Platelets in hemostasis and thrombosis:Novelmechanisms of fibrinogen-independent platelet aggregation and fibronectinmediated protein wave of hemostasis
Yan Hou,Naadiya Carrim,Yiming Wang,Reid C.Gallant,Alexandra Marshall, Heyu Ni,5,✉
1Department of Laboratory Medicine,Keenan Research Centre for Biomedical Science,Li Ka Shing Knowledge Institute, St.Michael's Hospital and Toronto Platelet Immunobiology Group,Toronto,M5B 1W8,Ontario,Canada;
2Jilin Provincial Center for Disease Control and Prevention,Changchun,Jilin,130062 China;
3Department of Laboratory Medicine and Pathobiology,University of Toronto,Toronto,Ontario M5S 1A1,Canada;
4Canadian Blood Services,Toronto,Ontario M5B 1W8,Canada;
5Department of Medicine and Department of Physiology,University of Toronto,Toronto,Ontario M5S 1A1,Canada.
Platelets in hemostasis and thrombosis:Novelmechanisms of fibrinogen-independent platelet aggregation and fibronectinmediated protein wave of hemostasis
Yan Hou1,2,△,Naadiya Carrim1,3,4,△,Yiming Wang1,3,4,Reid C.Gallant1,3,Alexandra Marshall1, Heyu Ni1,3,5,✉
1Department of Laboratory Medicine,Keenan Research Centre for Biomedical Science,Li Ka Shing Knowledge Institute, St.Michael's Hospital and Toronto Platelet Immunobiology Group,Toronto,M5B 1W8,Ontario,Canada;
2Jilin Provincial Center for Disease Control and Prevention,Changchun,Jilin,130062 China;
3Department of Laboratory Medicine and Pathobiology,University of Toronto,Toronto,Ontario M5S 1A1,Canada;
4Canadian Blood Services,Toronto,Ontario M5B 1W8,Canada;
5Department of Medicine and Department of Physiology,University of Toronto,Toronto,Ontario M5S 1A1,Canada.
Platelets are smallanucleate cells generated from megakaryocytes in the bone marrow.Although plateletgeneration,maturation,and clearance are stillnotfully understood,significantprogress has been made in the last1-2 decades.In blood circulation,platelets can quickly adhere and aggregate atsites ofvascularinjury,forming the platelet plug(i.e.the firstwave ofhemostasis).Activated platelets can also provide negatively charged phosphatidylserinerich membrane surface thatenhances cell-based thrombin generation,which facilitates blood coagulation(i.e.the second wave of hemostasis).Platelets therefore play centralroles in hemostasis.However,the same process of hemostasis may also cause thrombosis and vessel occlusion,which are the most common mechanisms leading to heart attack and stroke following ruptured atherosclerotic lesions.In this review,we will introduce the classical mechanisms and newly discovered pathways of platelets in hemostasis and thrombosis,including fibrinogen-independent platelet aggregation and thrombosis,and the plasma fibronectin-mediated‘protein wave’of hemostasis that precedes the classical first wave of hemostasis.Furthermore,we briefly discuss the roles of platelets in inflammation and atherosclerosis and the potential strategies to control atherothrombosis.
platelets,thrombosis and hemostasis,integrinαIIbβ3,fibrinogen,fibronectin
Platelets:evolution,generation, and clearance
Platelets are small anucleate cells in the circulation, with a diameter of approximately 1-2μm.They were firstidentified in 1874 by Osler[1];however,itwas the Italian physician,Bizzozero,who in 1881 established the role ofplatelets in hemostasis and thrombosis in his seminalpublications[2-3].By staining the granules of platelets(Wright stain),it was later demonstrated thatthese anucleate cells are generated from megakaryocytes in thebone marrow[4].In thefollowing century,intensive investigation of the cellular and molecular mechanisms of platelets and megakaryocytes[5-7]enabled the developmentofa number oftherapeutic agents forthe treatment and prevention of thrombotic disorders[8-11].
In non-mammalian vertebrates,he mostasis is mediated by nucleated cells called thrombocytes[12]. Anucleated platelets evolved only in mammals,and to the bestofourknowledge,there is no animalspecies that have been found thus far to have an intermediate state between thrombocytesand platelets.In addition,platelets are more effective than thrombocytes in forming occlusive thrombiunderarterialshearstress[13].Since platelets havean averagelifespan ofonly 8-10 daysin humansand approximately 5 days in mice,they are constantly being produced from megakaryocytes in the bone marrow. During maturation,megakaryocytesundergo DNAreplications withoutcelldivision(a process called endomitosis),leading to generation ofpolyploid megakaryocytes. The abundantgenomic DNA in the polyploid megakaryocytes enhances their ability to synthesize proteins and package them into specific plateletgranules[7,14].
The exactmechanism ofplateletrelease from megakaryocytes is stillunderdebate.In vitro studies demonstrated that platelet formation begins at one pole of megakaryocytes,then the whole cell is disintegrated, resulting in the generation ofnumerous proplatelets[15]. However,a recentintravitalmicroscopy study revealed thatmegakaryocytes extend long protrusions into bone marrow sinusoids and release proplatelets from the tip of the protrusions under shear stress,suggesting that platelet generation in vivo is drastically differentfrom in vitro cell culture conditions[16].Proplatelets then undergo further division to generate mature platelets in vivo[17].In addition to bone marrow,new discoveries suggest that megakaryocytes can also mature in the lung and shed platelets into the pulmonary vasculature[18-19].Interestingly,a recent study suggested that platelets are capable of celldivision and progeny generation even withouta nucleus[20],although more evidence is required to confirm this finding.
The process and mechanism ofplateletclearance is also notwellunderstood,butitisassumed thatthisoccurs in the reticuloendothelialsystem by macrophages.Aged platelets may express more phosphatidylserine(PS), which may attractmacrophages for clearance[21-23]. During red blood cellclearance,oldercells may induce more autoantibody binding[24];however,whether this occurs in aged platelets and whether the Fc portion of the autoantibody interacts with Fc receptors on macrophages leading to phagocytosis stillremains to be determined.A more recentstudy demonstrated thatantibody opsonization can activate platelets,leading to platelet desialylation[25,26],amechanism also involved in clearance ofchilled platelets[27-29].These desialylated platelets can then be destroyed in the liver via hepatocyte Ashwell-Morellreceptors[21,25].Whetherthis novelplateletclearance pathway[25,30]plays a role in the clearance of aged plateletshas yetto be investigated.
Platelets in hemostasis and thrombosis
Hemostasis is a criticalphysiologicalprocess to stop bleeding.Plateletaccumulation at the site of injury is considered the firstwave ofhemostasis and the second wave of hemostasis is mediated by the blood coagulation pathway[31].Platelets play a centralrole in a series of sequential events during the platelet accumulation (i.e.platelet adhesion,activation,and aggregation) and are also actively involved in cell-based thrombin generation,which markedly amplifies the blood coagulation cascade.Thus,platelets contribute to both the first and the second waves of hemostasis[6,7,32-35].
Platelet adhesion
Plateletadhesion to the injured vesselwallcan occur atboth low and high shearconditions butare mediated through distinctmechanisms.Low shearrates(20-200/s) are observed in the venous system whilsthigher shear rates are found in arteries(300-800/s)and stenotic vessels(800-10,000/s)[36-37].Following vascular injury, subendothelial matrix proteins such as collagens are exposed to the blood components.Plasma von Willebrand Factor(VWF),originated from endothelial cells,megakaryocytes,and platelets,can then anchor onto the collagen.The VWF receptoron platelets[glycoprotein(GP)Ibα],via interaction with the immobilized VWF,subsequently initiates platelettethering to the site ofinjury[38-39].This binding isessentialforplatelet adhesion at high shear(e.g.coronary arteries), although the GPIbα-VWF interaction may also contribute to plateletadhesion atlow shear[40,41].Following platelettethering,GPVIand integrinα2β1 may interact with collagen and deliver activation signals to platelets[38,42-43].Stable adhesion is subsequently mediated by binding of several integrins to their ligands on the vessel wall(e.g.integrinαIIbβ3 to fibrinogen/fibrin and fibronectin,α5β1 to fibronectin or collagen,and α2β1 to collagen,etc.)[6,42,44-46].Atlow shear(e.g.veins) the interactions between platelet integrins and their ligands(e.g.αIIbβ3 to fibrinogen/fibrin or fibronectin etc.)may directly initiate plateletadhesion[6,47].
In the last decade there have been significant advances in in vivo models of platelet adhesion and thrombus formation using intravital microscopy.VWF knockout(-/-)mice demonstrate decreased platelet adhesion[39,48],a phenotype that,interestingly,is notas severe as the GPIbα-/-mice,suggesting that GPIbαhas additionalhemostatic function[49].Mice lacking GPVI presentwith prolonged bleeding times[50]and similarly, mice deficientinα2 orβ1 integrins also have delayed thrombus formation,although these deficiencies are mild compared to GPIbα-/-mice[51].
Platelet activation and granule secretion
The primary interactions between platelet surface receptors(e.g.GPIbα,integrins)and their ligands (e.g.VWF,collagen,fibrinogen/fibrin,fibronectin, etc.),can lead to plateletactivation[7,38,52,53].In addition, following vascular injury,the coagulation system is activated[11,54-55],which generates the mostpotentplatelet activation factor,thrombin.Through cleavage of protease-activated receptors(PARs)and binding to GPIbα,thrombin activates platelets[56-59].
Plateletactivation exposes PS on the membrane surface thatdrives the cell-based thrombin generation[34,35]and facilitates further platelet activation[53,60-61]. Activation signals induced by thrombin,collagen,or ligands ofadhesion receptors with the addition ofshear stress,can lead to plateletgranule release.Plateletadhesion molecules,P-selectin[62],integrins,VWF,fibrinogen,fibronectin[63-64],vitronectin[65],multimerin[60], plateletfactor4,and approximately 300 otherproteins are contained within theα-granules[66].Dense granules release adenosine di-phosphate(ADP),which supports the second waveofplateletaggregation following integrin activation[67].The release of Ca2+from the endoplasmicreticulumand thedensegranules via the Ca2+sensor, stromalinteraction molecule(STIM)1,and the Ca2+channel,Orai,isalso a significantcontributorto platelet activation[68-69].There are many positive feedback loops during plateletactivation/granule release.Notably, ADP,likely via interaction with its receptors on platelets,initiatescell-based thrombin generation and further plateletactivation/granule release[61].These secretion eventsactassecondary messengersand,in combination with thegeneration ofthromboxane(Tx)A2and reactive oxygen species,amplify the activation process and integrinαIIbβ3 inside-outsignaling,which in turn recruits more platelets foraggregation[70-74].
Platelet aggregation:fibrinogen-dependent and-independent aggregation
Following plateletactivation,integrinαIIbβ3 binds fibrinogen and otherligands(i.e.fibrinogen-dependent and-independent pathways[31,39,61,75-76]),which leads to platelet aggregation.It is notable that following the engagementofligands,integrinαIIbβ3 can deliveroutside-in signals,which further enhance platelet activation,cytoskeleton rearrangement,and granule secretion.These signal events facilitate hemostatic plug and thrombus formation.
For more than half a century,fibrinogen was considered required forplateletaggregation[61].Through interaction withαIIbβ3 via itsγchain C-terminus, fibrinogen bridges adjacent activated platelets[22,77]. However,data from Ni etal.demonstrated thatthrombus formation still occurred in fibrinogen-/-mice and in VWF and fibrinogen double knock-out(DKO)mice[39], indicating that fibrinogen was not indispensable for this process.Further studies demonstrated that DKO platelet aggregation occurred in vitro in platelet-rich plasma and gel-filtered platelets withoutanti-coagulant treatment(i.e.in a more physiologicalcondition compared to anti-coagulated blood used in clinic and research).In contrast,integrinβ3-/-mice exhibit no significant platelet aggregation,which indicates an essential role forαIIbβ3 in platelet aggregation and suggests the existence of other unidentifiedαIIbβ3 ligand(s)[61].
In VWF-fibrinogen DKO mice,fibrinogen-/-mice,and fibrinogen C-terminalγchain mutantmice[77],aswellas in afibrinogenemic patients[22,78],plateletfibronectin(an αIIbβ3 ligand)contentwas increased 3-5 fold due to enhanced internalization of plasma fibronectin(p Fn) by integrinαIIbβ3.Conditional p Fn-/-mice have impaired thrombus growth atarterialshear[79],implying thatfibronectin may be a compensatoryαIIbβ3 ligand thatsupports plateletaggregation.Unexpectedly,however,further depletion of pFn in VWF-fibrinogen DKO mice enhanced,instead ofabolishing,plateletaggregation[31,75].These results suggestthatp Fn can switch between supporting and inhibiting plateletaggregation, depending on the presence offibrinogen/fibrin[31,79].
AnotherαIIbβ3 ligand,vitronectin,plays a dualrole; aggregation is enhanced by granule-released vitronectin butis inhibited by plasma vitronectin[65].These studies suggested thatlikely neitherfibronectin norvitronectin are theαIIbβ3 ligand thatmediates fibrinogen-independentplateletaggregation.Cadherin 6 containsa canonical"RGD"(arginine-glycine-aspartic acid)integrinbinding motifand increases its expression on platelets afterplateletactivation[76].Whilstcadherin 6 contributes to plateletaggregation,clearly otherplasma and platelet proteinsexistthatcan also mediate and facilitate fibrinogen-independentplateletaggregation[61].However,what they are and how they contribute to thrombosis and hemostasis in differentpathophysiologicalconditions requires further study.
Platelet-mediated cell-based thrombin generation and blood coagulation
In addition to their central roles in the platelet adhesion,activation,and aggregation(the first wave of hemostasis),platelets also contribute to coagulation pathway(the second wave of hemostasis).The blood coagulation cascade can be activated by either the extrinsic(tissue factor)or the intrinsic(contact activation)pathways in thrombosis[54,80].Thrombin,a vital product of the coagulation cascade,converts fibrinogen to fibrin,the end product of the coagulation cascade.
Besides these two classicalcoagulation pathways,the exposure of PS on platelets,following platelet activation,markedly potentiates thrombin generation by inducing a negatively charged surface that harbors the coagulation factors[34].Interestingly,in a study of platelet aggregation in fibrinogen and VWF DKO mice, Yang et al.found that ADP can induce thrombin generation that is required for platelet aggregation in the DKO mice[61].Recently,GPVI was also identified as a novel fibrin receptor involved in potentiating thrombin generation[81].Thrombin initiates robustdownstream signaling,through PAR1,PAR4[82]and GPIbα,leading to plateletactivation and further PS exposure,a positive feedback loop forthrombin generation and blood coagulation[57,83-84].Thus,there are many interactions between the firstwave(plateletaccumulation)and the second wave(blood coagulation)of hemostasis,which synergistically contribute to the arrestof bleeding.
Platelets and the"protein wave"of hemostasis:new discoveries
One of the mostrecentstudies revealed a novelconcept of a′protein wave′of hemostasis,where pFn deposition on the injured vesselwalloccurs priorto platelet accumulation(the first wave of hemostasis)and contributes to hemostasis[31,85].In mice lacking fibrinogen,further depletion of pFn markedly increased the mortality rate due to uncontrolled bleeding.Increased bleeding time was also observed in pFn conditional-/-mice,treated with heparin and other anti-coagulants, suggesting thatpFn is importantfor hemostasis in both genetic and drug-induced deficiencies ofblood coagulation.We observed that the p Fn deposition onto the injured vessel wallcan occur independently of fibrinogen,VWF,β3 integrin,and platelets.Itseems thatthe pFn-collagen interaction may play an importantrole in this process[31,85].pFn,likely via the covalentbinding to fibrin,increases the diameter of fibrin fibers and enhances the mechanical strength of the clot and this mechanism likely contributes to the pFn deposition onto the injured vessel walls of normal individuals where fibrin exists.Interestingly,in the absence of fibrin(a product of fibrinogen),pFn switches its function from promoting to inhibiting platelet aggregation. As fibrin is mainly formed atthe bottom of the hemostatic plug close to the vessel wall,pFn may support hemostasis at the base of the thrombi(likely through the formation of a pFn-fibrin complex)and switch to inhibiting excessive thrombus growth at the apical surface ofthrombi.Through this mechanism,pFn servesto control bleeding,while preventing excessive thrombus growth and vessel occlusion.Further investigation of the interaction between platelets and circulating/deposited pFn may reveal novel therapeutic targets for thrombotic disorders,as well as usage of pFn for transfusion to control bleeding disorders,particularly those patients in association with anti-coagulant therapy.Itwould also be interesting to investigate whether the markedly increased platelet fibronectin content in fibrinogen-/-mice and afibrinogenemic patients can be released onto the injured vessel wall and contribute to the protein wave of hemostasis(Fig.1).
Conclusion and future directions
Theprimary physiologicalfunction ofplateletsisto stop bleeding upon vascularinjury.Platelets,via theircontributionsto the"protein wave"and to theclassicalfirstand second waves ofhemostasis,play key roles in the arrestof bleeding[31].Thrombocytopenias caused by eithergenetic deficiencies[86]or autoimmune[87-89]and alloimmune responses lead to bleeding disorders[21,32-33,90-96].However, the same plateletaccumulation and coagulation may lead to thrombosis.Thrombotic eventsoccuratthesiteofa ruptured atheroscleroticplaqueand can resultin heartattack or stroke,the leading causes of mortality and morbidity worldwide.
In addition to thrombosis,the late stage of atherothrombosis,recent studies demonstrated that platelets are actively involved in the initiation of atherosclerosis[97,98].Plateletsaresensitive to environmentalchanges, such asfood products[99-101],lipids[102],and advanced glycation end products in diabetes[103-104],which may affect atherosclerosis.Furthermore,aswedemonstrated,platelets can respond to fibrinogen level changes.Through interaction with integrinαIIbβ3,platelets can use their residualmRNA to de novo synthesize P-selectin and otherproteins[62,105],which may also affectinflammation and directly orindirectly affectatherosclerosis and the stability of atherosclerotic plugs[106-108].
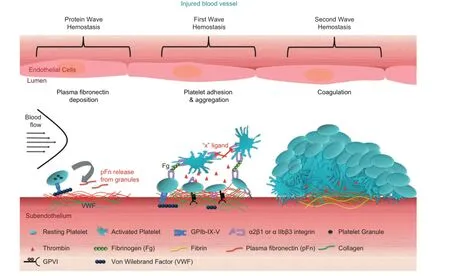
Fig.1 At the site of vascular injury plasma fibronectin deposition occurs even before platelets adhere.Platelets may release their internalized plasma fibronectin from intracellular granules.Platelet receptors then bind physiological ligands,such as VWF and collagen,activating integrinαIIbβ3 and resulting in fibrinogen binding and subsequentplateletaggregation.Thrombin is generated on the negatively charged plateletsurface and further activates platelets and contributes to the coagulation cascade.In a growing hemostatic plug/thrombus,the fibrin and fibronectin matrix is usually formed at the interface between the injured vessel wall and the platelet plug.
In this review,we described the conceptof fibrinogen/VWF-independent platelet aggregation,which was first noted in the early 2000s[61],and provided insightinto multiple and diverse interactions between platelets and their environment.Despite considerable efforts[62,76],the'x'ligand(s)of integrinαIIbβ3 has yet to be uncovered.These concepts are an example of how diverse platelets can be and demonstrate the need for further investigation into platelet interactions. Furthermore,whilst platelets play a pivotal role in hemostasis and thrombosis,they are also versatile cells and are involved in multiple functions,including inflammation,immune responses,lymphatic vessel development,angiogenesis,tumor metastasis,as well as atherosclerosis[106-109].Further elucidations of platelet versatilities willprovide insights into development of new methods to control not only thrombosis and hemostasis butalso inflammation,cancer,and immunologicaldisorders.
Acknowledgement
This work was supported in part by Canadian Institutes of Health Research(MOP 119540),National Natural Science Foundation of China-Canadian Institutes of Health Research(China-Canada Joint Health Research Initiative Program),Heartand Stroke Foundation of Canada(Ontario).This work was also supported by equipment Funds from St.Michael’s Hospital,Canadian Blood Services,and Canada Foundation for Innovation. Naadiya Carrim is a recipientof a Postdoctoral Fellowship from Canadian Blood Servicesand Health Canada. Yiming Wang is a recipientof a Ph.D.Graduate Student Fellowship from Canadian Blood Services and Meredith&Malcolm Silver Scholarship in Cardiovascular Studies,University of Toronto.Yan Hou is a recipient of a State Scholarship Fund from the China Scholarship Council.
[1]Osler W.An accountofcertain organisms occurring in the liquor sanguinis[J].Proc R Soc Lond,1874;391-398.
[2]Bizzozero G.Su di un nuovo elemento morfologico del sangue dei mammiferi e della sua importanza nella trombosie nella coagulazione[J].L’Osservatore,1881;785-787.
[3]Bizzozero G.Uber einen neuen Formbestandteil des Blutes und dessen Rolle bei der Thrombose und Blutgrinnung[J].Virchows Archiv,1882;261-332.
[4]Wright J.The origin and nature of blood platelets[J]. Boston Med Surg J,1906;643-645.
[5]Ruggeri ZM.Platelets in atherothrombosis[J].Nat Med, 2002,(8):1227-1234.
[6]Ni H,Freedman J.Platelets in hemostasis and thrombosis: role of integrins and their ligands[J].Transfus Apher Sci, 2003,(28):257-264.
[7]Wang Y,Andrews M,Yang Y,etal.Platelets in thrombosis an d hemostasis:old topic with new mechanisms[J]. Cardiovasc HematolDisord Drug Targets,2012,(12):126-132.
[8]Coller BS.Historical perspective and future directions in plateletresearch[J].J Thromb Haemost,2011,(9 Suppl1): 374-395.
[9]Jackson SP,Schoenwaelder SM.Antiplatelet therapy:in search of the'magic bullet'[J].Nat Rev Drug Discov, 2003;(2),775-789.
[10]Gachet C.Antiplatelet drugs:which targets for which treatments?[J].J Thromb Haemost,2015,(13):S313-322.
[11]Mack man N.Triggers,targ ets and treatments fo r thrombosis[J].Nature,2008,(451):914-918.
[12]Michelson AD.Platelets,2013;(3rd edition).
[13]Schmaier AA,Stalker TJ,Runge JJ,et al.Occlusive thrombi arise in mammals but not birds in response to arterialinjury:evolutionary insightinto human cardiovascular disease[J].Blood,2011,(118):3661-3669.
[14]Machlus KR,Italiano JE,Jr.The incredible journey:From megakaryocyte development to platelet formation[J]. J Cell Biol,2013,(201):785-796.
[15]Italiano JE,Jr.,Lecine P,Shivdasani RA,etal.Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes[J]. J Cell Biol,1999,(147):1299-1312.
[16]JuntT,Schulze H,Chen Z,etal.Dynamic visualization of thro mbop o iesis with in bone marro w[J].Scien ce, 2007,(317):1767-1770.
[17]Thon JN,Montalvo A,Patel-Hett S,et al.Cytoskeletal mech anics of pro platelet maturation and platelet release[J].J Cell Biol,2010,(191):861-874.
[18]Fuentes R,Wang Y,Hirsch J,et al.Infusion of mature megakaryocytes into mice yields functional platelets[J]. J Clin Invest,2010,(120):3917-3922.
[19]Wang Y,Hayes V,Jarocha D,etal.Comparative analysis of human ex vivo-generated platelets vs megakaryocytegenerated platelets in mice:a cautionary tale[J].Blood, 2015,(125):3627-3636.
[20]Schwertz H,Koster S,Kahr WH,etal.Anucleate platelets generate progeny[J].Blood,2010,(115):3801-3809.
[21]Li J,van der Wal DE,Zhu L,etal.Fc-independentphagocytosis:implications for IVIG and other therapies in immune-mediated thrombocytopenia[J].Cardiovasc Hematol Disord Drug Targets,2013,(13):50-58.
[22]Xu X,Wu J,Zhai Z,etal.A novelfibrinogen Bbeta chain frameshift mutation in a patient with severe congenital h ypo fibrin ogenaemia[J].Thromb Ha emo st,20 06, (95):931-935.
[23]Rand ML,Wang H,Bang KW,etal.Procoagulantsurface exposure and apoptosis in rabbit platelets:association with shortened survival and steady-state senescence[J]. J Thromb Haemost,2004,(2):651-659.
[24]Webster ML,Zhu G,Li Y,etal.Fc-independentphagocytosis:implications for intravenous IgG therapy in immune thrombocytopenia[J].Cardiovasc Hematol Disord Drug Targets,2008,(8):278-282.
[25]Li J,van der Wal DE,Zhu G,et al.Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia[J].Nat Commun,2015,(6):7737-7753.
[26]van Der Wal D,Zhu G,Li J,etal.Desialylation:A Novel Platelet Clearance Mechanism and a Potential New Therapeutic Target in Anti-GPIb Antibody Mediated Thrombocy top enia[J].54th ASH Annual Meetin g, 2012,(Session:311.Disorders of Platelet Number or Function:Biology of antibody mediated platelet destruction:HIT,NAIT and mechanisms of ivig).
[27]Sorensen AL,Rumjantseva V,Nayeb-Hashemi S,et al. Role of sialic acid for platelet life span:exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptorexpressing liver macrophages and hepatocytes[J].Blood, 2009,(114):1645-1654.
[28]Rumjantseva V,Hoffmeister KM.Novel and unexpected clearance mechanisms for cold platelets[J].Transfus Apher Sci,2010,(42):63-70.
[29]Jansen AJ,Josefsso n EC,Ru mjan tsev a V,et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase-mediated cleavage in mice[J].Blood,2012,(119):1263-1273.
[30]Jansen AJ,Peng J,Zhao HG,et al.Sialidase inhibition to increase platelet counts:A new treatment option for thrombocytopenia[J].Am J Hematol,2015,(90):E94-95.
[31]Wang Y,Reheman A,Spring CM,et al.Plasma fibronectin supports hemostasis and regulates thrombosis[J].J Clin Invest,2014,(124):4281-4293.
[32]Yougbare I,Lang S,Yang H,et al.Maternal anti-platelet beta3 integrins impair angiogenesis and cause intracranial hemorrhage[J].J Clin Invest,2015,(125):1545-1556.
[33]Vadasz B,Chen P,Yougbare I,etal.Platelets and platelet alloantigens:Lessons from human patients and animal models of fetaland neonatal alloimmune thrombocytopenia[J].Gene and Diseases,2015,(2):173-185.
[34]Roberts HR,Hoffman M,Monroe DM.A cell-based modelof thrombin generation[J].Semin Thromb Hemost, 2006,(32 Suppl1):32-38.
[35]Monroe DM,Hoffman M,Roberts HR.Platelets and thrombin generation[J].Arterioscler Thromb Vasc Biol, 2002,(22):1381-1389.
[36]Dopheide SM,Maxwell MJ,Jackson SP.Shear-dependent tether formation during platelet translocation on von Willebrand factor[J].Blood,2002,(99):159-167.
[37]Goto S,Salomon DR,Ikeda Y,et al.Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets[J]. J Biol Chem,1995,(270):23352-23361.
[38]Ruggeri ZM.Mechanisms initiating platelet thrombus formation[J].Thromb Haemost,1997,(78):611-616.
[39]Ni H,Denis CV,Subbarao S,et al.Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen[J].J Clin Invest, 2000,(106):385-392.
[40]Andre P,Denis CV,Ware J,et al.Platelets adhere to and translocate on von Willebran d facto r p resented by endo th eliu m in stimulated veins[J].Blood,20 00, (96):3322-3328.
[41]Lei X,Reheman A,Hou Y,et al.Anfibatide,a novel GPIb complex antagonist,inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis[J].Thromb Haemost,2014,(111):279-289.
[42]Savage B,Almus-Jacobs F,Ruggeri ZM.Specific synergy of multiple substrate-receptorinteractions in plateletthrombus formation under flow[J].Cell,1998,(94):657-666.
[43]Nieswan dt B,Brakebu sch C,Berg meier W,et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen[J].EMBO J, 2001,(20):2120-2130.
[44]Wilkins JA,Li A,Ni H,et al.Control of beta1 integrin function.Localization of stimulatory epitopes[J].J Biol Chem,1996,(271):3046-3051.
[45]Ni H,Li A,Simonsen N,et al.Integrin activation by dithiothreitol or Mn2+induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain[J].J Biol Chem,1998, (273):7981-7987.
[46]Ni H,Wilkins JA.Localisation of a noveladhesion blocking epitope on the human beta 1 integrin chain[J].Cell Adhes Commun,1998,(5):257-271.
[47]Bledzka K,Smyth SS,Plow EF.Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target[J].Circ Res,2013,(112):1189-1200.
[48]Denis C,Methia N,Frenette PS,etal.A mouse modelof severe von Willebrand disease:defects in hemostasis and thrombosis[J].Proc Natl Acad Sci U S A,1998,(95):9524-9529.
[49]Bergmeier W,Piffath CL,Goerge T,etal.The role ofplatelet adhesion receptor GPIbalpha far exceeds that of its main ligand,von Willebrand factor,in arterial thrombosis[J].Proc Natl Acad Sci U S A,2006,(103): 16900-16905.
[50]Nieswandt B,Schulte V,Bergmeier W,et al.Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice[J].J Exp Med,2001,(193):459-469.
[51]Nuyttens BP,Thijs T,Deckmyn H,etal.Plateletadhesion to collagen[J].Thromb Res,2011,(127):S26-29.
[52]Bergmeier W,Hynes RO.Extracellular matrix proteins in hemostasis and thrombosis[J].Cold Spring Harb Perspect Biol,2012,(4).
[53]Berndt MC,Metharom P,Andrews RK.Primary haemostasis:newer insights[J].Haemophilia,2014,(20):15-22.
[54]Gui T,Reheman A,Ni H,et al.Abnormal hemostasis in a knock-in mouse carrying a variantof factor IX with impaired binding to collagen type IV[J].J Thromb Haemost, 2009,(7):1843-1851.
[55]Gui T,Reheman A,Funkhouser WK,et al.In vivo response to vascular injury in the absence of factor IX: examination in factor IX knockout mice[J].Thromb Res, 2007,(121):225-234.
[56]Sambrano GR,Weiss EJ,Zheng YW,et al.Role of thrombin signalling in platelets in haemostasis and thrombosis[J].Nature,2001,(413):74-78.
[57]Celikel R,McClintock RA,Roberts JR,etal.Modulation of alpha-thrombin function by distinct interactions with p latelet g ly copro tein Ib alpha[J].Science,2003, (301):218-221.
[58]Ni H,Ramakrishnan V,Ruggeri ZM,et al.Increased thrombogenesis and embolus formation in mice lacking glycoprotein V[J].Blood,2001,(98):368-373.
[59]Li C,Piran S,Chen P,et al.The maternal immune response to fetal platelet GPIbalpha causes frequent miscarriage in mice that can be prevented by intravenous Ig G and anti-FcRn therapies[J].J Clin Invest,2011, (121):4537-4547.
[60]Reheman A,Tasneem S,Ni H,et al.Mice with deleted multimerin 1 and alpha-synuclein genes have impaired platelet adhesion and impaired thrombus formation that is corrected by multimerin 1[J].Thromb Res,2010, (125):e177-183.
[61]Yang H,Reheman A,Chen P,et al.Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo[J].J Thromb Haemost,2006,(4):2230-2237.
[62]Yang H,Lang S,Zhai Z,et al.Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression[J].Blood,2009,(114):425-436.
[63]Ni H.Unveiling the new face of fibronectin in thrombosis and hemostasis[J].J Thromb Haemost,2006,(4):940-942.
[64]Wang Y,Ni H.Fibronectin:extra domain brings extra risk?[J].Blood,2015,(125):3043-3054.
[65]Reheman A,Gross P,Yang H,etal.Vitronectin stabilizes thrombiand vesselocclusion butplays a dualrole in platelet aggregation[J].J Thromb Haemost,2005,(3):875-883.
[66]Rao AK.Inherited platelet function disorders:overview and disorders of granules,secretion,and signal trans d uction[J].Hematol On co l Clin No rth Am,20 1 3, (27):585-611.
[67]Johnston-Cox HA,Yang D,Ravid K.Physiological implications of adenosine receptor-mediated platelet aggregation[J].J Cell Physiol,2011,(226):46-51.
[68]Bergmeier W,Stefanini L.Novel molecules in calcium signaling in platelets[J].J Thromb Haemost,2009; (7 Suppl 1):187-190.
[69]Varga-Szabo D,Braun A,Nieswandt B.STIM and Oraiin platelet function[J].Cell Calcium,2011,(50):270-278.
[70]Jin J,Quinton TM,Zhang J,etal.Adenosine diphosphate (ADP)-induced thromboxane A(2)generation in human platelets requires coordinated signaling through integrin alp h a(IIb)b eta(3)an d ADP recep to rs[J].Blo o d, 2002,(99):193-198.
[71]Arthur JF,Gardiner EE,Kenny D,et al.Platelet receptor redox regulation[J].Platelets,2008,(19):1-8.
[72]Carrim N,Walsh TG,Consonni A,et al.Role of focal adhesion tyrosine kinases in GPVI-dependentplateletactivation and reactive oxygen species formation[J].PLoS One,2014,(9):e113679.
[73]Walsh TG,Berndt MC,Carrim N,etal.The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation[J].Redox Biol,2014,(13):178-186.
[74]Chen W,Thielmann I,Gupta S,etal.Orai1-induced storeoperated Ca(2+)entry enhances phospholipase activity and modulates canonicaltransientreceptor potentialchannel 6 function in murine platelets[J].J Thromb Haemost, 2014,(12):528-539.
[75]Reheman A,Yang H,Zhu G,et al.Plasma fibronectin depletion enhances platelet aggregation and thrombusformation in mice lacking fibrinogen and von Wille brand factor[J].Blood,2009,(113):1809-1817.
[76]Dunne E,Spring CM,Reheman A,etal.Cadherin 6 has a functional role in platelet aggregation and thrombus for mation[J].Arterioscler Thromb Vasc Biol,2012,(32): 1724-1731.
[77]Ni H,Papalia JM,Degen JL,et al.Control of thrombus embolization and fibronectin internalization by integrin alpha IIb beta 3 engagement of the fibrinogen gamma chain[J].Blood,2003,(102):3609-3614.
[78]Zhai Z,Wu J,Xu X,et al.Fibrinogen controls human platelet fibronectin internalization and cell-surface retention[J].J Thromb Haemost,2007,(5):1740-1746.
[79]Ni H,Yuen PS,Papalia JM,et al.Plasma fibronectin promotes thrombus growth and stability in injured art erioles[J].Proc Natl Aca d Sci U S A,2 00 3,(1 00): 2415-2419.
[80]Smith SA,Travers RJ,Morrissey JH.How it all starts: Initiation of the clotting cascade[J].Crit Rev Biochem Mol Biol,2015,(50):1-11.
[81]Mammadova-Bach E,Ollivier V,Loyau S,et al.Platelet glycoprotein VIbinds to polymerized fibrin and promotes thrombin generation[J].Blood,2015,(126):683-691.
[82]Alberelli MA,De Candia E.Functional role of protease activated recepto rs in v ascular biology[J].Vascu l Pharmacol,2014,(62):72-81.
[83]Dumas JJ,Kumar R,Seehra J,et al.Crystal structure of the GpIbalpha-thrombin complex essential for platelet aggregation[J].Science,2003,(301):222-226.
[84]Lechtenberg BC,Freund SM,Huntington JA.GpIbalpha interacts exclusively with exosite II of thrombin[J]. J Mol Biol,2014,(426):881-893.
[85]Wang Y,Carrim N,Ni H.Fibronectin orchestrates thrombosis and hemostasis[J].Oncotarget Journal,2015; (6):19350-19351.
[86]Nurden AT,Freson K,Seligsohn U.Inherited platelet disorders[J].Haemophilia,2012,(18):154-160.
[87]Peng J,Ma SH,Liu J,et al.Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia:a multicenter cohort study[J].J Thromb Haemost,2014,(12):497-504.
[88]Webster ML,Sayeh E,Crow M,etal.Relative efficacy of intravenous immunoglobulin G in ameliorating thrombocy topenia induced by antiplatelet GPIIb IIIa versus GPIbalpha antibodies[J].Blood,2006,(108):943-946.
[89]Zeng Q,Zhu L,Tao L,et al.Relative efficacy of steroid therapy in immune thrombocytopenia mediated by antiplatelet GPIIbIIIa versus GPIbalpha antibodies[J].Am J Hematol,2012,(87):206-208.
[90]Semple JW,Italiano JE,Jr.,Freedman J.Platelets and the immune continuum[J].Nat Rev Immunol,2011,(11):264-274.
[91]Chen P,Li C,Lang S,et al.Animal model of fetal and neonatal immune thrombocytopenia:role of neonatal Fc receptor in the pathogenesis and therapy[J].Blood, 2010,(116):3660-3668.
[92]Ni H,Chen P,Spring CM,etal.A novelmurine modelof fetal and neonatal alloimmune thrombocytopenia:re sponse to intravenous Ig G therapy[J].Blood,2006, (107):2976-2983.
[93]Sachs UJ.Fetal/neonatalalloimmune thrombocytopenia[J]. Thromb Res,2013;(131 Suppl1):S42-46.
[94]Liang Y,Qiu H,Glinka Y,etal.Immunity againsta therapeutic xenoprotein/Fc construct delivered by gene transfer is reduced through binding to the inhibitory receptor FcgammaRIIb[J].J Gene Med,2011,(13):470-477.
[95]Tiller H,Killie MK,Chen P,et al.Toward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine model[J].Transfusion,2012,(52):1446-1457.
[96]Tiller H,Killie MK,Husebekk A,etal.Plateletantibodies and fetalgrowth:maternal antibodies againstfetal platelet antigen 1a are strongly associated with reduced birthweight in boys[J].Acta Obstet Gynecol Scand,2012, (91):79-86.
[97]Murphy AJ,Bijl N,Yvan-Charvet L,et al.Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis[J].Nat Med,2013, (19):586-594.
[98]Lindemann S,Kramer B,Seizer P,et al.Platelets,inflammatio n and atherosclerosis[J].J Th romb Haemost, 2007;(5 Suppl 1):203-211.
[99]Yang Y,Andrews MC,Hu Y,et al.Anthocyanin extract from black rice significantly ameliorates platelet hyperactivity and hypertriglyceridemia in dyslipidemic rats induced by high fat diets[J].J Agric Food Chem,2011, (59):6759-6764.
[100]Yang Y,Shi Z,Reheman A,etal.Plantfood delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis:novel protective roles against cardiovascular diseases[J].PLoS One,2012,(7):e37323.
[101]Song F,Zhu Y,Shi Z,et al.Plant food anthocyanins inhibit platelet granule secretion in hypercholestero laemia:Involving the sig nalling path way of PI3 KAkt[J].Thromb Haemost,2014,(112):981-991.
[102]Podrez EA,Byzova TV,Febbraio M,et al.Platelet CD36 links hyperlipidemia,oxidant stress and a prothrombotic phenotype[J].Nat Med,2007,(13):1086-1095.
[103]Ni H.The platelet"sugar high"in diabetes[J].Blood, 2012,(119):5949-5951.
[104]Zhu W,Li W,Silverstein RL.Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36[J].Blood,2012; (119):6136-6144.
[105]Weyrich AS,Schwertz H,Kraiss LW,etal.Protein synthesis by platelets:historical and new perspectives[J]. J Thromb Haemost,2009,(7):241-246.
[106]Xu X,Carrim N,Lavalle C,et al.Platelets Are Versatile Cells:Emerging new roles in inflammation,immune response,angiogenesis,and atherosclerosis[J].Critical Reviews in Clinical Laboratory Sciences,In press.
[107]Li C,Li J,Li Y,etal.Crosstalk between Platelets and the Immune System:Old Systems with New Discoveries[J]. Adv Hematol,2012,(2012):384685-384699.
[108]Nording HM,SeizerP,LangerHF.Plateletsin inflammation and atherogenesis[J].FrontImmunol,2015,(6):98-109.
[109]Bertozzi CC,Schmaier AA,Mericko P,etal.Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling[J].Blood,2010,(116):661-670.
△These authors contributed equally to this work.
✉Corresponding author:Heyu Ni,MD,PhD Professor,Department of Laboratory Medicine and Pathobiology,Department of Medicine,and Departmentof Physiology,University of Toronto;Scientistat Canadian Blood Services;Platform Director for Hematology,Cancer,and Immunological Diseases,St.Michael's Hospital,Room 420,LKSKI-
Keenan Research Centre,209 Victoria Street,Toronto,Ontario,M5B 1W8,Canada.Tel:416-847-1738;,E-mail:nih@smh.ca.
Received 26 August2015,Accepted 12 October 2015,Epub 30 October 2015
R331.1+43 Document code:A
The authors reported no conflict of interests
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20150121
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Retinolbinding protein 4 correlates with and is an early predictor of carotid atherosclerosis in type 2 diabetes mellitus patients
- CC-chemokine receptor 7 and its ligand CCL19 promote mitralvalve interstitialcellmigration and repair
- Effectofburden and origin sites ofpremature ventricular contractions on left ventricular function by 7-day Holter monitor
- Prevention ofatrialfibrillation with renin-angiotensin system inhibitors on essentialhypertensive patients:a meta-analysis of randomized controlled trials
- Epirubicin-gold nanoparticles suppress hepatocellular carcinoma xenograft growth in nude mice
- A susceptibility locus rs7099208 is associated with non-obstructive azoospermia via reduction in the expression of FAM160B1
