Epirubicin-gold nanoparticles suppress hepatocellular carcinoma xenograft growth in nude mice
William C.S.Meng,Yunlong Pan,Xiaoxu Zhao
1Department of Surgery,Matilda Internatinal Hospital,Hong Kong,China;
2Department of General Surgery,the First Affiliated Hospital of Jinan University,Guangzhou 510632,China.
Epirubicin-gold nanoparticles suppress hepatocellular carcinoma xenograft growth in nude mice
William C.S.Meng1,✉,Yunlong Pan2,Xiaoxu Zhao2
1Department of Surgery,Matilda Internatinal Hospital,Hong Kong,China;
2Department of General Surgery,the First Affiliated Hospital of Jinan University,Guangzhou 510632,China.
We soughtto investigate the effects ofepirubicin-nanogold compounds(EPI-AuNP)on hepatocellularcarcinoma xenograftgrowth in nude mice.EPI-AuNP was prepared and hepatoma xenograftmodelwas established in nude mice.The mice were then randomly divided into four groups:the control group with injection of saline,the AuNP treatmentgroup,the EPItreatmentgroup and the EPI-AuNP treatmentgroup.Aftertwo weeks,the hepatoma weightand volume ofthe xenografts were assessed.Ourtransmission electron microscopy revealed thatepirubicingold nanoparticles caused significantly more structural changes of hepatocellular carcinoma cells HepG2.The tumorweightin the Epi-AuNP treatmentgroup(0.80±0.11 g)was significantly lowerthan thatofthe controlgroup (2.48±0.15 g),the AuNP treatmentgroup(1.67±0.17 g),and the EPItreatmentgroup(1.39±0.10 g)(P<0.01). Furthermore,the tumorvolume ofmice in the EPI-AuNP treatmentgroup(0.27±0.06 cm3)was significantly smallerthan thatofthe controlgroup(2.23±0.34 cm3),the AuNP treatmentgroup(1.21±0.25 cm3)and the EPItreatment group(0.81±0.11 cm3)(P<0.01).In conclusion,epirubicin-nanogold compounds(EPI-AuNP)have significantinhibitory effects on the growth of hepatocellular carcinoma cells in vivo.
epirubicin,nanogold,hepatoma xenograft,HepG2 cells,HUVEC
Introduction
Epirubicin is an anthraquinone antitumor drug.It has greatpotentialin cancer treatmentbutlacks specificity.The complications ofmyocardialand bone marrow toxicity have limited its clinical application[1]. Epirubicin could be combined with a nano-carrier, forming a synthetic drug delivery system,to improve the specificity of the drug.This system improves epirubicin targeting and prolongs in vivo bioavailability, thereby enhancing the drug's efficacy[2].Furthermore, epirubicin has the specialproperty ofbeing fluorescent under ultraviolet light.When combined with nanoparticles,this fluorescence is decreased and,hence,is an effective way to identify successful combination of the compound.
Gold nanoparticles are spherical,with a mean diameter of 10 nm.They have the advantages of having a well-established synthesis process,good biocompatibility,optimal tissue permeability,and relatively easy modification of the surface[3].Preliminary studies showed that gold nanoparticles possess an antiangiogenesis effect by itself[4].The proposed mechanism is binding by gold nanoparticles to the heparin receptor sites and vascular endothelial growth factor (VEGF165),which would in turn block the activationof VEGFR2 and hence inhibit the activation and proliferation of tumor vascular endothelial cells.As a result,gold nanoparticles prohibitangiogenesis[5]
In addition,gold nanoparticles can serve as a''drug delivery system''.It can be combined with anti-tumor drugs by means ofphysicaladsorption,ionic orcovalent bonding[6].This system would have the functions ofantiangiogenesis as well as anti-tumor cell proliferation. Hence,it can exert its anti-tumor effect via multiple levels and multiple targets.In the currentstudy,we first synthesized gold nanoparticlesand then investigated the in vivo effectofepirubicin-gold nanoparticleson human hepatocellularcarcinoma xenografts in nude mice.
Materials and methods
Reagents and instruments
Epirubicin was commercially available(Sigma,St. Louis,MO,USA).Gold nanoparticles were prepared with gold chloride and sodium citrate(Sinopharm Chemical Reagent Co.,Ltd.).RPMI-1640 medium,L-glutamine, penicillin and streptomycin(Hyclone,USA)were purchased.M199 medium,fetal bovine serum,and trypsin (Gibco Le.)were commercially available.Human hepatocellular carcinoma cellline HepG2 was maintained in our own laboratory.Human umbilicalvein endothelialcells (HUVECs)were obtained by primary isolation and culture[7].Ultraviolet-visible(UV-Vis)spectrophotometer (Lambda 45,Perkin Elmer,USA)and transmission electron microscope(TECNAI10,Philips Ltd.),fullwavelength multifunctionalmicro-plate reader(Safire II,Bio-Rad,Hercules,CA,USA),atomic force microscopy (American Thermo-microscope,Ltd.)and micro-titer plates(Costar)were used forthe in vitro study.
Animals
Six to eight week-old BALB/c nude mice with body weight ranging from 15-22 g were purchased from the Sun Yat-sen University Laboratory Animal Center (Guangzhou,China)and kept in the Laboratory Animal Center of Jinan University under conditions recommended by the Environmental Standards of China GB14925-2007,at20-27°Cand the IS07 levelof cleanliness.The study protocolwasapproved by the localinstitutionalreview boards and animalstudy was carried out in accordance with the established institutionaland state guidelines on experimentaluse ofanimals.
Synthesis and identification of gold nanoparticles
Gold chloride was mixed with citric acid and Au3+in gold chloride was reduced to Au.All glassware was cleansed in aq ua regia(3 p arts HCl an d 1 part HNO3).Then,5 mL of sodium citrate(38.8 mmol/L) was adde d to 50 m L of boiling gold chloride (1 mmol/L)solution.The resultant suspension was then cooled down to room temperature.It was subsequently filtered with a 0.22μm filter to remove large aggregates and then stored at 4°C.
Synthesis and identification of epirubicingold nanoparticles
Four mL of the gold nanoparticle solution was diluted with 8 mL of ultrapure water.Sodium hydroxide(1 mol/L NaOH)solution was added and titrated to pH 8.0.Then,2 mL of epirubicin was added.The solution was placed overnight in an air shaker(172 g) at37°C.Aftercentrifugation(28,720 g)for 40 minutes, the supernatantwas discarded and the epirubicin-gold nanoparticles were washed thrice with 0.01 mol/L phosphate buffered saline with 0.2%bovine serum albumin and then analyzed[8]by UV-Vis absorption spectrophotometry,fluorescence quenching,dynamic laser light scattering(DLS),and zeta potentialanalyzer.
Atomic force microscopy(AFM)
Cellularsuspensions of HepG2 cells and HUVECs at 1×104cells/mL were plated in 6-well plates with 22 mm×22 mm sterile cover slips,respectively,and then cultured for24 hours.Thecoverslips,adsorbed with HepG2 cells and HUVECs,were gently rinsed with PB solution(0.1 mol/L,pH=7.4)three timesfor5 min each. Then,they were immersed in 4%paraformaldehyde for 15 minutes and subsequently rinsed three times with ultrapure water.They were then air-dried atroom temperature.Finally,AFM was used forscanning.The setting of 100μm scanner,contact mode,and silicon probe,with resonance frequency of 250-320 Hz was used.The images were enhanced using Proscan Image Processing Software Version 2.1.
Mouse hepatocellular carcinoma xenograft model
HepG2 cellsin 0.2 mL(containing 5×106cells)were implanted subcutaneously in the rightaxilla of six to eight-week-old BALB/c nude mice.When the transplanted tumordiameterreached 6-8 mm,the nude mice were randomly divided into four groups of eightand marked for identification.The four groups were processed as:(1)the controlgroup(normalsaline),(2)the Au NP(2.5μg/kg)treatment group,(3)the EPI (5 mg/kg)treatment group and(4)the EPI-AuNP (AuNP 2.5μg/kg+EPI 5.0 mg/kg)treatmentgroup. The drugs were injected as 0.2 mL solution via the tail vein every otherday fortwo consecutive weeks.
Formeasurementoftumorvolumeandweight,thenude mice were sacrificed and the tumor xenografts were removed en bloc and measured using the Verniercaliper by oneperson to eliminate observervariation.Thelongest diameter(a)and perpendiculardiameter(b)were measured and thetumorvolumewascalculated(tumorvolume =ab2/2).The weightofthe tumorxenograftswere measured with electronic analyticalbalance.
Statistical analysis
Continuous variables were checked for normal distribution and presented as mean and standard deviation or median and range as appropriate.Comparison of continuous variables was performed by using Student's ttest for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. One-way analysis of variance(one-way ANOVA)was used to compare means of two or more samples with least significant difference(LSD)as post hoc test.All tests of significance were at the 5%significance level. Analyses were conducted using SPSS version 11.0.
Results
Epirubicin-gold nanoparticles caused apparent structural changes of hepatocellular carcinoma cells
Transmission electron microscopy(TEM)(Fig.1) revealed that epirubicin-gold nanoparticles were spherical,uniform in size and about10 nm in diameter. UV-Vis spectrophotometry displayed the maximum absorption peak of gold nanoparticles at 520 nm, which was markedly reduced when epirubicin-gold nanoparticles were used.DLS demonstrated thatgold nanoparticles were(14.34±0.75 nm),which was significantly smaller than epirubicin-gold nanoparticles (18.54±1.84 nm).Zeta potentialanalyzershowed that gold nanoparticles had a zeta potentialof-21.19±0.64 mV,which was markedly differentfrom thatof epirubicin-gold nanoparticles(-15.34±0.72 mV).

Fig.1Transmission electron microscope images of epirubicinnanogold compounds.Nanogold compounds were prepared as described in Methods and they appearspherical,uniform in size and about 10 nm in diameter.(Bar=100 nm)
AFM was used to visualize the morphological changes in the hepatoma xenografts and in turn compared the treatmenteffect.Asshown in Fig.2,the surface of normal Hep G2 cells was smooth with turgid cytoplasm.In comparison to the control group,in AuNP-treated cells,there was invagination of cell membrane and there was an,increase in surface pore size with the more rough surface morphology.EPItreated HepG2 cells showed obviousholes ofvarying sizes on the cell surface,which may signify cellular injury due to EPI.This was more apparently aggravated in EPI-AuNP treated HepG2 cells whose cellmembrane appeared rougher with uneven larger pores with the maximum diameter of up to about2μm.
Epirubicin-gold nanoparticles markedly suppress hepatocellular carcinoma xenograft growth in nude mice
We observed significant difference in the tumor weightofhepatocellularcarcinoma xenograftin the differentgroups ofnude mice(Fig.3).The tumorweight in the Epi-Au NP treatment group(0.80±0.11 g) was significantly less than that of the control group(2.48±0.15 g)and the Au NP treatmentgroup (1.67±0.17 g),and the EPI treatment group (1.39±0.10 g)(P<0.01).Furthermore,there were significantdifferences in the tumorvolume ofhepatocellular carcinoma xenograftin the differentgroups of nude mice.The tumorvolume ofmice in the EPI-AuNP treatmentgroup(0.27±0.06 cm3)was significantly smaller than that of the control group(2.23±0.34 cm3),the AuNP treatment group(1.21±0.25 cm3)and the EPI treatmentgroups(0.81±0.11 cm3)(P<0.01).
Discussion
Epirubicin isan effective antitumordrug butitslack of specificityhasprohibited itswidespread use.Theintroduction ofnano-biotechnology may provide a possible solution forits use as a targeted therapy.In the currentstudy, we successfully created epirubicin nanogold particles. The specialnature ofepirubicin being fluorescentunder ultravioletlightis also a convenience.When combined with nano-particle,this fluorescence is quenched.This can serveasasimpleand effectiveway to confirm success in the creation ofthe compound.
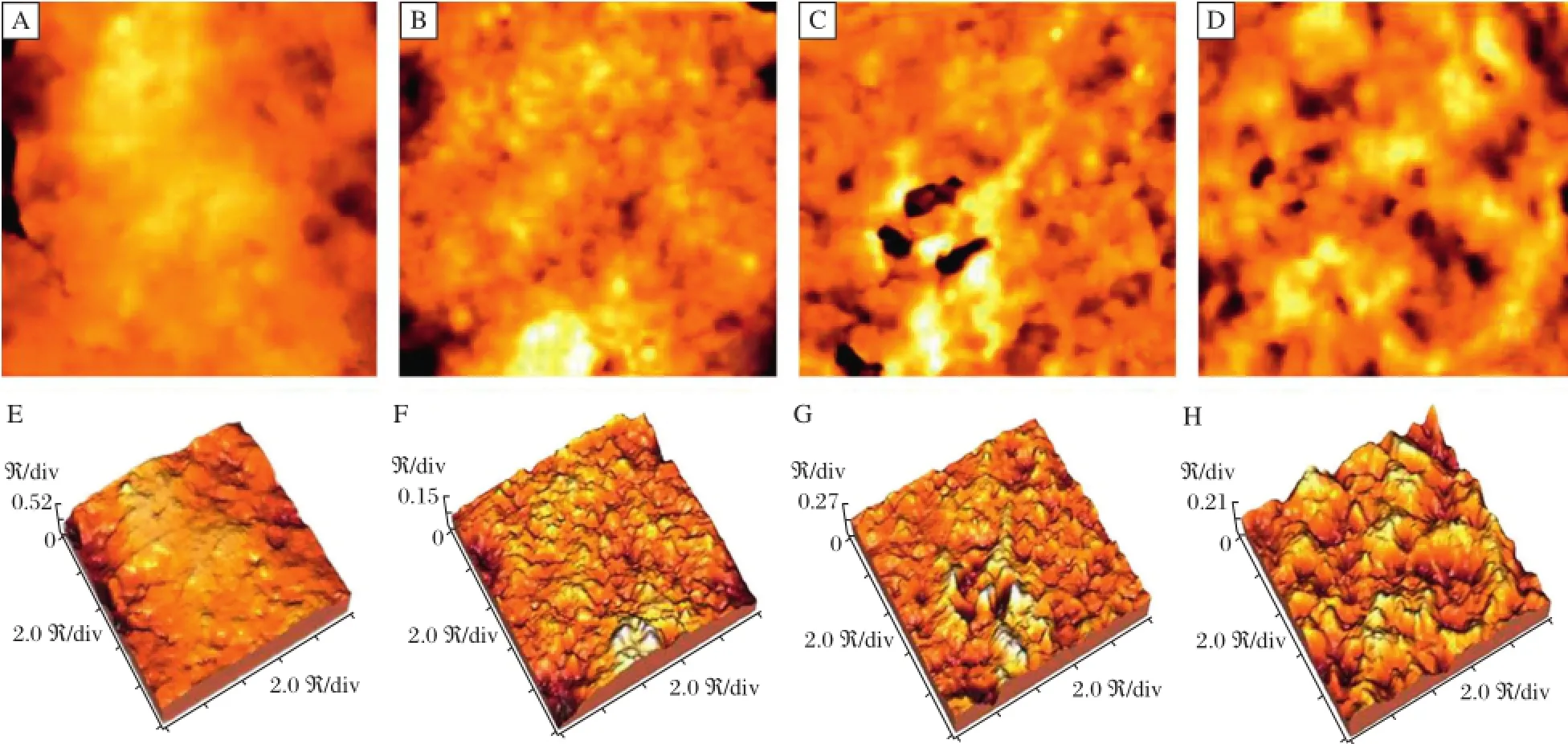
Fig.2 AFM image of HepG2 cell surface morphology A:Control;B:AuNP(nanogold compounds)treatment group;C:EPI(epirubicin) treatmentgroup;D:EPI-AuNP(epirubicin-nanogold compounds)treatmentgroup.Photos(E)to(H)are corresponding three-dimensionalreconstruction (image size:10μm×10μm).
Gold nanoparticle itself can prohibit angiogenesis. When combined with epirubicin,apart from the anti-tumor effect,it can increase the uptake of epirubicin into tumor cells.In turn,epirubicin willact by intercalating DNA strands,resulting in a complex formation which inhibits DNA and RNA synthesis.In addition,by triggering DNA cleavage by topoisomerase II,cytotoxic effects and free radicals wilallcontribute to the cytotoxic effect.In our study,we confirmed the successful synthesis of EPI-AuNP compound by several ways.Firstly,ultraviolet-visible absorption spectrophotometer(UV-Vis)can measure the absorbance of light in visible and ultraviolet ranges in organic compounds.A shift in the maximum absorption denotes successful combination of compounds, altering the permittivity,which is how the electric field is affected by a dielectric medium.Secondly, fluorescence spectroscopy can detect the fluorescence intensity of EPI,which is greatly diminished in EPIAu NP.Thirdly,DLS analyzes the Brownian motion ofparticlesormolecules in suspension thatcauseslaser lightto be scattered atdifferent intensities.These intensity fluctuations yield the velocity of the Brownian motion and hence the particle size using the Stokes-Einstein relationship.Finally,Zeta Potential Analyzer measures the electro-kinetic potential of colloid. Colloids with high zeta potential(negative or positive) are electrically stabilized while colloids with low zeta potentials tend to coagulate or flocculate.
In the results ofthe in vivo effecton hepatoma xenografts in nude mice,there was a clear anti-tumoreffect both in terms ofweightand volume as compared to the controlfor allthree limbs.There is also an increase in anti-tumor effect in an ascending order for AuNP,EPI and EPI-AuNP.EPI-AuNP can evade phagocytosis of the reticuloendothelial system and can maintain epirubicin biologicalactivity untilitreaches the tumorsite[9]. Through vascular permeability and,also,intercellular communication channels,EPI-AuNP can concentrate atthe tumor cells and decrease the side effects on the heart and the bone marrow.This targeted therapy will increase the safety of its use.
After the release of epirubicin,AuNP itself could still have an anti-tumor angiogenesis effect by reducing the tumor’s nutrient and oxygen supply,and prohibiting tumor growth.Furthermore,it is capable of binding to VEGF165,thereby blocking signaltransduction and,in the end,inhibiting endothelial cell proliferation and tumor angiogenesis.Finally, it can inhibit human hepatoma cells Hep G2 for VEGF expression and secretion.
AuNP is a multifunctional inorganic nano-particle for imaging,targeting,and drug delivery[10].It can efficiently carry anticancer drugs,plasmid DNA or small interfering RNA to reach therapeutic targets.It has the potentialto play a significantrole in cancer chemotherapy[11],radiation therapy[12]or even gene therapy.Furthermore,Au NP can function as a new generation of contrast agents for diagnostic imaging of tumormorphology.In addition,itcan be combined with tumor marker antibody and may serve as a sensitive diagnostic modality[13].
In conclusion,in vitro studies ofepirubicin-nanogold compound(EPI-AuNP),when compared to AuNP alone or EPI alone,showed an increase in morphological changes signifying cellular damage in Hep G2 cells and HUVECs.EPI-AuNP also had an increase in accumulation in cells compared with epirubicin alone. Furthermore,in vivo studies of hepatoma xenografts in nude mice showed that EPI-AuNP has enhanced antitumor effects,when compared to AuNP alone or EPI alone in both tumorvolume and weight.
[1]Minotti G,Menna P,Salvatorelli E,et al.Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity[J].Pharmacol Rev, 2004,56:185-229.
[2]Peer D,Karp JM,Hong S,etal.Nanocarriers as an emerging platform for cancer therapy[J].Nat Nanotechnol,2007, 2(12):751-760.
[3]Dan iel MC,Astruc D.Go ld nan o p articles:Assembly,supramolecular chemistry,quantum-size-related p roperties,and applicatio ns toward b iology,catalysis,and nanotechnology[J].Chem Rev,2004,104(1): 293-346.
[4]Mukherjee P,Bhattacharya R,Wang P,et al.Antiangiogenic Properties of Gold Nanoparticles[J].Clin Cancer Res,2005,11:3530-3534
[5]Pan YL,Qiu SY,Qin L,et al.Nanogold inhibits angiogenesis and growth of liver cancer:experiment with mice[J].Zhonghua Yi Xue Za Zhi(in Chinese),2009,89(12): 800-804.
[6]Chen YH,Tsai CY,Huang PY,etal.Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model[J].Mol Pharm,2007,4(5):713-722.
[7]Marin V,Kaplanski G,Gre`s S,et al Bongrand P.Endothelialcellculture:protocolto obtain and cultivate human umbilical endothelial cells[J].J Immuno l Methods,2001, 254:183-190.
[8]Zhao XU,Pan YL,Hu YX,etal.In vitro antitumor effects of epirubiin-nanogold compounds[J].J Sun Wat-sen Univ (Med Sci),2012,33(2):172-177.
[9]Giljohann DA,Seferos DS,Daniel WL,et al.Gold nanoparticles for biology and medicine[J].Angew Chem Int Ed,2010,49(19);3280-3294.
[10]Liong M,Lu J,Kovochich M,etal.Multifunctionalinorganic nanoparticles for imaging,targeting,and drug delivery[J].Acs Nano,2008,2(5):889-896.
[11]Sharma,J,Chhabra,R,Cheng,A,et al.Control of Self-Assembly of DNA Tubules Through Integration of Gold Nanoparticles[J].Science,2009,323(5910):112-116.
[12]Nam J,Won N,Jin H,et al.pH-Induced Aggregation of Gold Nanoparticles for Photothermal Cancer Therapy[J]. J Am Chemical Soc,2009,131(38):13639-13645.
[13]Kim D,Jeong,YY,Jon S.A Drug-Loaded Aptamer-Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer[J].Acs Nano,2010,4(7): 3689-3696.
✉Corresponding author:William C.S.Meng,MD,Department of Surgery,Our Lady of Maryknoll Hospital,118 Shatin Pass Road,Wong Tai Sin,Kowloon,Hong Kong,China.Tel/Fax:+852-2354-2440+852-23/+862-2327-6852,E-mail:wmeng@netvigator.com.
Received 07 March 2014,Revised 29 April2014,Accepted 11 October 2014,Epub 05 August 2015
R735.7 Document Code:A
The authors reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140044
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Platelets in hemostasis and thrombosis:Novelmechanisms of fibrinogen-independent platelet aggregation and fibronectinmediated protein wave of hemostasis
- Retinolbinding protein 4 correlates with and is an early predictor of carotid atherosclerosis in type 2 diabetes mellitus patients
- CC-chemokine receptor 7 and its ligand CCL19 promote mitralvalve interstitialcellmigration and repair
- Effectofburden and origin sites ofpremature ventricular contractions on left ventricular function by 7-day Holter monitor
- Prevention ofatrialfibrillation with renin-angiotensin system inhibitors on essentialhypertensive patients:a meta-analysis of randomized controlled trials
- A susceptibility locus rs7099208 is associated with non-obstructive azoospermia via reduction in the expression of FAM160B1
