
[129] Ma D Y, Hu Y Y, Wang J Y, et al. Effects of antibacterials use in aquaculture on biogeochemical processes in marine sediment [J]. Science of the Total Environment, 2006, 367(1): 273-277
[130] Córdova-Kreylos A L, Scow K M. Effects of ciprofloxacin on salt marsh sediment microbial communities [J]. The ISME Journal, 2007, 1(7): 585-595
[131] 唐璐, 牛成镇, 吕镇梅. 土霉素对活性污泥微生物群落结构的影响 [J]. 浙江大学学报(农业与生命科学版), 2013, 39(5): 545-555
Tang L, Niu C Z, Lv Z M. Dynamic change of microbial community structure of activated sludge influenced by oxytetacycline [J]. Journal of Zhejiang University (Agriculture & Life Sciences), 2013, 39(5): 545-555 (in Chinese)
[132] Pruden A, Pei R T, Storteboom H, et al. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado [J]. Environmental Science & Technology, 2006, 40(23): 7445-7450
[133] Davies J, Davies D. Origins and evolution of antibiotic resistance [J]. Microbiology and Molecular Biology Reviews, 2010, 74(3): 417-433
[134] Allen H K, Moe L A, Rodbumrer J, et al. Functional metagenomics reveals diverse beta-lactamases in a remote Alaskan soil [J]. The ISME Journal, 2009, 3(2): 243-251
[135] D'Costa V M, King C E, Kalan L, et al. Antibiotic resistance is ancient [J]. Nature, 2011, 477(7365): 457-461
[136] Lang K S, Anderson J M, Schwarz S, et al. Novel florfenicol and chloramphenicol resistance gene discovered in Alaskan soil by using functional metagenomics [J]. Applied and Environmental Microbiology, 2010, 76(15): 5321-5326
[137] Wu N, Qiao M, Zhang B, et al. Abundance and diversity of tetracycline resistance genes in soils adjacent to representative swine feedlots in China [J]. Environmental Science & Technology, 2010, 44(18): 6933-6939
[138] Peak N, Knapp C W, Yang R K, et al. Abundance of six tetracycline resistance genes in wastewater lagoons at cattle feedlots with different antibiotic use strategies [J]. Environmental Microbiology, 2007, 9(1): 143-151
[139] Chagas T P G, Seki L M, Cury J C, et al. Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro, Brazil [J]. Journal of Applied Microbiology, 2011, 111(3): 572-581
[140] Kotzamanidis C, Zdragas A, Kourelis A, et al. Characterization of vanA-type Enterococcus faecium isolates from urban and hospital wastewater and pigs [J]. Journal of Applied Microbiology, 2009, 107(3): 997-1005
[141] Schwartz T, Kohnen T, Jansen B, et al. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms [J]. FEMS Microbiology Letter, 2003, 43(3): 325-335
[142] Frye J G, Lindsey R L, Meinersmann R J, et al. Related antimicrobial resistance genes detected in different bacterial species co-isolated from swine fecal samples [J]. Foodborne Pathogens and Disease, 2011, 8(6): 663-679
[143] Kobashi Y, Hasebe A, Nishio M, et al. Diversity of tetracycline resistance genes in bacteria isolated from various agricultural environments [J]. Microbes and Environments, 2007, 22(1): 44-51
[144] Byrne-Bailey K G, Gaze W H, Kay P, et al. Prevalence of sulfonamide resistance genes in bacterial isolates from manured agricultural soils and pig slurry in the United Kingdom [J]. Antimicrobial agents and Chemotherapy, 2009, 53(2): 696-702
[145] Chee-Sanford J C, Aminov R I, Krapac I J, et al. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities [J]. Applied and Environmental Microbiology, 2001, 67(4): 1494-1502
[146] Koike S, Krapac I G, Oliver H D, et al. Monitoring and source tracking of tetracycline resistance genes in lagoons and groundwater adjacent to swine production facilities over a 3-year period [J]. Applied and Environmental Microbiology, 2007, 67(15): 4813-4823
[147] Li J, Wang Thanh, Shao B, et al. Plasmid-mediated quinolone resistance genes and antibiotic residues in wastewater and soil adjacent to swine feedlots: Potential transfer to agricultural lands [J]. Environmental Health Perspectives, 2012, 120(8): 1144-1149
[148] Cesare A D, Vignaroli C, Luna G M, et al. Antibiotic-resistant Enterococci in seawater and sediments from a coastal fish farm [J]. Microbial Drug Resistance, 2012, 18(5): 502-509
[149] Hoa P T P, Nonana L, Viet P H, et al. Detection of the sul1, sul2, and sul3 genes in sulfonamide-resistant bacteria from wastewater and shrimp ponds of North Vietnam [J]. Science of the Total Environment, 2008, 405(1-3): 377-384
[150] 梁惜梅, 聂湘平, 施震. 珠江口典型水产养殖区抗生素抗性基因污染的初步研究 [J]. 环境科学, 2013, 34(10): 4073-4080
Liang X M, Nie X P, Shi Z. Preliminary studies on the occurrence of antibiotic resistance genes in typical aquaculture area of the Pearl River Estuary [J]. Environmental Science, 2013, 34(10): 4073-4080 (in Chinese)
[151] Yang Y, Zhang T, Zhang X X. Quantification and characterization of β-lactam resistance genes in 15 sewage treatment plants from East Asia and North America [J]. Applied Microbiology and Biotechnology, 2012, 95(5):1351-1358
[152] Du J, Ren H Q, Geng J J. Occurrence and abundance of tetracycline, sulfonamide resistance genes, and class 1 integron in five wastewater treatment plants [J]. Environmental Science and Pollution Research, 2014, 21(12): 7276-7284
[153] Munir M, Wong K, Xagoraraki I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan [J]. Water research, 2011, 45(2): 681-693
[154] Börjesson S, Melin S, Matussek A, et al. A seasonal study of the mecA gene and Staphylococcus aureus including methicillin resistant S.aureus in a municipal wastewater treatment plant [J]. Water research, 2009, 43(4): 925-932
[155] Auerbach E A, Seyfried E E, McMahon K D. Tetracycline resistance genes in activated sludge wastewater treatment plants [J]. Water Research, 2007, 41(5): 1143-1151
[156] Zhang T, Zhang M, Zhang X X, et al. Tetracycline resistance genes and tetracycline resistant lactose-fermenting Enterobacteriaceae in activated sludge of sewage treatment plants [J]. Environmental Science & Technology, 2009, 43(10): 3455-3460
[157] Stoll C, Sidhu J P S, Tiehm A, et al. Prevalence of clinically relevant antibiotic resistance genes in surface water samples collected from Germany and Australia [J]. Environmental Science & Technology, 2012, 46(17): 9716-9726
[158] Luo Y, Mao D, Rysz M, et al. Trends in antibiotic resistance genes occurrence in the Haihe River, China [J]. Environmental Science & Technology, 2010, 44(19): 7220-7225
[159] Pei R T, Kim S C, Carlson K H, et al. Effect of river landscape on the sediment concentrations of antibiotics and correspond ing antibiotic resistance genes (ARG) [J]. Water Research, 2006, 40(12): 2427-2435
[160] Chee-Sanford J C, Aminov R I, Krapac I J, et al. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities [J]. Applied and Environmental Microbiology, 2001, 67(4): 1494-1502
[161] Koike S, Krapac I G, Oliver H D, et al. Monitoring and source tracking of tetracycline resistance genes in lagoons and groundwater adjacent to swine production facilities over a 3-year period [J]. Applied and Environmental Microbiology, 2007, 73(15): 4813-4823
[162] Dalkmann P, Broszat M, Siebe C, et al. Accumulation of pharmaceuticals, Enterococcus, and resistance genes in soils irrigated with waste water for zero to 100 years in Central Mexico [J]. PLoS ONE, 2012, 7: e45397. doi:10.1371/journal.pone.0045397
[163] Schmitt H, Stoob K, Hamscher G, et al. Tetracyclines and tetracycline resistance in agricultural soils: microcosm and field studies [J]. Microbial Ecology, 2006, 51(3): 267-276
[164] Zhu Y G, Johnson T A, Su J Q, et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms [J]. Proceedings of the National Academy of Sciences, 2013, 110(9): 3435-3440
[165] Gilbert Y, Veillette M, Duchaine C. Airborne bacteria and antibiotic resistance genes in hospital rooms [J]. Aerobiologia, 2010, 26(3): 185-194
[166] Sapkota A R, Ojo K K, Roberts M C, et al. Antibiotic resistance genes in multidrug-resistant Enterococcus spp and Streptococcus spp recovered from the Indoor Air of a large-scale swine-feeding operation [J]. Letter in Applied Microbiology, 2006, 43(5): 5534-540
◆
基金项目:国家自然科学基金(No. 41173102;31200396);天津市科技计划项目(No.12ZCZDSF01400);天津理工大学教学改革项目(YB11-26);天津市自然科学基金(No. 14JCQNJC08500);海洋公益性项目(201005026-05)
作者简介:葛兴彬(1990-),男,硕士研究生,研究方向为纳米毒理学,E-mail: gexingbin0504@163.com;
*通讯作者(Corresponding author), E-mail: litielong@nankai.edu.cn
DOI: 10.7524/AJE.1673-5897-20140227004
葛兴彬,王振虹,郭楚奇, 等. 纳米零价铁的生态毒性效应研究进展[J]. 生态毒理学报,2015, 10(3): 28-37
Ge X B, Wang Z H, Guo C Q, et al. Review of the ecotoxicity of nanoscale zero-valent iron [J]. Asian Journal of Ecotoxicology, 2015, 10(3): 28-37
(in Chinese)
The Antibiotic in Environment and Its Ecotoxicity: A Review
Xu Yonggang, Yu Wantai*, Ma Qiang, Zhou Hua, Jiang Chunming
Institute of Applied Ecology, Chinese Academy of Sciences, Shanyang, 110016, China
Received 11 August 2014 accepted 23 October 2014
In recent years, more and more antibiotic drugs were used in the medical treatment, livestock breeding and aquaculture. Since antibiotic drugs could not be completely absorbed by the body of humans and animals, their parent compounds or related metabolites would be returned back to the aquatic and soil environment via urine or faece, causing their residue in environment. Residual antibiotics drugs could lead to the potential environmental risks, of which the most serious one is to induce and spread antibiotic resistance genes (ARGs), subsequently producing threat to human health. This paper mainly described the source, fate and occurrence of antibiotic drugs in the environment, and then we reviewed the ecotoxicity and ARGs caused by antibiotic. In addition, we also pointed out the problems existing in the present studies and prospected the future research work.
antibiotics; ecotoxicity; ARGs
国家科技部支撑计划项目(2012BAD05B01)和国家自然科学基金(31100465)
徐永刚(1984-),男,博士,研究方向为微生物生态学,E-mail:xuyonggang1228@163.com;
*通讯作者(Corresponding author), E-mail: wtyu@iae.ac.cn
10.7524/AJE.1673-5897-20140811001
2014-08-11 录用日期:2014-10-23
1673-5897(2015)3-011-17
X171.5
A
宇万太(1965-),男,研究员,博士生导师,主要研究方向为土壤养分循环及农业环境。
徐永刚,宇万太,马强, 等. 环境中抗生素及其生态毒性效应研究进展[J]. 生态毒理学报,2015, 10(3): 11-27
Xu Y G, Yu W T, Ma Q, et al. The antibiotic in environment and its ecotoxicity: a review [J]. Asian Journal of Ecotoxicology, 2015, 10(3): 11-27 (in Chinese)

