非典型心房扑动治疗的实用方法
Jeffrey Munro Win-Kuang Shen 刘晓宇 译 王如兴审校
非典型心房扑动治疗的实用方法
Jeffrey Munro Win-Kuang Shen 刘晓宇 译 王如兴审校
心房扑动是一类具有不同表现的折返性房性心律失常,包括典型心房扑动和非典型心房扑动,其中典型心房扑动是以下腔静脉-三尖瓣峡部为关键传导区域形成的折返性心律失常,而非典型心房扑动的折返环多存在于左心房或右心房且与瘢痕和慢传导区域有关。近年来,随着心脏外科手术和房颤导管消融手术例数的增加,非典型心房扑动的发病率也逐渐升高。典型心房扑动的消融通常是沿下腔静脉-三尖瓣峡部进行线性消融,而非典型心房扑动消融通常需要多种技术联合应用以明确关键传导区域的位置,这些技术包括采用三维标测系统行电解剖电压标测和激动标测及采用拖带技术确定非典型心房扑动发生的关键传导区域。本文以一例既往行房颤导管消融术后发生非典型心房扑动的患者为例,介绍我们是如何确定非典型心房扑动折返环的组成,尤其是如何发现非典型心房扑动发生的关键传导区域并予以消融成功。
非典型心房扑动;电解剖电压标测;激动标测;拖带;折返性心律失常;心房颤动消融
1 引言
心房扑动(房扑),是一种具有折返机制的心律失常,通常分为两种类型:典型房扑和非典型房扑(表1)。典型房扑,也称为下腔静脉-三尖瓣峡部(cavo-tricuspid isthmus,CTI)依赖型房扑,整个心动过速折返环在右心房内,CTI是折返环路中必要组成部分。从心尖角度来看,典型房扑是沿三尖瓣环形成的折返,且多呈逆时针方向而顺时针方向少见。在折返环路较低的情况下,折返环仍以CTI作为传导的关键区,激动围绕下腔静脉传导并通过界脊中间隙,而不是围绕整个三尖瓣环进行折返。沿房间隔和右心房侧壁传导的上行扑动波,与下位折返环心房扑动在高位右心房发生碰撞。目前已经在解剖学上明确了典型房扑的环路,且绝大多数典型房扑可沿 CTI予以成功地线性消融[1-4]。所谓“非峡部依赖”的房扑出现较少,其可以在心房内任何部位形成折返。尽管非典型房扑比典型房扑少见,但随着先天性心脏病、冠心病和瓣膜性心脏病患者心房切开术例数,以及心房颤动导管消融术造成心房瘢痕的患者数的增加,非典型房扑的发生率正不断提高。相比于典型房扑的标准治疗方法(即沿CTI行线性消融)较高的成功率,非典型房扑消融治疗仍然具有挑战性,主要原因是非典型房扑折返环的确定需要进行复杂的标测和电生理拖带操作,故其消融成功率通常低于典型房扑[5-7]。

表1 心房扑动的分类:根据是否下腔静脉-三尖瓣峡部依赖主要分为典型房扑和非典型房扑两类
典型心房扑动体表心电图的特点是出现没有等电位线节段的持续不断的锯齿样扑动波,且其在心电图下壁导联Ⅱ、Ⅲ、aVF呈显著的负向偏移;而逆钟向心房扑动心电图表现为扑动波在心前区导联V1呈正向偏移,顺钟向(“逆向的”)心房扑动心电图则表现为扑动波在下壁导联呈正向偏移[图1A:典型的沿三尖瓣环逆时针方向折返的心房扑动,表现为下壁导联(Ⅱ、Ⅲ、aVF)扑动波倒置;图1B:典型的沿三尖瓣环顺时针方向折返的心房扑动,表现为下壁导联扑动波直立]。在体表心电图上,非典型房扑的扑动波形态极其多变,对预测折返环的定位作用有限,而在室性心动过速心电图中,则可根据心电图形态大致定位室性心动过速的出口。此外,利用体表心电图对有巨大折返环的非典型心房扑动与局灶性房性心动过速进行辨别,临床价值不是很大,原因在于这两类扑动波都可能存在较明显的等电位线[图2:12导联体表心电图提示为非典型房扑伴2︰1房室传导(A);瘢痕相关性右心房非典型房扑(B);房颤消融患者行肺静脉隔离和线性消融后在左心室形成的非典型房扑折返环(C)。围绕二尖瓣环周围的心房肌和左下肺静脉附近的二尖瓣峡部是二尖瓣峡部依赖性房扑折返环的重要组成部分。沿房顶部线性消融所形成的间隙可能也是非典型房扑重要的折返传导区域]。尽管借助体表心电图来定位非典型房扑折返环存在不足,但目前已发现了一些利用体表心电图定位非典型房扑的基本方法,然而精确定位仍需要采用更先进的技术[8]。
在本文中,我们以一例非典型房扑患者的导管消融为例,介绍用于确定不典型房扑消融关键峡部的多种现代标测技术,包括电压标测、激动标测和拖带标测。最后我们结合文献总结了目前对于非典型房扑的病因、标测和消融的认识。
2 一例非典型房扑
患者男,52岁,既往有原发性高血压、采用持续正压通气(continuous positive airway pressure,CPAP)治疗阻塞性睡眠呼吸暂停综合征和约5年的持续性心房颤动病史。2012年年初患者在外院行微创迷宫手术治疗,包括肺静脉电隔离和心外膜神经节丛消融并恢复正常窦性心律,同时使用40 mm封闭器行左心耳封堵。消融术后不久,患者心房颤动复发并出现非典型房扑,先予以药物治疗6个月;2012年6月,在其他医院接受了第二次消融治疗,包括冷冻消融和左心房线性消融,但术后不久,房性心律失常再次复发。在以后的两年中,患者持续应用氟卡尼、地尔硫卓及一次同步电复律以维持窦性心律,但患者仍反复发作有症状的、持续性非典型房扑,伴有心悸、运动量下降和呼吸困难。
患者于2014年6月来我院住院,我们对患者的房性快速性心律失常进行了评估和并决定采取相应的治疗方案。由于当时患者仍存在运动量下降的表现,故可供选择的治疗方案包括:继续控制患者心室律、再次电复律或心内膜导管射频消融。由于患者仍存在明显症状及既往电复律无效。最终,由于出现活动受限症状及之前尝试电复律失败,患者选择再次进行导管射频消融。
术前心电图(图2)为具有等电位线及2︰1房室传导的房性快速性心律失常。对具有不连续P波且伴有等电位线的房性心动过速心电图的鉴别诊断,应考虑局灶机制或折返所致的非典型房扑。该患者心动过速周长为240 ms且恒定。在患者冠状静脉窦放置二十极冠状窦导管(Orbiter,Bard),发现心房最早激动点靠近冠状窦导管近端,并向冠状窦导管远端传导。电生理检查第一步通常是从右房到左房进行多部位拖带标测(表2)。电生理诊断起搏导管分别置于高位右心房(图3:在高位右心房对一例非典型房扑进行显性拖带,其中,ABL:消融导管;CS:冠状窦导管)、右心房房间隔上部、右心房侧壁和三尖瓣峡部。在所有部位成功进行显性拖带,结果发现起搏后间期与心动过速周长之差(PPI-TCL)均>20 ms,且右心房侧壁的PPI-TCL最长,而越接近房间隔,PPITCL逐渐变短,提示右心房不在折返环所在的区域。进行房间隔穿刺前,在冠状窦口进行拖带标测,结果显示隐匿拖带伴有长PPI-TCL,提示此处是接近关键峡部的慢传导区,但不是折返环的关键传导区。在冠状静脉窦中部进行拖带标测时,PPI-TCL较短,刺激波与扑动波之间的间期为零,且和腔内心电图(EGM)显示刺激到扑动波的间期相同,提示拖带处为出口且折返环包含左心房。

表2 本例患者非典型心房扑动不同部位拖带标测结果
通过两次房间隔穿刺,分别将消融导管和二十极Lasso导管送入左心房。Lasso导管分别送入至四根肺静脉,证实肺静脉完全隔离。接着,利用消融导管在左心房不同部位进行拖带标测,其中左心房后壁为显性拖带,而左心耳残端,二尖瓣瓣环10点、12点位置及左心房房间隔下部均表现为隐匿性拖带,PPI-TCL较短并逐渐缩短至接近体表扑动波周期,所有腔内心电图显示的扑动波周期均与体表心电图扑动波周期一致。在二尖瓣环11点位置,我们发现一个低振幅的碎裂电位,且符合瘢痕处慢传导区和电位传导间隙的表现(图4:沿二尖瓣环11点钟位置标测到碎裂低振幅双极电位,提示为慢传导区,予以成功消融)。
在二尖瓣环10点位置的腔内局部电位距离体表心电图扑动波为84 ms,此时心动过速周长为241 ms,因此占心动过速周长的35%(图5:沿二尖瓣环10点位置的隐匿性拖带,其中,ABL:消融导管;CS:冠状窦电极)。二尖瓣环12点位置和左心房间隔下部腔内局部电位到体表扑动波的距离分别为159 ms和54 ms,分别占心动过速周长的66%和22%。
应用CARTO三维标测系统(Biosense Webster)进行激动标测和电压标测[图6:左房激动标测(A)和左房电压标测(B);从二尖瓣环11点位置至右上肺静脉进行线性消融,在此过程中房扑发作终止;然后再沿慢传导区存在潜在间隙的低电压区,包括房顶线、左上肺静脉至二尖瓣环线和右下肺静脉至二尖瓣环线进行补充线性消融]。激动标测图表明激动传导是占心动过速周期大部分且围绕二尖瓣环的大折返环,而不是局部最早激动并向外围扩散的局灶性房性心动过速。电压标测图表明;环绕肺静脉开口处存在许多低电压区域;除了左心房顶部以外,从肺静脉到二尖瓣环11点和3点位置之间存在线性低电压区。
二尖瓣峡部依赖的非典型房扑的诊断是在左心耳基底进行隐匿性拖带标测,刺激波到体表心房扑动波之间的长度占心动过速周长的66%,则为折返形成的关键峡部。消融应用3.5 mm灌注消融导管(SmartTouch,Biosense Webster),输出功率35 W,温度上限45℃。消融策略为沿二尖瓣游离壁峡部进行线性消融,消融损伤从二尖瓣环至左上肺静脉,与前次环肺静脉消融瘢痕处相连(图6A)。第一次线性消融结束后,心动过速周长从240 ms延长到254 ms。第二次线性消融是由二尖瓣环11点前方开始向右上肺静脉进行,消融过程中心动过速周长延长至320 ms并最终停止(图7:从右上肺静脉至二尖瓣环线性消融中,不典型房扑开始减慢和终止图形,房扑终止并恢复窦性心律)。接着,继续延长第二消融线,消融方向是从左心房房顶到左心耳基底部(图6B)。消融结束后,进行心房程序刺激,给予多达三次期前刺激也无法诱发任何房性快速性心律失常。
激动标测图表明本例患者心动过速似乎是围绕二尖瓣环折返并为二尖瓣峡部依赖性。第一次行二尖瓣峡部线性消融后,患者心动过速周长延长,考虑可能的原因是折返环内环被阻滞,但没有影响关键传导区,折返激动顺序改变,折返可能沿左心房后壁进行,或形成“8”字形的复合折返。最终,在二尖瓣环11点处消融,终止了心动过速的发作,此处有先前电压标测时发现的碎裂低电压电位,表明对关键峡部进行了消融,从而不能再次诱发非典型房扑。
3 非典型心房扑动的文献回顾
3.1 病因和发病率
非典型房扑,有时也被称为非局灶性房性心动过速,具有折返机制,通常见于有心房瘢痕形成病史的患者,这些瘢痕往往是由心脏外科手术,以及外科或导管消融治疗心房颤动所致(图2B和图2C)。有报道称,心房颤动射频消融术后的左房扑动患者,77%有器质性心脏病,23%无明显器质性心脏病[9]。在最近一个多中心回顾性研究分析中,Coffey等[5]报道在91例非典型心房扑动患者中,65%的患者曾接受心房导管消融治疗;27%的患者既往有心脏手术治疗史,其中12%的患者接受迷宫手术,8%的患者存在与非典型房扑相关的特发性瘢痕,提示这8%的患者可能有心房心肌病。
随着先天性心脏病外科手术例数的增多和心房颤动射频消融术的日益普及,非典型房扑的发生率可能会相应升高。在一项前瞻性研究中,67例患者接受了心房颤动射频消融术,手术方式为环肺静脉隔离和线性消融,其中,43%的患者术后有房性心动过速发生,其中69%是以折返为机制的非典型房扑;在消融后的第3、第6和第12个月,应用连续7 d Holter进行监测,发现31%的患者存在持续性房性快速性心律失常[6]。尽管包括非典型房扑在内的房性心动过速发生率较高,但经过心房颤动导管消融治疗后,相当比例的房性心动过速表现出自限性。Chugh等[7]报道,在接受心房颤动射频消融的349例患者中,有24%在约1年的随访期间发生以大折返为机制的房性心动过速,其中33%的房性心动过速有自愈性且通常发生在最初消融术后的5个月内,因此他们建议术后短期内对这类患者予以密切观察。
3.2 标测
应用于非典型心房扑动的主要标测方法有拖带标测、电压标测和激动标测。使用拖带标测技术的目的是寻找隐匿性拖带位点,并确认非典型房扑折返环的关键峡部。通过构建右心房和/或左心房电压标测图,有助于确定低电压区,这些区域往往代表作为折返环边界的瘢痕区域。激动标测则可以将三维标测系统中折返性心动过速的大部分周长与心房解剖结构关联起来。另外,双电位或高度碎裂的局部电位所在区域,可能分别代表阻滞线、阻滞区或各向异性传导。每种标测技术均有自身的局限性,因此把采用这些技术所得到的信息整合起来,对成功标测和消融非常重要。
3.2.1 拖带标测 拖带标测已成为用于明确折返环存在的重要手段,研究证明其有助于确定阵发性室上性心动过速和非典型房扑折返环的构成,并用于室性心动过速的标测。拖带标测的基本操作方法是,当心动过速发作时从感兴趣位点以短于心动过速周长10~30 ms的间期进行心房超速起搏,确认拖带后停止起搏,心动过速仍继续存在。Waldo等[10]提出了拖带标测的4条准则,并报道了拖带方法在典型心房扑动标测中的应用。
在心房的某个区域对非典型房扑进行拖带,起搏后周期与心动过速周长之差(PPI-TCL)<20 ms,则认为该拖带区域在折返环路上。此外,当出现隐匿性拖带时,即体表心电图自身扑动波和腔内心电图心房波激动顺序与拖带后的扑动波相同,则认为起搏导管顶端位于关键性慢传导区域内。起搏标测部位(PPI-TCL)<20 ms,呈隐匿性拖带且刺激波到体表扑动波的间期逐渐缩短,表明起搏标测点逐渐靠近折返环的出口。
拖带标测对非典型房扑折返环的定位提供了一个总体思路,但应该与其他标测方法联合使用。Morton等[11]对这些标测方法应用于典型房扑消融的敏感性和特异性进行了研究。以比扑动波周期短10 ms的起搏间期进行隐匿性拖带标测,对关键性峡部判断的敏感性和特异性分别为100%和54%;如果逐渐缩短起搏间期,敏感性下降、特异性提高[11]。Triedman 等[12]将舒张期局部低电压碎裂电位鉴别技术和拖带标测技术应用于先天性心脏病术后发生房性折返性心动过速的射频消融治疗,急性期成功率为73%;随访平均4个月,复发率为53%。虽然这些研究没有针对非典型心房扑动进行研究,但他们指出了拖带标测在准确预测峡部位置和充分定位折返环构成方面还存在问题,而心动过速终止和心动过速周期变化也限制了拖带标测应用于存在多个折返环和弥散传导机制的患者。
3.2.2 电解剖(电压和激动)标测 非典型房扑患者的心房中通常存在瘢痕组织,这些组织几乎没有任何电活动,表现为低电压区及局部电信号消失。在心房内电压标测中,通常建议将瘢痕组织或无电活动区域定义为一些双极电压振幅<0.05 mV,且输出电流为20 mA时无法夺获心房组织的点[9]。瘢痕和低电压区可以在三维标测系统的心房解剖结构模型中标测出来,这对阻滞区域边界和可能的慢传导区域的判断提供了指导,而这些区域可能就是非典型房扑发生的关键性峡部。在局部阻滞区域经常可以发现双电位,这对寻找线性消融的消融线之间的间隙提供了进一步指导。此外,复杂的碎裂电位往往提示慢传导区,在标测中可以将其作为关键性峡部的候选区域予以标记。
激动标测应用三维标测系统,在心动过速发作时显示相对于参考电极上某一点的局部电位激动时间,参考电极通常选择冠状窦电极。局部电位激动时间与参考电极之间的时间差被指定为颜色光谱中的某种颜色,红色表示最早激动时间而紫色代表最晚激动时间,这样可鉴别占心动过速周期至少90%的激动时间间隔。在精确激动标测过程中,可能的关键峡部可通过慢传导区加以鉴别,在激动标测图上表现为在一定的区域内颜色的快速变化。在激动标测过程中,一项有用的发现是当最早的局部激动固定在一个较小的区域内,则提示该心律失常的发生机制可能是局灶或局部折返所致,且最早激动点即为消融靶点。多极导管和高密度标测的发展为快速标测非持续性房性心动过速提供了有效方法。
Nakagawa等[13]报道了利用电压和激动标测技术对13例有先天性心脏病修补术病史的患者行射频消融,在这些患者中共发现15个持续性的大折返性右心房房性心动过速。应用电解剖标测,他们确定了瘢痕区以及折返通路,并以这些通路作为消融靶点进行治疗,最终15个心动过速均成功终止,所有患者在射频消融术后均不能诱发持续性房性心动过速。应用电解剖标测系统对心房颤动消融术后的非典型房扑做进一步研究,所得结果与上述类似[5-7,14-15]。
3.3 折返环特点
非典型房扑折返环可根据许多变量进行分类,这些变量包括折返环的位置、大小、形态以及与之相关的既往线性消融产生的间隙。与既往消融相关的非典型房扑通常可根据折返环大小进一步分类:大折返环定义为折返环>2~3 cm或涉及两个或更多的心房节段,并且利用激动标测可对占心动过速周期至少90%的折返环进行定位识别;而小折返环定义为折返环<2~3 cm或折返位于术者定义的单一心房节段。实际上,局灶性房性心动过速在激动标测中表现为有一个最早激动点,且激动以该点为中心以离心性扩散的方式传导[6,9,16]。
心房扑动折返环的解剖定位几乎包括左房或右房中的任何结构。在有心房颤动消融病史的患者中,以大折返为机制的非典型房扑,其关键峡部在二尖瓣峡部的占25% ~42%,在左心房顶部的占13%~20%,在间隔的占13%;而微折返或局灶机制的房性心动过速常见于肺静脉前庭、左心房前后壁、左心耳基底部、左心房间隔和冠状窦[14-15]。
常见的折返环形态是单环折返和多环折返以及微折返。Linton等[17]采用在两个不同位置依次进行超速起搏,通过测量这两个位置的起搏后间期,鉴别折返环是单一环路还是双重环路。在双环路折返的情形下,首先在具有最短起搏后间期(拖带过程中几乎和心动过速周期相等)的峡部进行消融;如果心动过速没有终止,则在下一个最短起搏后间期部位消融。
折返环穿过之前消融线的房性心动过速称为间隙相关性房性心动过速,据文献[7]报道其占折返性房性心动过速的96%以上。这充分说明在线性消融过程中,对消融线完全阻滞可潜在避免这些心律失常的发生。当然,虽然线性消融损伤能提高持续性心房颤动患者的消融成功率,但随之带来的是术后随访期间房性心动过速发生率升高[18]。
3.4 消融终点和成功率
总体说来,不典型房扑导管消融急性期成功率较高,但长期随访成功率稍差。消融靶点在房间隔区域时,急性和长期成功率明显偏低。研究报道消融成功率还与所使用的导管不同有关。房扑折返环的房间隔部位之所以难以消融,是因为该区域解剖结构关系复杂且可能难以输送适当的射频能量。Coffey等[5]进行回顾性病例分析,射频消融术后发生房性心律失常的患者进行(16±12)个月的随访,急性期成功率97%、长期成功率77%,而非间隔部和间隔部的成功率分别为82%和67%。同时,他们还发现,长期成功率的不同与瘢痕可能的成因有关:消融治疗外科手术形成的瘢痕相关性房性心动过速,长期成功率为88%;导管消融术形成的瘢痕为75%;特发性心房瘢痕为57%。其他研究表明,这类房性心动过速导管消融的急性和长期成功率均较低,原因可能是这些研究通常使用消融导管进行激动标测而非多电极导管标测,由此可能造成标测时所取点的密度较低[7,14-15]。
消融导管类型的选择也可能影响非典型房扑射频消融的急性和长期成功率。一项随机研究表明,应用3.5 mm冷盐水灌注导管和8 mm非灌注导管对非典型房扑进行消融,急性期成功率分别为94%和77%;随访10个月,采用48 h动态心电图在3、6和9个月进行检查,发现长期成功率分别为92%和59%,所以盐水灌注消融导管成功率更高[19]。
4 结论
随着接受心房颤动射频消融的患者以及在心脏手术中切开心房的患者尤其是先天性心脏病患者越来越多,非典型房扑正在成为一种越来越常见的心律失常。综合运用包括拖带标测和高级标测在内的系统性方法,以及随着人们对电解剖基质认识的提高,射频消融术治疗上述颇具临床挑战性的心律失常的成功率可达90%。影响消融结果的因素较多,包括患者的手术和消融病史、折返环的位置和特点以及标测技术和消融导管。尽管目前对于非典型房扑消融还无具体研究报道,但消融成功率和并发症的发生率,通常与术者的临床经验及各中心开展这种复杂性心律失常的手术数量有关。
(图和参考文献请查阅前面的英文原文)
【致谢】 衷心感谢南京医科大学附属无锡人民医院心内科的刘晓宇博士和王如兴教授在百忙中分别承担了本文的翻译和审校工作。
A practical approach to atypical atrial flutter
Jeffrey Munro Win-Kuang Shen
Atrial flutter is a heterogeneous group of re-entrant atrial arrhythmias including typical atrial flutter where the cavo-tricuspid isthmus is a critical zone of conduction and atypical atrial flutter in which the re-entrant circuit can exist in the right or left atrium associated with scar and a slow zone of conduction.The prevalence of atypical atrial flutter is increasing as the number of cardiac surgery and catheter based ablations of atrial fibrillation is more widespread.Typical atrial flutter ablation typically involves a linear ablation along the cavo-tricuspid isthmus as opposed to atypical atrial flutter usually requiring an integrated approach of electroanatomical voltage mapping and activation mapping in a computer based 3D mapping system as well as entrainment techniques for determining critical zones of conduction.In this article we present a case of atypical atrial flutter that we have employed these tools to determine the components of an atypical atrial flutter circuit,specifically the critical zone of conduction which was targeted with radiofrequency ablation for termination of atypical atrial flutter in a patient with a prior ablation for atrial fibrillation.
atypical atrial flutter;electroanatomical voltage mapping;activation mapping;entrainment;re-entrant arrhythmias;atrial fibrillation ablation
10.13308/j.issn.2095 -9354.2015.04.001
R541.75
A
2095-9354(2015)04-0229-16
10.13308/j.issn.2095 -9354.2015.04.001
【编者按】 近年来,随着心脏外科手术和房颤导管消融手术例数的增加,非典型心房扑动的发病率逐渐升高。本期特邀世界顶级医院美国梅奥医学中心(Mayo Clinic)心电生理专家Jeffrey Munro博士和Win-Kuang Shen(沈文光)教授结合临床病例,探讨在非典型心房扑动消融中如何联合运用电解剖电压标测、激动标测及拖带技术等多种技术,明确关键传导区域位置。“Brugada拟表型”是2012年由Adrian Baranchuk等首次提出的一个新术语,其有助于鉴别先天Brugada综合征,可防止学术上概念的混淆,且准确识别Brugada综合征与Brugada拟表型,有助于合理治疗,令更多的患者获益。本期奉上该术语的首次提出者,加拿大金斯顿综合医院的Adrian Baranchuk教授的最新综述,首次向国内读者全面介绍Brugada拟表型的相关前沿问题,以期推动我国心血管病研究领域对该问题的关注。
2015-05-12)
(本文编辑:顾艳)
海外论坛
1 Introduction
Atrial flutter,an arrhythmia with a reentrant mechanism,is often classified by subdividing it into two types:typical and atypical(Tab.1).Typical atrial flutter,otherwise known as cavo-tricuspid isthmus(CTI)dependent,with the entire tachycardia circuit within the right atrium uses the CTI as an obligatory component of the reentrant circuit.The wavefront in typical atrial flutter rotates around the tricuspid annulus in a counterclockwise or less commonly clockwise direction,from the perspective of the apex.In the case of lower loop
Author Unit:85054 Phoenix,Arizona,U.S.,Department of Cardiovascular Diseases,Division of Cardiac Electrophysiology,Mayo Clinic
Author Brief Introduction:Jeffrey Munro,D.O.,fellow of clinical cardiac electrophysiology;research interests:atrial fibrillation,atypical atrial flutter,ventricular tachycardiafibrillation,E-mail:Munro.Jeffrey@mayo.edureentry,the reentrant circuit again involves the CTI as the critical zone of conduction and the wavefront propagates around the inferior vena cava with conduction occurring through a gap in the crista terminalis rather than around the entire tricuspid annulus.The ascending wavefront along the septum and lateral right atrium collide in the high right atrium with lower loop reentry atrial flutter.The anatomic barriers of the circuit have been well defined and the site of successful ablation almost always involves a linear ablation along the CTI[1-4].Less commonly,atrial flutter can involve a circuit anywhere else within the atrium,the so called“non-isthmus dependent”atrial flutter.Although less common than typical atrial flutter,the atypical variant is increasing in prevalence with an increasing number of individuals with an atriotomy from surgery related to congenital heart disease,coronary disease and valvular heart disease as well as atrial scar related to atrial fibrillation ablation procedures.In contrast to the well-defined approach,with a high success rate,of linear ablation along the cavo-tricuspid isthmus for typical atrial flutter,ablation procedures targeting atypical atrial flutter remains a challenging procedure involving complex mapping and entrainment maneuvers to define components of the circuit at times with success rates generally less than those seen with typical atrial flutter[5-7].

Tab.1 A classification of atrial flutter:two main types of atrial flutter are typical and atypical atrial flutter,as determined by the role of the cavo-tricuspid isthmus
Typical atrial flutter is characterized on the surface electrocardiogram as having a continuously undulating flutter wave without an isoelectric segment with a predominantly negative deflection in the inferior ECG leads(Ⅱ,Ⅲ,aVF)and a positive deflection in precordial lead V1for the counterclockwise variant and positive deflection in the inferior leads in the case ofthe clockwise(“reverse”)variant(Fig.1A,Fig.1B).The flutter wave morphology on a surface ECG for atypical atrial flutter is highly variable and is limited in its role for prediction of the circuit location as opposed to ventricular tachycardia where ECG morphology is useful for a general localization of exit sites.Additionally,using the surface ECG to distinguish atypical atrial flutter with a macro-reentrant circuit from a focal atrial tachycardia is not particularly valuable as the flutter waves of either type may have significant isoelectric intervals(Fig.2).Despite the shortcomings of surface ECG for prediction of circuit location some general rules have been developed but precise determination requires more sophisticated techniques[8].
In this article we present a case of atypical atrial flutter that demonstrates many of the contemporary techniques for mapping including voltage,activation and entrainment to identify the critical isthmus for ablation.A literature review follows summarizing the current understanding of atypical atrial flutter,including etiology,mapping and ablation.
2 A case of atypical atrial flutter
Our case is of a 52 year old male with a history of essential hypertension,obstructive sleep apnea treated with continuous positive airway pressure(CPAP)therapy,and persistent atrial fibrillation for approximately 5 years.He had undergone a minimally invasive Maze procedure at an outside institution in early 2012 including pulmonary vein isolation,ganglionated plexus ablation epicardially with restoration of normal sinus rhythm and left atrial appendage closure with a 40 mm atrial clip.Shortly after the ablation procedure the patient had recurrence of atrial fibrillation and atypical atrial flutter and was treated medically for 6 months.He underwent a second ablation procedure at another outside institution in mid-2012 with cryoablation and a linear ablation within the left atrium,but again had recurrent atrial arrhythmias shortly after the procedure.Over the following 2 years,treatment with flecainide and diltiazem was continued for rhythm control in addition to a synchronized external cardioversion but the patient continued to have persistent symptomatic atypical atrial flutter with palpitations,reduced functional capacity and dyspnea.
He presented to us in mid-2014 for evaluation and management of his atrial tachyarrhythmias.At that time he was still experiencing reduced functional capacity and alternatives were discussed including a continued rate control approach,an additional attempt at cardioversion orendocardialradiofrequency energy catheter ablation.The patient opted for a repeat ablation because of the limiting symptoms and failed prior attempt at cardioversion.
The initial ECG(Fig.2)showed an atrial tachyarrhythmia with an isoelectric interval and 2︰1 AV conduction.The differential-diagnosis of discrete P waves with an isoelectric interval includes an atrial tachycardia with a focal mechanism or an atypical flutter with a reentrant mechanism.The cycle length of the tachycardia was 240 ms with no variability of the cycle length.A duodecapolar catheter(Orbiter,Bard)was placed in the coronary sinus and atrial activation was the earliest in the proximal poles with activation proceeding distally.The first maneuver in the electrophysiology lab was entrainment mapping from multiple locations throughout the right atrium and left atrium(Tab.2).The diagnostic pacing catheter was positioned at the high right atrium(Fig.3),superior right atrial septum,and lateral right atrium and cavo-tricuspid isthmus and entrainment maneuvers performed.All sites showed manifest entrainment with post pacing interval minus tachycardia cycle length interval(PPI-TCL)>20 ms with the greatest interval at the lateral right atrium and progressively shorter intervals moving closer to the septum suggesting that the right atrium was not part of the reentrant circuit.Prior to transeptal puncture,entrainment was performed at the coronary sinus ostium showing concealed entrainment but long PPI-TCL suggestive of a slow zone of conduction contiguous with the critical isthmus but not a zone of critical conduction of the reentry circuit.Entrainment at the mid coronary sinus had a short PPI-TCL with a stimulus to flutter wave interval that was zero,equaling the local electrogram(EGM)to flutter wave,consistent with an exit site and a circuit that involved the left atrium.
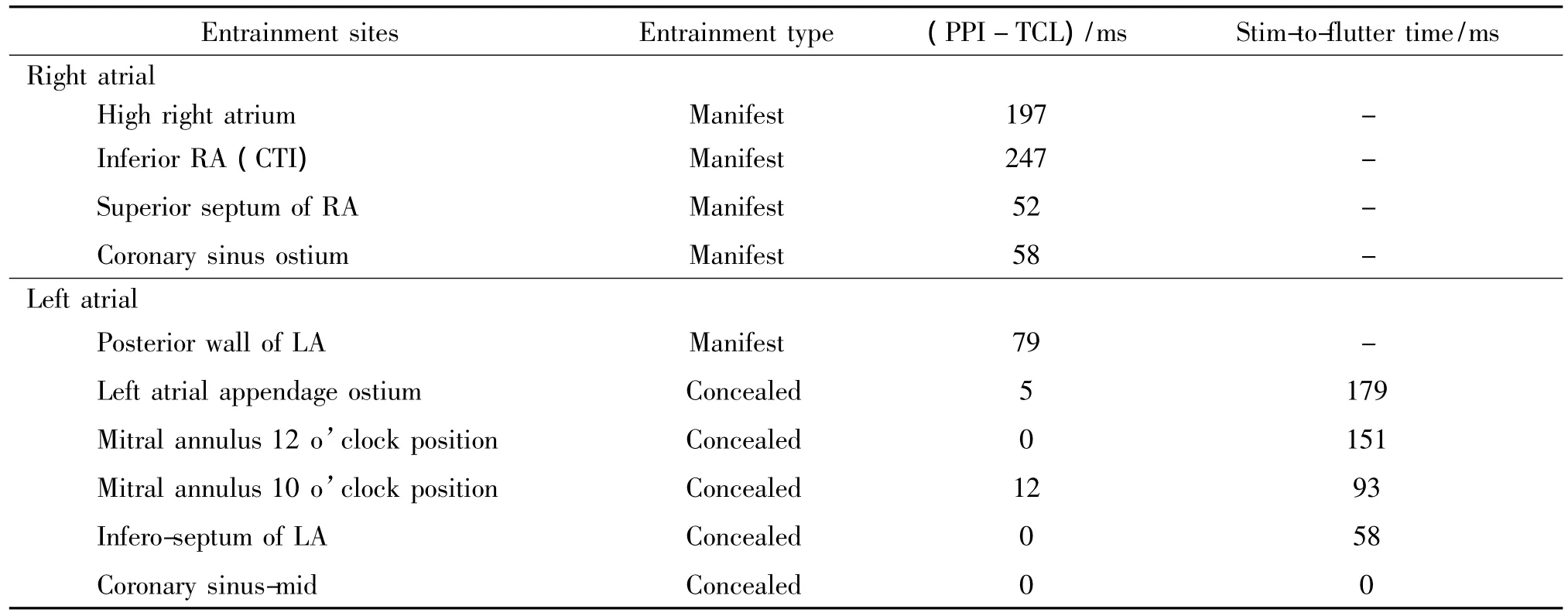
Tab.2 Entrainment mapping at different sites for the presented case of atypical atrial flutter
Two transeptal punctures were made for advancing the ablation catheter and duodecapolar Lasso catheter into the left atrium.The Lasso catheter was advanced into each of the pulmonary veins demonstrating isolation of all four.Next,the ablation catheter was positioned and entrainment maneuvers were performed at the posterior wall of the left atrium,with manifest entrainment,at the base of the left atrial appendage remnant,both 12 o’clock and 10 o’clock positions of the mitral annulus,and inferior septum of the left atrium(LA)with concealed entrainment and short PPI-TCL with progressively shorter stimulus to surface flutter wave intervals,all matching the local EGM to surface flutter wave intervals.A low-amplitude fractionated electrogram was identified at the 11 o’clock position of the mitral annulus consistent with a slow zone of conduction and potential conduction gap in scar(Fig.4).
The local EGM to the surface flutter wave at the 10 o’clock mitral annulus position was 84 ms at which time the tachycardia cycle length was 241 ms,hence representing 35%of the tachycardia cycle length(Fig.5).The 12 o’clock mitral annular position and LA inferoseptal local EGM’s to surface flutter waves were 159 ms and 54 ms,representing 66%and 22%of the tachycardiac cycle length,respectively.
Both an activation map and voltage map was constructed using the CARTO 3D-mapping system(Biosense Webster)(Fig.6).The activation map demonstrated an activation sequence consistent with a macro-reentrant circuit with the majority of the tachycardia cycle length identified around the mitral annulus,rather than a discrete area of early activation and centrifugal spread as one would expect with a focal tachycardia.The voltage map demonstrated a number of low voltage areas around the ostia of the pulmonary veins and linear areas from the pulmonary veins to the mitral annulus at the 11 o’clock and 3 o’clock areas in addition to along the roof of the LA.
Mitral-isthmus dependent atypical atrial flutter was diagnosed with concealed entrainment at the base of the left atrial appendage having a stimulus to surface flutter wave interval representing 66%of the tachycardia cycle length,consistent with a critical isthmus site.A 3.5 mm irrigated tip ablation catheter(SmartTouch,Biosense Webster)was used for all ablation lesions with a power output of 35 W and temperature limit of 45℃.Ablation was performed along the mitral isthmus with the creation of a linear ablation lesion from the mitral annulus to the left superior pulmonary vein,connecting to the scar of prior circumferential ablation(Fig.6A).With completion of the first linear ablation,the tachycardia cycle length increased from 240 ms to 254 ms.A second linear ablation lesion was made from the mitral annulus at 11 o’clock anteriorly toward the right superior pulmonary vein with lengthening of the tachycardia to 320 ms followed by termination(Fig.7).This linear lesion was extended across the roof to the base of the left atrial appendage(Fig.6B).Post ablation,programmed stimulation of the atrium using up to triple extra-stimuli was unable to induce any further atrial tachyarrhythmias.
The activation map demonstrated a tachycardia that appeared to involve the mitral annulus and was mitral isthmus dependent.After the initial ablation along the mitral isthmus,the lengthening of the tachycardia cycle length was consistent with an alteration in the circuit with possibilities being ablation of an inner loop but not the critical zone of conduction,changing the activation of the circuit to then include the posterior wall,or a multiple loop reentry circuit such as a figure of 8.Finally,ablation at the 11 o’clock position of the mitral annulus with previously identified fractionated low-voltage electrograms had terminated the tachycardia,consistent with ablation of the critical isthmus and resultant noninducibility.
3 Literature review on atypical atrial flutter
3.1 Etiologies and incidence
Atypical atrial flutter,sometimes called non-focal atrial tachycardia(AT),has a reentrant mechanism and most commonly presents with a history of cardiac surgery,or prior atrial ablation procedure from either a surgical or catheter based approach with presence of atrial scar(Fig.2B,Fig.2C).In a report of patients with left atrial flutter prior to widespread use of catheter ablation for atrial fibrillation 77%had evidence of structural heart disease with the remaining 23%having no obvious structural heart disease[9].In a more contemporary retrospective multi-center review of 91 patients with atypic al atrial flutter,Coffey et al[5]reported that 65%had prior catheter ablation of the atrial tissue,27%had prior cardiac surgery including a Maze procedure in 12%and idiopathic scar related atypical atrial flutter was found in 8%suggestive of an atrial cardiomyopathy.
The incidence of atypical atrial flutter is likely to increase with more individuals living with congenital heart disease after surgery as well as catheter ablation of atrial fibrillation becoming an increasingly common procedure.In a prospective study of 67 patients undergoing catheter ablation of atrial fibrillation with pulmonary vein isolation and linear ablation,43%of patients had experienced atrial tachycardia,of which 69% had a reentrant mechanism consistent with an atypical atrial flutter,and 31%had sustained atrial tachyarrhythmias using 7-day Holter monitors at 3,6 and 12 months post ablation for screening[6].Although there is a significant incidence of atrial tachycardias including atypical atrial flutter,after catheter ablation of atrial fibrillation a significant proportion of them are self-limited.Chugh et al[7]reported an experience of 349 patients who underwent catheter ablation for atrial fibrillation with 24%developing macro-reentrant atrial tachycardia during follow-up of approximately 1 year,with 33%of these being are self-resolving,commonly within 5 months after the original ablation procedure,and a strategy for shortterm monitoring may be considered.
3.2 Mapping
The primary tools for mapping an atypical atrial flutter are entrainment,voltage,and activation mapping.The entrainment technique is used to identify sites of concealed entrainment and define the critical isthmus of the circuit for atypical atrial flutter.Constructing a voltage map of the right and/or left atrium help define the low voltage areas that represent scar and can serve as boundaries for the re-entrant circuit.Activation mapping helps define the majority of the cycle length of a reentrant tachycardia on a 3D-mapping system in relation to the atrial anatomy.Additionally areas of double potentials or highly fractionated local electrograms may define lines or block or slow anisotropic conduction,respectively.Each of these techniques has its limitations and an approach integrating all of the information available is important for successful mapping and ablation.
3.2.1 Entrainment mapping Entrainment mapping has become an important tool for interrogating reentrant circuits,and has proven helpful in paroxysmal supraventricular tachycardias as well as defining components of atypical atrial flutter circuits similar to the employment of this technique in mapping ventricular tachycardia.Atrial overdrive pacing from a site of interest during the tachycardia at a cycle length of 10-30 ms shorter than the tachycardia with cessation of pacing and resumption of the tachycardia is the basic maneuver involved in entrainment.Waldo et al[10]proposed 4 criteria for entrainment and has reported the use of entrainment for mapping typical atrial flutter.
Entrainment of an atypical atrial flutter at a location within the atria with the difference between the post-pacing intervaland tachycardia cycle length(PPI-TCL)less than 20 ms is suggestive of the location being within the re-entrant circuit.Furthermore,if there is concealed entrainment where the spontaneous flutter waves on the surface electrocardiogram and activation sequence of the intracardiac atrial electrograms are identical to those seen while pacing during entrainment,the position of the pacing catheter tip is within the critical slow zone of conduction.Mapping locations with a(PPI-TCL)of less than 20 ms,concealed entrainment and progressively shorter stimulus to surface flutter wave intervals is consistent with points progressively closer to exit site of the circuit.
Entrainment provides a general idea of the location of the components of an atypical atrial flutter circuit but should be used in conjunction with other mapping modalities.The sensitivity and specificity of these maneuvers for typical atrial flutter have been investigated by Morton et al[11].The sensitivity and specificity for concealed entrainment,and hence isthmus identification,pacing a cycle length 10 ms shorter than the flutter cycle length were 100%and 54%respectively,with declining sensitivity and improved specificity with progressively shorter cycle lengths of overdrive[11].Triedman et al[12]reported acute success rates of 73%and recurrence rates of 53%at a mean of 4 months for ablation of intraatrial reentrant tachycardias in patients with congenital heart disease using identification of low-voltage fractionated diastolic electrograms and entrainment mapping techniques.Although these studies have not addressed atypical atrial flutter specifically,they raise questions about the usefulness of entrainment to accurately predict the location of the isthmus and fully define the circuit involved.Termination of the tachycardia and alteration of the cycle length with entrainment maneuvers also limit its utility in patients with multiple reentrant circuits and diffuse substrate.
3.2.2 Electroanatomical(voltage and activation)mapping Atypical atrial flutters usually have areas of scar tissue that have little or no electrical activity and are identified by the very low voltage or absence of local electrograms.It has been suggested that scar,or electrically silent areas,for a voltage map within the atria be defined as points with a bipolar amplitude of<0.05 mV and inability to capture atrial tissue with a power output of 20 mA[9].Visualization of scar and low voltage areas can be achieved by annotating these areas on a computer generated anatomical 3D-map and provides a guide for boundaries of block and potential zones of slow conduction that could represent a critical isthmus.Double potentials are often found at zones of local block and can provide further guidance when assessing gaps in linear ablation lines.Additionally,complex fractionated electrograms representing slow conduction zones can be marked within the map as candidates for a critical isthmus.
Activation mapping uses a 3D-mapping computer system to display local activation time during tachycardia of the atrium relative to a reference point,usually a catheter in the coronary sinus.The difference in the local activation time as compared to the reference point is assigned a color based on the color spectrum with red representing the earliest activation time and violet the latest activation time,with identified activation times spanning 90%or more of the tachycardia cycle length.With an accurate activation map,a potential critical isthmus may be identified by slow zone of conduction,seen on an activation map by a rapid change in color in a confined area.A useful finding of activation mapping is when the earliest local activation is confined to a very small area,suggestive of a focal mechanism or localized reentry circuit which can be approached with targeting ablation at the earliest activation point.Multipole catheters have been developed and provide an effective means for rapidly mapping non-sustained atrial tachycardias as well as high density mapping.
Nakagawa et al[13]reported using voltage and activation mapping for ablation of 15 sustained macroreentrant right atrial tachycardias in 13 patients with a history of surgical repair of congenital heart disease.With electroanatomical mapping they were able to identify areas of scar as well as channels for propagation which were targeted with radiofrequency ablation and successful termination of all 15 tachycardias and inability to induce sustained atrial tachycardia in any patient post-ablation.Further studies using electroanatomical mapping systems for atypical atrial flutter after atrial fibrillation ablation have yielded similar results[5-7,14-15].
3.3 Circuit characteristics
The circuits of atypical atrial flutter can be categorized by a number of variables including circuit location,circuit size,morphology,and association with a gap in a previous linear ablation.Atypical atrial flutter related to a prior ablation procedure is often subdivided by size of the circuit with macro-reentrant being defined as a re-entrant circuit size >2-3 cm or involving 2 or more atrial segments with activation mapping identifying 90% or more of the tachycardia cycle length,small reentrant defined the tachycardia cycle length is mapped in an area <2-3 cm or within 1 atrial segment depending on author.Truly focal atrial tachycardias have a point source and centrifugal spread of the wavefront on activation mapping[6,9,16].
Anatomical location of a flutter circuit can involve nearly any structure within the left or right atrium.In patients having a history of atrial fibrillation ablation the isthmus is identified most commonly at the mitral isthmus in 25%-42%,left atrial roof in 13%-20%and septal in 13%for macro-reentrant mechanisms;at the antrum of the pulmonary veins,the anterior and posterior wall,base of the LAA,left septum and coronary sinus for small reentrant circuits and focal AT[14-15].
The common circuit morphologies are single-loop reentry and multiple-loop reentry as well as microreentrant circuits.Linton et al[17]have developed a technique for distinguishing whether a circuit involves a single or double loop reentrant mechanism by measuring the post pacing intervals at two separate locations after sequentially overdrive pacing at each of these locations.In the case of double-loop reentry,the isthmus having the shortest post-pacing interval approximating the tachycardia cycle length with entrainment is ablated initially and the next shortest site targeted if termination of the tachycardia is not achieved.
Reentrant circuits involving conduction across a prior ablation line have been termed gap-related AT’s with up to 96%of re-entrant AT’s being reported as gaprelated[7].This highlights the importance of complete block across a linear ablation to potentially avoid occurrence of these arrhythmias.Certainly,linear ablation lesions potentially offer higher success rates in patients with persistent atrial fibrillation,but come with the additional cost of higher AT occurrence on follow-up[18].
3.4 Ablation end-points and success rates
In general,acute success rates are high with freedom from recurrence with long-term follow-up only marginally worse.Septal locations are associated with significantly lower acute and long-term success rates for ablation.Different success rates have also been reported depending on ablation catheter type used.A septal location of flutter circuits are often more resistant to ablation with complex anatomical relationships and potential difficulty of delivering adequate radiofrequency energy in those locations.Coffey et al[5]reported in their review of cases,an acute success rate of 97%and longterm success rates of 77%,being any post-procedure AT during(16±12)months follow-up,of 82%and 67%for non-septal and septal location atrial tachycardias,respectively.Furthermore they found that long-term success rates varied depending on the likely etiology of scar with freedom from AT of 88%with surgical scar,75%for those with a history of catheter ablation and 57%for individuals with idiopathic atrial scarring.Other studies have reported lower acute and long-term success rates but often used the ablation catheter for activation mapping rather than a multi-electrode catheter which may have resulted in a lower density of points when mapping[7,14-15].
Choice of catheter type may also play a role in acute and long-term success rates with radiofrequency ablation of atypical atrial flutter.A randomized study comparing 3.5 mm open-irrigated tip catheters to 8 mm non-irrigated tip catheters for ablation of atypical atrial flutter showed that acute(94%vs.77%)and long-term success at 10 months follow-up,using 48-hour Holter monitors at 3,6,and 9 months,(92%vs.59%)was higher with the irrigated tip catheter[19].
4 Conclusion
Atypical atrial flutter is becoming an increasingly prevalent problem as more atrial fibrillation ablations are performed and a larger number of individuals are living after cardiac surgery with atriotomy especially in patients with congenital heart disease.With a systematic approach including entrainment,advanced mapping,and improved understanding of the electroanatomical substrate,the success rates of radiofrequency ablation can approach 90%for this clinically challenging arrhythmia.A number of factors are related to ablation outcomes,including the patient’s surgical and ablation history,location and characteristics of the reentrant circuits,and mapping and ablation catheters.Although not specifically reported for the atypical atrial flutter ablation,success and complications outcomes are often associated with the experience of the operator and the procedural volumes of the medical center for these complex interventional procedures.
[1]Kalman JM,Olgin JE,Saxon LA,et al.Activation and entrainment mapping defines the tricuspid annulus as the anterior barrier in typical atrial flutter[J].Circulation,1996,94(3):398-406.
[2]Kalman JM,Olgin JE,Saxon LA,et al.Electrocardiographic and electrophysiologic characterization of atypical atrial flutter in man:use of activation and entrainment mapping and implications for catheter ablation[J].J Cardiovasc Electrophysiol,1997,8(2):121 -144.
[3]Cosio FG,López-Gil M,Goicolea A,et al.Radiofrequency ablation of the inferior vena cava-tricuspid isthmus in common atrial flutter[J].Am J Cardiol,1993,71(8):705-709.
[4]Feld GK,Fleck RP,Chen PS,et al.Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter:identification of a critical zone in the reentrant circuit by endocardial mapping techniques[J].Circulation,1992,86(4):1233-1240.
[5]Coffey JO,d’Avila A,Dukkipati S,et al.Catheter ablation of scar-related atypical atrial flutter[J].Europace,2013,15(3):414-419.
[6] Deisenhofer I,Estner H,Zrenner B,et al.Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation:incidence,electrophysiological characteristics,and results of radiofrequency ablation[J].Europace,2006,8(8):573-582.
[7]Chugh A,Oral H,Lemola K,et al.Prevalence,mechanisms,and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation[J].Heart Rhythm,2005,2(5):464 -471.
[8]Medi C,Kalman JM.Prediction of the atrial flutter circuit from the surface electrocardiogram[J].Europace,2008,10(7):786-796.
[10]Waldo AL.Atrial flutter:entrainment characteristics[J].J Cardiovasc Electrophysiol,1997,8(3):337 -352.
[11] Morton JB,Sanders P,Deen V,et al.Sensitivity and specificity of concealed entrainment for the identification of a critical isthmus in the atrium:relationship to rate,anatomic location and antidromic penetration[J].J Am Coll Cardiol,2002,39(5):896 -906.
[12]Triedman JK,Bergau DM,Saul JP,et al.Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease[J].J Am Coll Cardiol,1997,30(4):1032 -1038.
[13]Nakagawa H,Shah N,Matsudaira K,et al.Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease:isolated channels between scars allow“focal”ablation[J].Circulation,2001,103(5):699-709.
[14]Chae S,Oral H,Good E,et al.Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation:mechanistic insights,results of catheter ablation,and risk factors for recurrence[J].J Am Coll Cardiol,2007,50(18):1781-1787.
[15]Jas P,Matsuo S,Knecht S,et al.A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation:importance of localized reentry[J].J Cardiovasc Electrophysiol,2009,20(5):480 -491.
[16]Yokokawa M,Latchamsetty R,Ghanbari H,et al.Characteristics of atrial tachycardia due to small vs large reentrant circuits after ablation of persistent atrial fibrillation[J].Heart Rhythm,2013,10(4):469-476.
[17]Linton NW,Wilton SB,Scherr D,et al.A practical criterion for the rapid detection of single-loop and double-loop reentry tachycardias[J].J Cardiovasc Electrophysiol,2013,24(5):544 -552.
[18]Hassaguerre M,Sanders P,Hocini M,et al.Catheter ablation of long-lasting persistent atrial fibrillation:critical structures for termination[J].J Cardiovasc Electrophysiol,2005,16(11):1125-1137.
[19]Bai R,Fahmy T,Patel D,et al.Radiofrequency ablation of atypical atrial flutter after cardiac surgery or atrial fibrillation ablation:a randomized comparison of open-irrigation-tip and 8-mm-tip catheters[J].Heart Rhythm,2007,4(12):1489-1496.
学术动态

