The role of estrogen in the development of colorectal cancer
·综述·
The role of estrogen in the development of colorectal cancer
张玲王吉凌朱圣韬史学森
作者单位:010040包头,内蒙古包头市中心医院消化科(张玲、王吉凌、史学森);100070 北京,首都医科大学附属北京友谊医院北京临床医学研究所(朱圣韬)
Cancer is a major public health problem in the world.With the progress of medical technology,mean 5-year survival rates of colorectal cancer(CRC)have doubled over the past 40 years[1].However,in 2013,the International Agency for Research on Cancer(IARC)released data on cancer incidence,mortality and prevalence worldwide.The IARC’s online database provides the estimates for 28 types of cancer in 184 countries worldwide.The data demonstrate that colorectal cancer is the third most common cancer in men(746000 cases,10.0% of the total)and the second in women(614,000 cases,9.2% of the total)worldwide.Almost 55% of the cases occur in more developed regions.At present,the patients with CRC are often not treated until late stage[2].Therefore,in most cases,the mean 5-year survival rate is relatively low.
All over the world,the incidence of CRC is lower in women than men.This epidemiologic observation can be explained by differences in the colorectal cancer-related risk factors between men and women such as obesity,smoking,dietary exposures,and physical activity.However,the gender differences in different racial groups across the world,suggest that sex hormones play a critical role in the pathogenic pathways of CRC[3-4].In addition,studies from relationship between gender and mortality of colorectal cancer consistently show lower mortality for women,especially for premenopausal women.So the the above results hint thatestrogen is most likely to play protective roles in tumorigenesis of CRC.However the pathogenesis is uncertain.So we summarize the role of estrogen and estrogen receptors and how their signaling pathways play a critical role in colorectal cancer development.
1.Epidemiological study of estrogen and colorectal cancer
In 1932,Lacassagne’s research demonstrated that estrogen administration to mice increased the incidence of mammary cancer.Since then,more and more studies have demonstrated sex hormones to be involved in the risk and development of many malignant tumor.The initial study on the relationship between estrogen with colorectal cancer began in 1969.Fraumeni and colleagues,studying a group of nuns,noticed that in addition to an elevated breast cancer risk these women were also more likely to develop colon cancer[5].Nuns are an unusual group compared to the majority of the female population as they are nullipara ;their menstrual cycles not disrupted,equatating to them having a longer lifetime estrogen exposure.These ideas were challenged by McMichael and Potter in 1980.Their investigations confirmed that long-term hormonal changes associated with pregnancy would result in a lower CRC risk[6].At that time,the relationship between estrogen and colorectal became a outstanding problem.In the next 10 years,lots of research were taken.One of most famous research results from the Women’s Health Initiative(WHI),this randomized control trial reported a 40 % reduced risk of CRC among women using estrogen plus progestin formulations compared to the placebo group[7-8].Further analysis of the WHI data suggested a possible association between an increase in CRC risk and endogenous circulating estrogen concentrations in postmenopausal women.In agreement with this finding,a study by Cleveland et al.showed that disruption of estrogen signaling increased intestinal neoplasia in Apc(Min/C)mice[9].The association between HRT and a decrease in CRC risk seems to have been conclusively proven by a recent comprehensive meta-analysis covering 2661articles,four randomised controlled trials,eight cohort and eight case-control studies indicating that the previous and current use of oestrogen and progestin or the current use of exogenous oestrogen is significantly linked to a decrease in CRC risk[10].
2.Estrogen receptors in CRC
Estrogens are members of the steroid hormone family and are traditionally associated with female reproductive development.The most abundant and most potent estrogen in humans is 17-estradiol(E2),which is synthesized in granulose cells of the ovary,Leydig cells of the testis[11],adiposetissue[12],and brain[13].Studies have shown that E2can bind to the estrogen receptor,and its binding affinity is higher than other estrogens[14].Other natural estrogens include estrone(E1)and estriol(E3),metabolites of E2.Estrogenis famous for its role in growth and differentiation of the breast,ovary and uterus in females,and testes and prostate in males.As the estrogen study carried out widely,studies demonstrated that estrogen is also required for proper functioning of the cardiovascular,immune,gastrointestinal,musculoskeletal,and nervous systems as well as skin.Although the colon might not be considered one of “conventional” E2 target tissue,this pleiotropic hormone exerts various actions on the organs which assemble gastrointestinal apparatus.The impact of E2 on colon physiology became evident when considering that several gastrointestinal disorders show considerable gender-specific incidence[15].
The classical actions of estrogen are mainly mediated via the two nuclear estrogen receptors(ERs),ERand ER.They are members of the nuclear receptor superfamily which are defined as ligand-activated transcriptional factors.ERα and ERβ are localized in the cytoplasm and in the nucleus of E2-target cells.Estrogens regulate these cellular effects through their intracellular receptors.ERand ERare transcribed from two genes(ESR1 andESR2 respectively)that are located on different chromosomes[16].About more than 50 years ago,ERwas discovered by Elwood Jensen and the gene was cloned in 1985[17],ERwas discovered in 1996 by cross-hybridization of a consensus DNA-binding domain(DBD)probe in a rat prostate cDNA library[18].ESR1 and ESR2 producing different receptor isoforms from alternative splicing result in three ERand five ERvariants.At present,research about ER variants have been taken.Studies manifested that ERprotein composed of 595 amino acids,and the amino acid-long of ERis 530.ERand ERare architecturally similar with six regions(A-F)in the primary amino acid sequence.Overall there are three functional domains: N-terminal domain(NTD),DNA binding domain(DBD),and ligandbinding domain(LBD).The two ERs share -97% similarity in their DBD and 59% in LBD,whereas the NTD is merely 16% similar(Fig.1)[19].The differences in LBD influence the shape of the ligand-binding pocket and that lead to change the binding affinities for the each receptor.ERbinds better than ERto estrogen,which has been regards as a health-promoting and anti-cancer agent.
Some studies demonstrated thatERis minimally expressed in normal colon mucosa and colon cancer cells[20],Similar expression levels of ERare observed between normal and cancerous colon tissue,implying ERhas limited role in CRC.ERmRNA was also detected throughout the human gastrointestinal tract,including the colon,the effect of estrogen on colon cancer susceptibility could be mediated by ER.Acconcia F et al[21]showed that ERβ is the predominant ER subtype in the human colon,In accordance,other authors[22]showed that ERβ expression was significantly lower in colon cancer cells than in normal colonic epithelium,and that there was a progressive decline in ERβ expression.In Adenomatosis polysis coli mice model,E2-induced prevention of APC associated tumor formation was correlated with an increase in ERβ protein[23].Altogether,these results strongly suggest that the presence of ERβ could justify the E2 effects against colon carcinogenesis.Study on ERs has been carried out many years,the viewpoint of different institution are difference(Tab.1)[1]The majority of studies indicate that E2acts via ERexpressed within CRC cells to induce cell cycle arrest and apotosis.
3.Estrogen pathway in CRC
The protective role of the estrogen pathway in development of colon cancer has been studied and established in animal models.Fiorelli andhis colleagues determined that E2 treatment inhibited HCT-116,DLD-1,and LoVo proliferation in vitro[24]which is mediated by ERβ.The resultswere later supported by other groups[25].Proposals have been made suggesting E2 signalling is dominantly controlled by ERβ[26].However,the downstream pathway of ERβ is still obscure.
Apoptotic induction by ERβ via DNA fragmentation in COLO205 colon cancer cells has been described[27].Other work has noted that ERβ induces apoptosis in LoVo cells due to increased p53 signalling,up-regulation of caspase 8 and 9,and a reduction in β-catenin.Trinei M and colleagues reported that p66Shc acts as a downstream target of the p53 and is indispensable for the ability of stress-activated p53 to induce elevation of intracellular oxidants,cytochromecrelease and apoptosis[28].It is well-known that reactive oxygen species(ROS)are produced by different intracellular redox reactions,which involve molecular oxygen as the final electron acceptor,and are the most recognized mediators of cell damage.Recently,Galimov ER studied effects of p66Shc on oxidative stress induced by hydrogen peroxide or by serum deprivation in human carcinoma cell line RKO.Results indicate that p66shc participates in mitochondrial ROS production in human colon cancer cells[29].But the mechanisms are poorly understood.
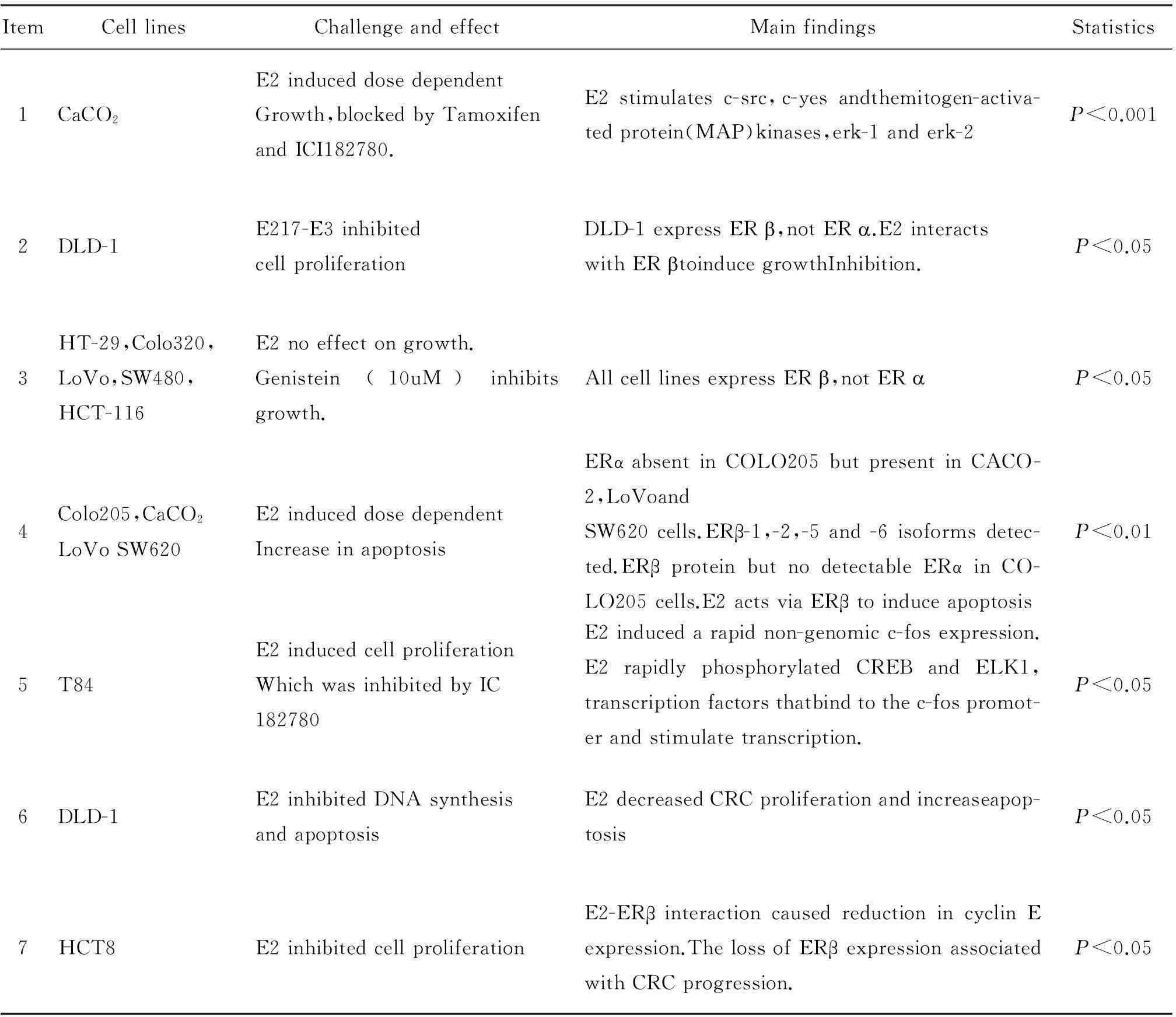
Table 1 Genomic effects of estrogen in a range of CRC cell lines
In addition,estrogen and its receptors can activate several signaling pathways without direct interaction with DNA,including protein kinase C(PKC),intracellular Ca2+,cytosolic cAMP,nitric oxide,and MAPK[30].Edvardsson and his colleagues have shown that transfection of colon cancer cell lines with ERaffects the MAPK signaling pathway.Furthermore,epidermal growth factor receptor(EGFR)and insulin-like growth factor receptor(IGFR)can activate the serine kinases,ERK and Akt,which in turn catalyze the phosphorylation and activation of ERs.The fields of estrogen pathway are still uncertain,it has the potential to be targeted for preventive and therapeutic strategies in CRC.
4.Conclusions
Epidemiological evidence has implicated estrogens as protective against CRC since late 1960s.This results encouraged further research into the estrogenic nature of this disease.Cellular and animal models clearly indicate genomic actions mediated by ER-estrogen binding.Meanwhile,studies demonstrate that ERplay a critical role on the development and progression.This review highlights that estrogen is an inverse risk factor for CRC,and refer to the ERβ implicated in inducing apoptosis and inhibiting cell cycle protein transcription.The downstream pathways of ERβ-E2 need to be expanded.Besides,intracellular ROS induced the apoptosis of colon cancer line.But the mechanism of the ROS on CRC are needed to identify.Future therapies should look to manipulate these pathways in order to identify new CRC treatments.More focused studies on estrogen mechanisms in human cells would provide valuable clues for treatment and prevention of accelerated aging.
REFERENCES
[1]Foster PA.Oestrogen and colorectal cancer:mechanisms and controversies.Int J Colorectal Dis,2013,28(6):737-749.
[2]Brenner H,Bouvier AM,Foschi R,et al.Progress of colorectal cancer survival in Europe,from the late 1980s to the early21st century: the EUROCARE study.IntJ Cancer,2012;131(7):1649-1658.
[13]Hendifar A,Yang D,Lenz F,et al.Gender disparities in metastatic colorectal cancer survival.Clin Cancer Res,2009,15(20):6391 -6397.
[4]Fernandez E,Bosetti C,La Vecchia C,et al.Sexdifferences in colorectal cancer mortality in Europe,1955-1996.Eur J Cancer Prev,2000,9(2):99-104.
[5]Fraumeni JF Jr,Lloyd JW,Smith EM,et al.Cancer mortality among nuns:role of marital status in aetiology of neoplastic disease in women.J Natl Cancer Inst,1969;42(3):455-468.
[6]Delellis HK,Duan L,Sullivan-Halley J,et al.Menopausal hormone therapy use and risk of invasive colon cancer.Am J Epidemiol,2010,171(4):415-425.
[7]Ritenbaugh C,Stanford JL,Wu L,et al.Conjugated equine estrogens and colorectal cancer incidence and survival:the Women’s Health Initiative randomized clinical trial.Cancer Epidemiol Biomarkers Prev,2008,17(10):2609-2618.
[8]Rossouw JE,Anderson GL,Prentice RL,et al.Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial.J Am MedAssocia,2002,288(3):321-333.
[9]Cleveland AG,Oikarinen SI,Bynote KK,et al.Disruption of estrogen receptor signaling enhances intestinal neoplasiain Apc(Min/+)mice.Carcinogenesis,2009,30(9):1581-1590.
[10]Lin JK,Cheung WY,Lai JY-C,et al.The effect of estrogen vs.combined estrogen progestogen therapy on the risk of colorectal cancer.Int J Cancer,2012,130(2):419-430.
[11]Stocco C.Tissue physiology and pathology of aromatase.Steroids,2012;77(1):27-35.
[12]Rice S,Patel B,Bano G,et al.Aromatase expression in abdominal omental/visceral and subcutaneous fatdepots:a comparison of pregnant and obese women.Fertility and Sterility,2012,97(6):1460-1466.
[13]Boon WC,Chow JD,Simpson ER.The multiple roles of estrogens and the enzyme aromatase.Prog Brain Res,2010,181: 209-232.
[14]Dey P,Barros RP,Warner M,et al.Insight into the mechanisms of action of estrogen receptorin the breast,prostate,colon,and CNS.J Mol Endocrine,2013,51(3):61-74.
[15]Bòttner M,Thelen P,Jarry H.Estrogen receptor beta:tissue distribution and the still largely enigmatic physiological function.J Steroid Biochem Mol Biol,2014,139:245-251.
[16]Deroo BJ,Buensuceso AV.Minireview:estrogen receptor-beta:mechanistic insights from recent studies.Mol Endocrinol,2010,24(9):1703-1714.
[17]Walter P,Green S,Greene G,et al.Cloning of the human estrogen receptor cDNA.PNAS,1985,82(23):7889-7893.
[18]Kuiper GG,Carlsson B,Grandien K,et al.Comparison of the ligand binding specificity and transcripttissue distribution of estrogen receptorsand.Endocrinology,1997,138(3): 863-870.
[19]Pettersson K,Gustafsson JA.Role of estrogen receptorb in estrogenaction.Annual Review Physiol,2001,63(3):165-192.
[20]Campbell-Thompson M,Lynch IJ,Bhardwaj B.Expression of estrogen receptor(ER)subtypes and ERbeta isoforms in colon cancer.Cancer Res,2001,61(2): 632-640.
[21]Acconcia F,Marino M.The effects of 17β -estradiol in cancer are mediated by estrogen receptor signaling at the plasma membrane.Frontiers in Physiology,2011,2(30):301-308.
[23]Marino M,Pellegrini M,Acconcia F,et al.Susceptibility of estrogen receptor rapid responses to xenoestrogens:Physiological outcomes.Steroids,2012,77(10):910-917.
[24]Fiorelli G,Picariello L,Martineti V,et al.Estrogen synthesis in human colon cancer epithelial cells.J Steroid Biochem Mol Biol,1999,71(5-6):223-230.
[25]Maria Marino.Xenoestrogens challenge 17β-estradiol protective effects incolon cancer.World J Gastrointest Oncol,2014,6(3):67-73.
[26]Koehler KF,Helquero LA,Haldosén LA,et al.Reflections on the discovery and significance of estrogen receptor beta.Endocr Rev,2005,26(3):465-478.
[27]Qui Y,Waters CE,Lewis AE,et al.Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta.J Endocrinol,2002,174(3):369-377.
[28]Trinei M,Giorgio M,Cicalese A,et al.A p53-p66Shc signalling pathway controls intracellular redox status,levels of oxidation-damaged DNA and oxidative stress-induced apoptosis.Oncogene,2002,21(24):3872-3878.
[29]Galimov ER,Chernyak BV,Sidorenko AS,et al.Prooxodant properties of p66Shc are mediated by mitocho- ndria in human cells.PLos one,2014,9(3):1-10.
[30]Barzi A,Lenz AM,Labonte MJ,et al.Molecular Pathways:Estrogen Pathway in Colorectal Cancer.Clin Cancer Res,2013,19(21):5842-5848.
(本文编辑:马天翼)
张玲,王吉凌,朱圣韬,等.The role of estrogen in the development of colorectal cancer [J/CD].中华结直肠疾病电子杂志,2015,4(2):173-176.
(收稿日期:2015-03-07)
通信作者:史学森,Email:1962shixuesen@163.com
基金项目:国家自然科学基金资助项目(81372155)
DOI:10.3877/cma.j.issn.2095-3224.2015.02.14

