Prevention ofatrialfibrillation with renin-angiotensin system inhibitors on essentialhypertensive patients:a meta-analysis of randomized controlled trials
Di Zhao,Ze-Mu Wang,Lian-Sheng Wang
Department of Cardiology,The First Affiliated Hospital of Nanjing Medical University,Nanjing,Jiangsu 210029,China.
Prevention ofatrialfibrillation with renin-angiotensin system inhibitors on essentialhypertensive patients:a meta-analysis of randomized controlled trials
Di Zhao,Ze-Mu Wang,Lian-Sheng Wang✉
Department of Cardiology,The First Affiliated Hospital of Nanjing Medical University,Nanjing,Jiangsu 210029,China.
We aimed to investigate the effectiveness and safety of angiotensin-converting enzyme inhibitors(ACEIs) or angiotensin II receptor blockers(ARBs)on preventing atrial fibrillation in essential hypertensive patients. Systematic literature retrieval was carried out to obtain randomized controlled trials on the effects of ACEI/ ARBs on essential hypertensive patients before December,2013.Data extraction and quality evaluation were performed.Meta-analysis was performed by Review Manager 5.2.3.Ten high quality studies(11 articles) with a total of 42,892 patients(20,491 patients in the ACEI/ARBs group and 22,401 patients in theβ-blocker or the calcium antagonist group)met the inclusion criteria and were included in the meta-analysis.The results showed that ACEI/ARBs reduced the incidence of atrial fibrillation(AF)recurrence compared to calcium antagonists(RR=0.48;95%CI,0.40-0.58;P<0.00001)orβ-blockers(RR=0.39;95%CI,0.20-0.74; P=0.005)in long-term follow-up,respectively.Furthermore,ACEI/ARBs reduced the incidence of congestive heartfailure(RR=0.86;95%CI,0.77-0.96;P=0.007).However,no significanteffects were observed on the incidence of new AF,cardiac death,myocardial infarction,and stroke.Our results suggest that ACEI/ ARBs may reduce the incidence of AF recurrence and congestive heart failure,with fewer serious adverse effects.
angiotensin-converting enzyme inhibitors,angiotensin II receptor blockers,hypertension,atrial fibrillation,meta-analysis
Introduction
Hypertension is one of the mostprevalentand powerful contributors to cardiovasculardiseases,especially stroke, the leading cause ofdeath allovertheworld[1].Atrialfibrillation(AF),a common complication ofhypertension,is associated with an increased risk ofmorbidity and mortality[2].Although medication for hypertention has been well developed,alarge numberofwell-controlled hypertensive patients stillsufferfrom AF.Therefore,finding a more effective way ofpreventing AF isimportantforimproving the prognosis of patients with essentialhypertension.
Angiotensin-converting enzyme inhibitors(ACEIs) and angiotensin IIreceptorblockers(ARBs)are two commonly used antihypertensives,which preventcardiac structuralremodeling and electricalremodeling.AF activates the renin-angiotensin system(RAS),which in turn promotes atrialfibrosis,atrialelectrophysiologicalandstructuralremodeling,and subsequently facilitates the recurrence of AF[3].Thus,ACEI/ARBsmay theoretically attenuate deleterious cardiac remodeling and reduce the recurrence of AF[4].However,the results ofdifferentstudies are controversial.Jibrini etal.[5]found thatpatients with hypertension benefited from treatmentof ACEI/ ARBs on reducing the relative risk of AF by 23%,while the othertwo groups found no benefits[6-7].
To furtherinvestigate the efficacy and safety of RAS inhibitorsin preventing AF,we performed ameta-analysis ofrandomized controlled trials.Ourresults may provide more powerfulevidence forclinicians.
Methods
Literature search
Following the methodologicalguidelinesin Cochrane Reviewer's Handbook(Version 5.1.0),3 databasesincluding PubMed(1966-2013.12),Embase(1974-2013.12) and the Cochrane Library(Issue 12,2013)were searched with the following words:"Angiotensin-Converting Enzyme Inhibitors"[Mesh/Emtree],Angiotensin Converting Enzyme Inhibitor*,"Angiotensin IIType 1 Receptor Blockers"[Mesh/Emtree],"angiotensin receptor",ACE,ACEI,ACE-I,ACEs,captopril,enalapril,fosinopril,lisinopril,perindopril,ramipril,quinapril, benazepril,cilazapril,trandolapril,spirapril,delapril, moexipril,zofenopril,imidapril,AT 2 receptorblock*, AT 2 receptorantagon*,ARB,ARBs,candesartan,eprosartan,irbesartan,losartan,olmesartan,telmisartan,valsartan,Hypertension,"Hypertension"[Mesh/Emtree],"Atrial Fibrillation"[Mesh/Emtree],and"AtrialFlutter"[Mesh/ Emtree].The process did notsetlimit.In addition,the references ofthe retrieved literature were also manually checked to filterpotentially eligible studies.Lastsearch reached December,2013.
Criteria for considering trials for this review
Inclusion criteria
Randomized control trials(RCTs)only,detailed information aboutrandom sequence generation,allocation concealmentand blinding were notconsidered.All patients entering the studies needed to meetthe following criteria with no restriction of age suffering from essentialhypertension,which was defined as systolic blood pressure(SBP)≥140 mmHg and/or diastolic blood pressure(DBP)≥90 mmHg.The patients should remain in sinus rhythm butwith atleastone electrocardiogram(ECG)-documented episode of symptomatic or paroxysmal AF during 6 months before randomization.Particularly,we also included trials in which patients suffered essential hypertension without AF. These patients were thus at risk of developing AF. The ACEI/ARB group received ACEIs or ARBs,and the controlgroup received placebo or positive drugs, such asβ-blockers and calcium antagonists.Primary endpoints:incidence of new AF or AF recurrence during follow-up.Secondary endpoints:cardiovascular events,including cardiac death,myocardialinfarction, cerebral infarction,congestive heart failure,and adverse effects(bradycardia,atrialflutter,intolerable and unproductive cough,peripheraledema and dizziness)during follow-up.
Exclusion criteria
Trials in the following categories were excluded, including non-randomized controlled trials,subjects who were not treated with ACEIor ARB,and trials with no mentioning of AF prevention.
Data extraction
According to previously defined data-extraction form,2 investigators(D-Z and Z-MW)independently read the titles,abstracts and fulltexts,using the following steps:(1)examining titles and abstracts to remove obviously irrelevantstudies,(2)retrieving the fulltexts of potentially relevant trials,(3)examining fulltexts for compliance of studies with eligibility criteria,and (4)making finaldecisions on study inclusion and proceeding to data collection.Baseline information of patients and detailed methods of study designs were extracted from included studies.Disagreement was solved by discussion with others(D-Z and Z-MW).
Quality evaluation
Evaluation of methodological quality was based on criteria described in Cochrane Reviewer's Handbook 5.1.0.It contains random sequence generation,allocation concealment,blinding,and incomplete outcome data.Each study was subjected to quality assessment by 2 investigators(D-Z and Z-MW).Forunclearinformation forstudy design ordata,investigatorcontacted the author by E-mail.
Statistical analysis
Differences were expressed as risk ratios(RRs)and odd ratio(ORs)with 95%confidence intervals (95%CIs)for dichotomous outcomes and standardized mean differences(SMDs)with 95%CIs for continuous outcomes.Heterogeneity across studies was tested by using the I2statistic,which is a quantitative measure of inconsistency across studies.Studies with an I2statisticof 25%-50%were considered as low heterogeneity, those with an I2statistic of50%-75%had moderate heterogeneity,and those with an I2statistic of>75%had a high degree of heterogeneity[8].An I2value>50%indicated significant heterogeneity.A fixed-effects model was used,and a random-effects modelwas used in the case ofsignificantheterogeneity(I2>50%)[9].We further conducted sensitivity analyses to explore possible explanations for heterogeneity on the overallpooled estimate and to examine influence ofvariousexclusion criteria on the overallpooled estimate.Differences were considered statistically significantat P<0.05.Allstatisticalanalyses were performed by Review Manager Software(Version 5.2.3,Cochrane Collaboration).
Results
Process for included trials
As shown in Fig.1,a totalof863 potentially relevant studies were identified and screened forretrieval.After reading titles and abstracts,433 studies were excluded due to duplications,reviews,case reports and animal experiments.Then,380 studies were excluded afterreading the abstracts in more detail.Among the remaining 50 studies,39 studies were excluded because they included non-hypertensive patients or did notreportinteresting outcomes.Finally,11 studies[10-20]were included in ourreview.AsthestudiesofJulius etal.[18]and Schmieder etal.[20]are the same trialin differenttime,we included them as one study.
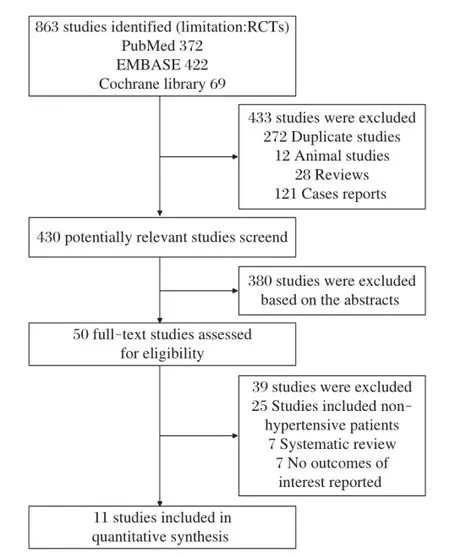
Fig.1 Flowchart of studies included in the meta-analysis.
Characteristics of included trials and quality evaluation
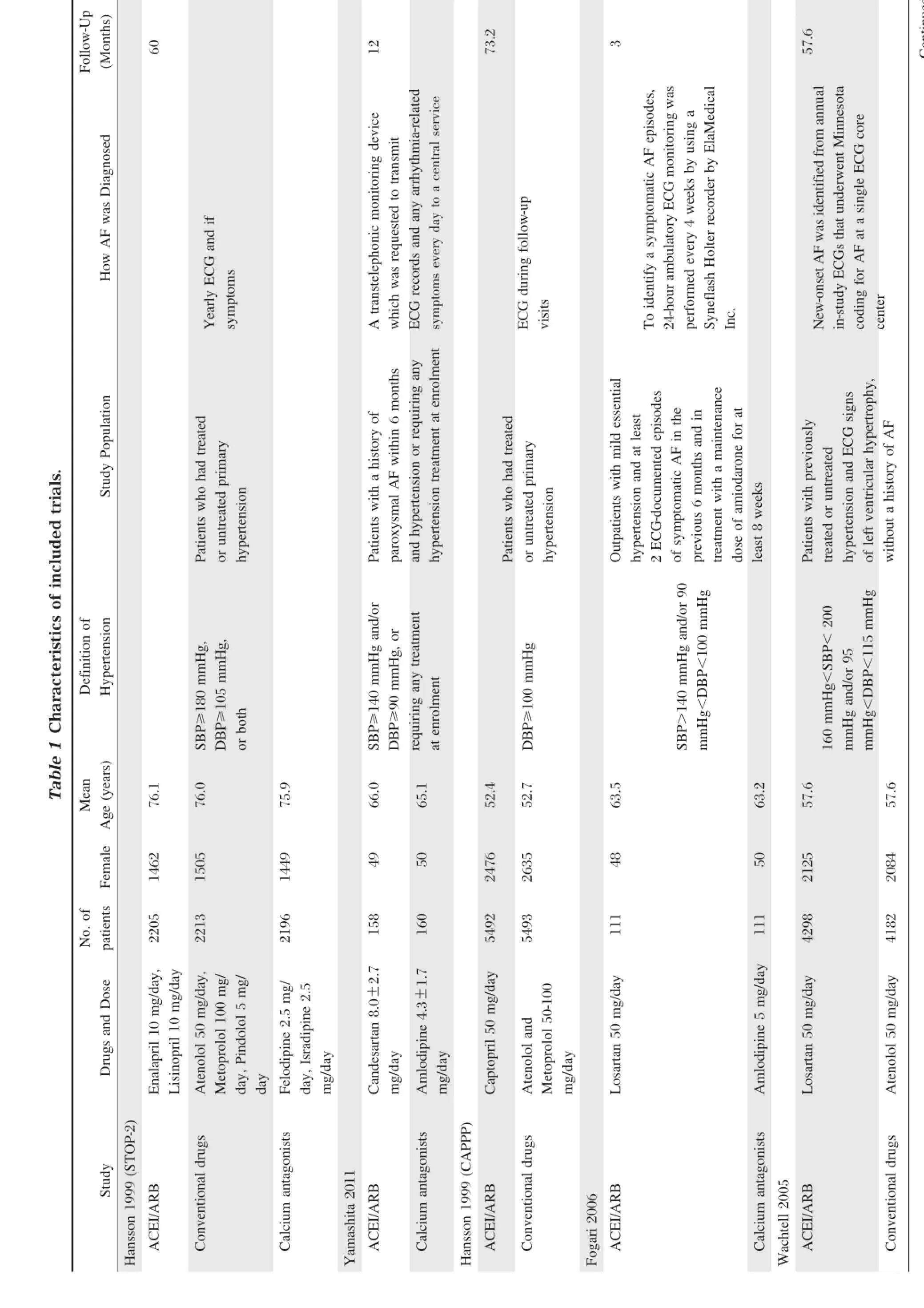
Main characteristics ofthe trials included in ourmetaanalysisareshown in Table1.Therewere 20,491 hypertensive patients in the ACEI/ARBs group,and 22,401 patients in theβ-blocker orcalcium antagonistgroup. Six studies[11-15,20]included outpatients with mild essential hypertension and atleastone ECG-documented episode of symptomatic or paroxysmal AF in the previous 6 monthsbeforerandomization.Fourstudies[10,17-19]included hypertensive patientswithouta history of AF.Seven studies[11-15,18-19]compared the efficiency between ACEI/ ARBsand calcium antagonists,and 4 studies[10,16-17,20]compared the efficiency between ACEI/ARBs andβ-blockers. Duration of follow-up varied from 3 months to 73.2 months.
Table2showsthatthe quality ofstudies in thismetaanalysis was good.Five studies[11-12,17-19]reported random sequence generation,which was from computerized randomization,and the restwere randomized controlled trials.Allocation concealmentin detailwas only reported in one study[18].Six studies[10-12,16,17,19]had open-label design.Six studies[10,11,13-16,18]used the double-blind method,1 study the single-blind method,and 2 studies[17,19]applied masked-endpointforevaluation.A total of428 patientswerelostto follow-up in 9 studies[11-17,19-20].
Meta-analysis results
Primary endpoints
As shown in Fig.2,ACEI/ARBs decreased the incidence of AF recurrence at3 months(RR=0.49;95%CI, 0.34-0.72;P=0.0003)and long-term follow-up (RR=0.47;95%CI,0.39-0.47;P<0.00001),and the tests for heterogeneity in those subgroups were I2=0%, P=0.89 and I2=0%,P=0.65,respectively.However, ACEI/ARBs did notchange the incidence ofnew AF in long-term follow-up(RR=0.86;95%CI,0.69-1.07; P=0.19),with a high heterogeneity(I2=81%,P=0.001).
We further performed sensitivity analyses to explore the stability ofourresults.Afterremovalof2 studies[12,16]with modestsample sizes(n≤150),we stillfound that ACEI/ARBs decreased the incidence of AF recurrence in long-term follow-up(RR=0.49;95%CI,0.40-0.59; P<0.00001)with low heterogeneity(I2=0%,P=0.48). Changing effectsize did notinfluence the pooled results substantially:AF recurrence at3 months(OR=0.45;95%CI,0.29-0.69;P=0.0003),AF recurrence in long-term follow-up(OR=0.34;95%CI,0.27-0.44; P<0.00001)and new AF in long-term follow-up (OR=0.86;95%CI,0.68-1.08;P=0.19),and the heterogeneity was(I2=0%,P=0.93),(I2=0%,P=0.80),and (I2=81%,P=0.001),respectively.
?
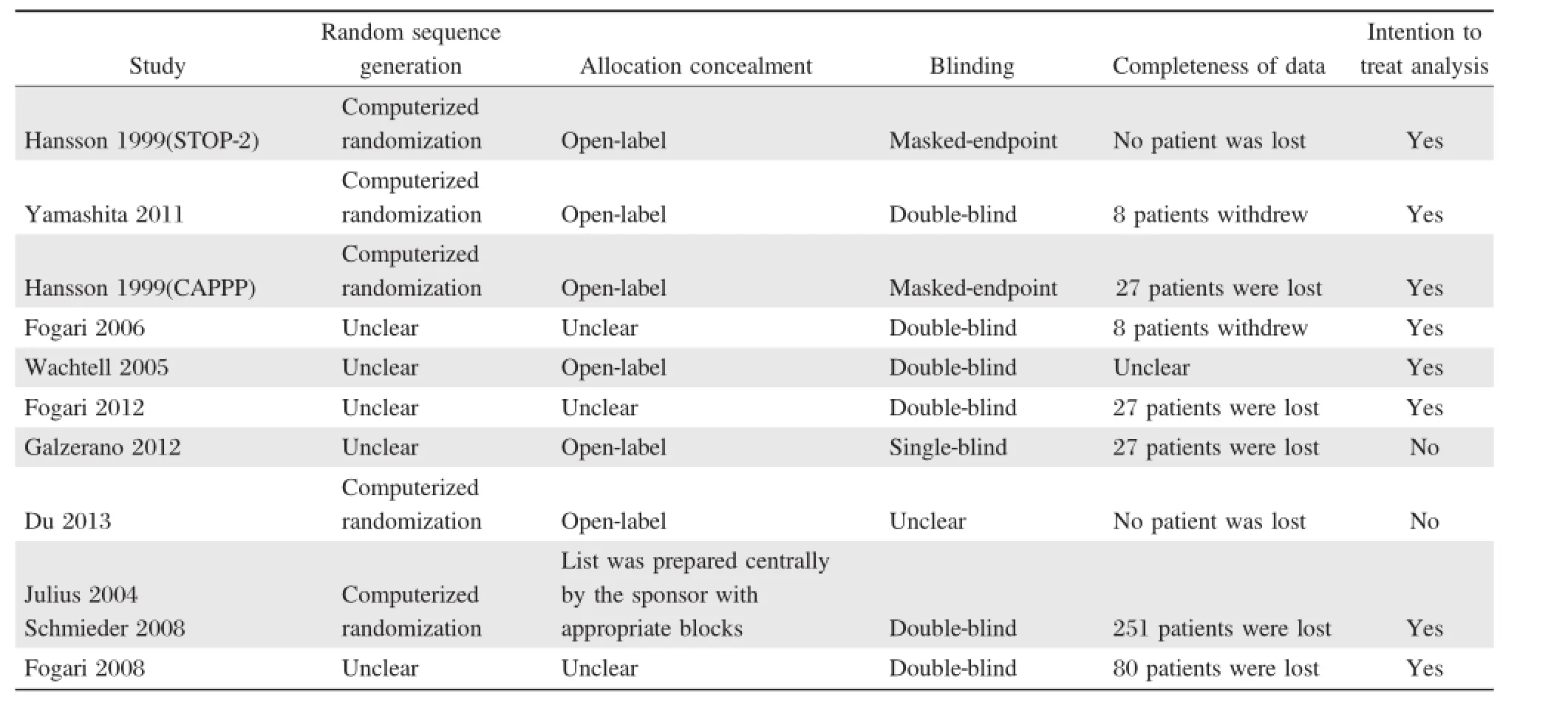
Table 2 Quality evaluation of the studies in this meta-analysis.
When compared to thedifferentcontrolgroups,the incidence of AF recurrence was lowerin patients receiving ACEI/ARBs than in those receiving calcium antagonists in long-term follow-up(RR=0.48;95%CI,0.40-0.58; P<0.00001;Fig.3)with low heterogeneity(I2=0%, P=0.57).However,ACEI/ARBs did notreduce new AF in long-term follow-up(RR=0.96;95%CI,0.74-1.24; P=0.75;Fig.3)with high heterogeneity(I2=76%, P=0.04).Similarly,ACEI/ARBs reduced the incidence of AF recurrence(RR=0.39;95%CI,0.20-0.74; P=0.005;Fig.3),butnotnew AF(RR=0.87;95%CI, 0.62-1.21;P=0.40;Fig.3)with high heterogeneity (I2=86%,P=0.0007),when compared toβ-blockers.
Median time of AF recurrence was reported only in 4 studies[12-15].Du etal.[12]reported thatmedian time of AF recurrence had no significantdifferences between the nifedipine group and the telmisartan group.However, the other 3 studies reported that ARBs postponed AF recurrence.Therefore,preliminary comparison of these data without statistics did not reveal tendency that ACEI/ARBs could postpone AF recurrence.
Secondary endpoints
We also compared the cardiovasculareventsin the follow-up,which included cardiac death,myocardialinfarction,stroke,and congestive heartfailure.Cardiovascular events were reported in three large-scale studies[17-19]. When compared toβ-blockers and calcium antagonists, ACEI/ARBs did notreduce cardiac death(RR=1.00; 95%CI,0.90-1.12;P=0.94),myocardial infarction (RR=1.00;95%CI,0.81-1.23;P=0.98),and stroke (RR=1.01;95%CI,0.70-1.47;P=0.94;Fig.4). Heterogeneities were(I2=0%,P=0.47),(I2=78%, P=0.001),and(I2=94%,P<0.00001),respectively. ACEI/ARBs reduced the incidence ofcongestive heart failure(RR=0.86;95%CI,0.77-0.96;P=0.007; Fig.4),with low heterogeneity(I2=0%,P=0.56).
Data of adverse effects(bradycardia,atrial flutter, intolerable and unproductive cough,peripheraledema and dizziness)during follow-up were reported in 6 studies[12-15,17-18].Four studies[12-15]reported adverse effects requiring discontinuation due to bradycardia,atrialflutter,intolerable and unproductive cough,and the aggregated results of these studies suggested that ACEI/ ARBs cou ld d ecrease these adv erse effects (RR=0.44;95%CI,0.21-0.89;P=0.02;Fig.5)with low heterogeneity(I2=0%,P=0.63).In the studies of Hansson et al.(STOP-2)[17]and Julius et al.[18],they compared the incidence ofperipheraledema and dizziness,the pooled outcomes showed that ACEI/ARBs reduced peripheral edema(RR=0.47;95%CI,0.42-0.53;P<0.00001)with high heterogeneity(I2=57%, P=0.13),but incre a se d the risk of dizz in ess (RR=1.11;95%CI,1.02-1.20;P=0.01;Fig.6)with high heterogeneity(I2=51%,P=0.15).
Publication bias
Publication biaswasassessed,even though only 10 studieswere included in this analysis.The resultsillustratedthattheprobability ofpublication biaswaspossibledueto asymmetry(Fig.7).
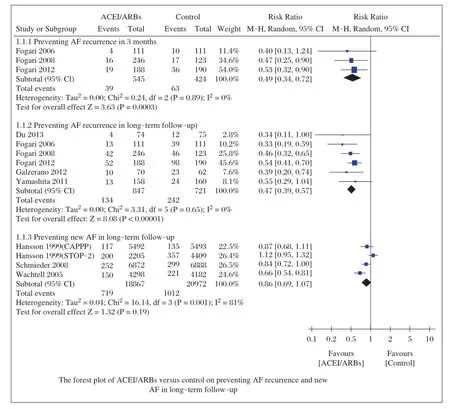
Fig.2Forest plot of ACEI/ARBs versus control on preventing AF recurrence and new AF in long-term follow-up.
Discussion
Regarding the effects of ACEI/ARBs on hypertensive patientsand AF,theresultsofindividualtrialsareconflicting.Here,we performed a meta-analysisofavailable data to define the conditions and circumstances in which ACEI/ARBs may be a promising preventive therapy. The pooled resultsfrom 10 RCTs using a random effects modelsuggested that ACEI/ARBs decreased AF recurrence rate by 7%in 3 months,and 17%in long-term follow-up.In subgroups,ACEI/ARBs reduced more AF recurrence rate by 17%than calcium antagonists and 23%thanβ-blockers.However,ACEI/ARBs did not decrease the rate of new AF.Compared to the control group,ACEI/ARBsdid notreducecardiacdeath,myocardialinfarction orstroke,excepting congestive heartfailure.ACEI/ARBs cutdown adverse effects,but may increase dizziness.
Ourmeta-analysis indicated that ACEI/ARBs could decrease the incidence of AF recurrence at3 months and in long-term follow-up.However,ACEI/ARBscould notreduce the incidence ofnew AF in long-term followup.The heterogeneitieswere greatin subgroupsanalyses. We found thatheterogeneities come from Hansson etal. (STOP-2)[17].The blood pressuresofpatientsin thisstudy were higherthan those in otherstudies,with SBP≥180 mmHg and/or DBP≥105 mmHg.Diuretics,amiloride and fixed-ratio hydrochlorothiazide were used in theβblockergroup,which may also contributeto heterogeneity.When compared to the differentcontrolgroups,the incidence of AF recurrence was lowerin patients receiving ACEI/ARBsthan in those receiving calcium antagonists orβ-blockers in long-term follow-up;however,ACEI/ ARBs did notreduce new AF in long-term follow-up when compared to calcium antagonists andβ-blockers. Median time to AF recurrence was described without pooled data,which did notrevealtendency that ACEI/ ARBs could postpone AF recurrence.
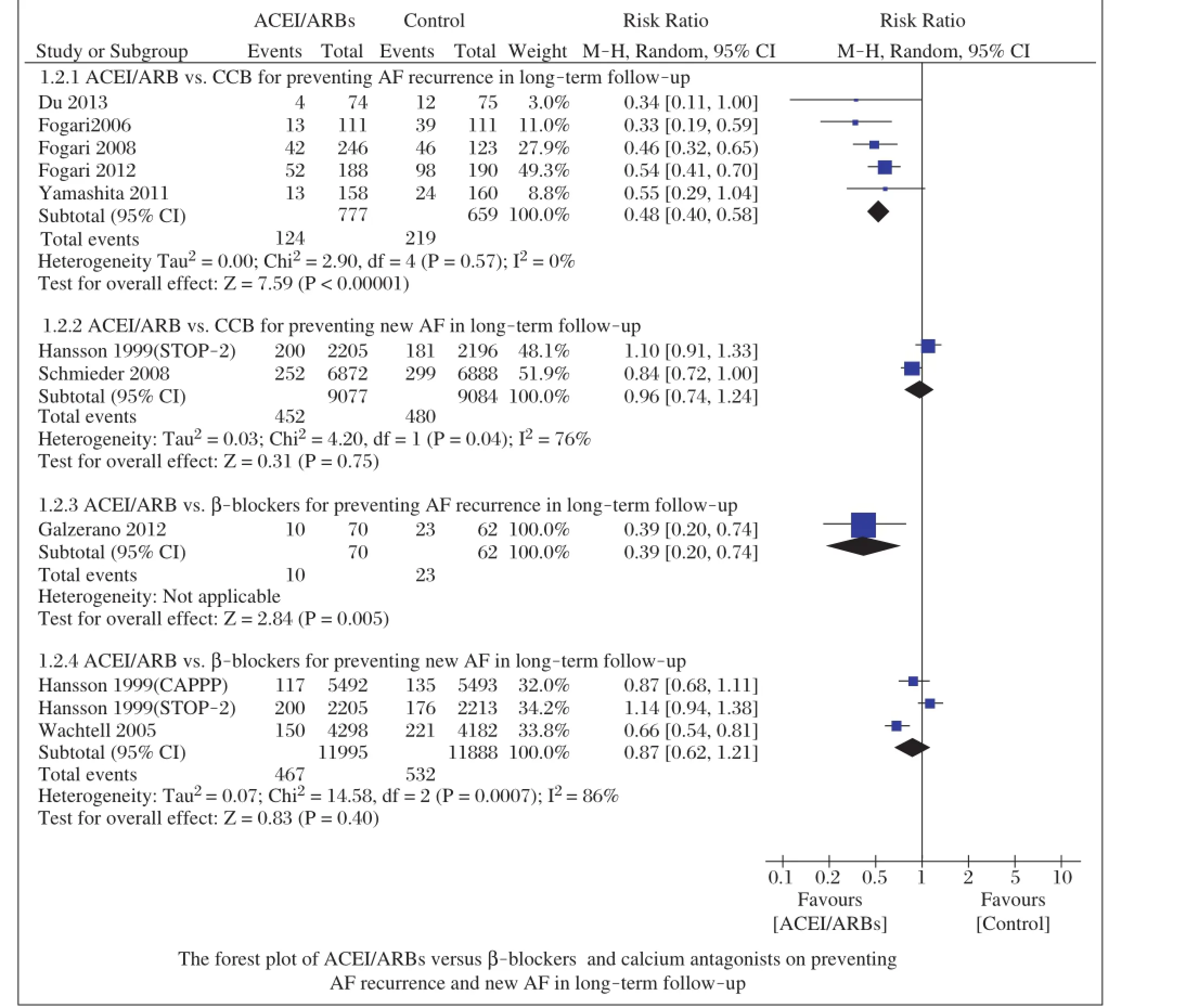
Fig.3Forest plot of ACEI/ARBs versusβ-blockers and calcium antagonists on preventing AF recurrence and new AF in longterm follow-up.
Cardiovascularevents were assessed,and the results showed that ACEI/ARBs could reduce the incidence of congestive heartfailure,butnotcardiac death,myocardialinfarction,or stroke,comparing toβ-blockers and calcium antagonists.Although ACEI/ARBsare generally regarded as safe and welltolerated drugs in most populations,it should be careful that ACEIs may induce non-productive cough and peripheraledema.
Our results are partly similar to the last 2 metaanalyses[21-22].Huang et al.[21]reported that ACEIs/ ARBs were effective for new AF and AF recurrence. Han etal.[22]also demonstrated that ACEI/ARBs prevented AF recurrence.In our presentanalysis,considering the close relation between hypertension and AF, we specifically included hypertensive patients for review.We found that ACEI/ARBs did not prevent new AF in hypertensive patients.The results are differentfrom Huang etal.[21],which may resultfrom different included patients.In their study,patients wereincluded as follows:myocardial infarction,coronary heart disease,hypertension and chronic heart failure, withoutany subgroup analysis.Furthermore,ourstudy also investigated the role of ACEI/ARBs in cardiovascular events and adverse effects,which may provide more powerful evidence for clinicians.
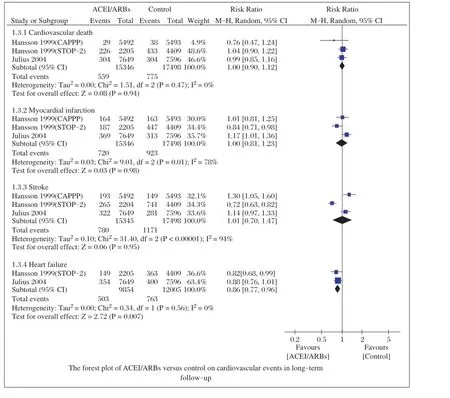
Fig.4Forest plot of ACEI/ARBs versus control on cardiovascular events in long-term follow-up.
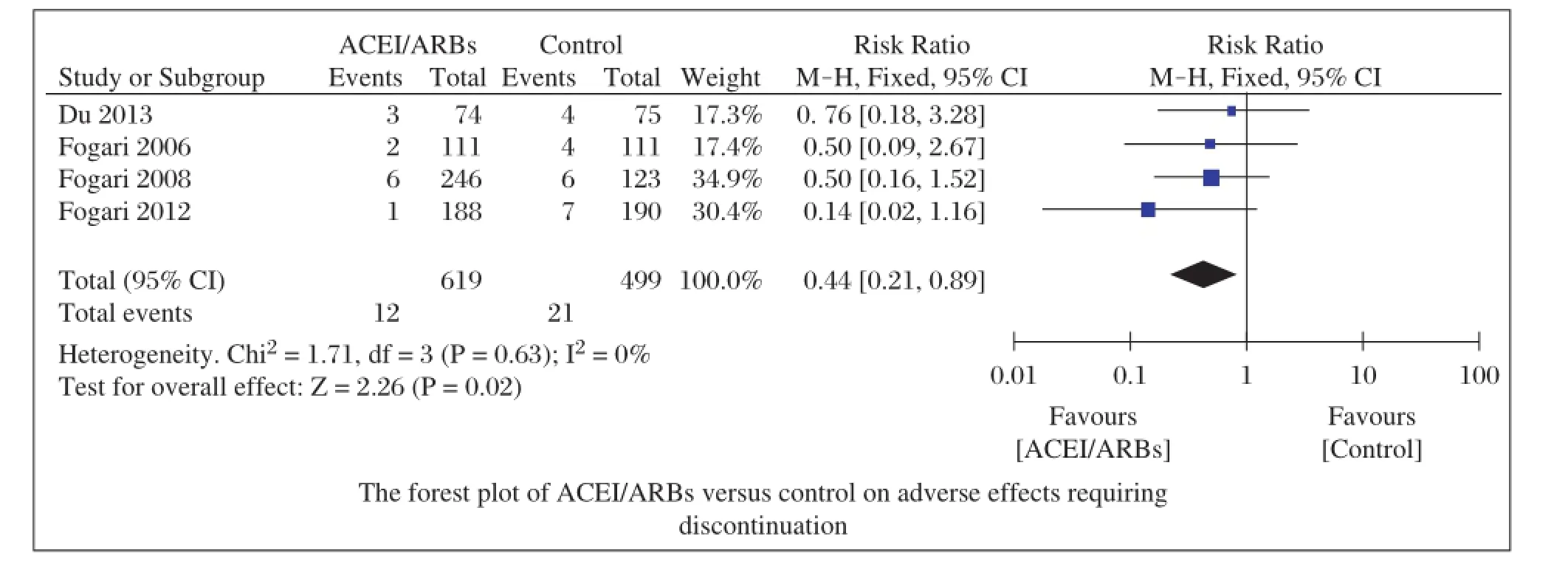
Fig.5 Forest plot of ACEI/ARBs versus control on adverse effects requiring discontinuation.
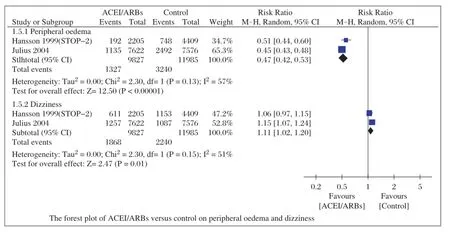
Fig.6Forest plot of ACEI/ARBs versus control on peripheral oedema and dizziness.
Fig.7 Funnel plot of ACEI/ARBs versusβ-blockers and calcium antagonists on preventing AF recurrence and new AF in long-term follow-up.
Ourmeta-analysishasseveralpotentiallimitations that should be taken into account.First,even though we analyzed calcium antagonists andβ-blockers in subgroups, theircharacteristics are different,and the effectmay be unequal.In the randomized controlled trials,thecharacteristics ofhypertensive patients were notbased on a unified level,which varies in the range of SBP≥140 mmHg and DBP≥90 mmHg.These factors may have potential impacton ourresults.Second,follow-up varies from 3 months to 73.2 months.Finally,as many ACEI/ARBs drugs,involving enalapril,lisinopril,ramipril,captopril, candesartan,losartan,valsartan and telmisartan,wereused in ourincluded studies,and we are notsure to assess the impactof ACEI/ARBs basing on meaningfulendpoints.
In conclusion,our results suggest that ACEI/ARBs may reduce the incidence of AF recurrence,heartfailure,with less serious adverse effects.Further unified protocol and well-designed randomized controlled trials on this topic are still needed.
Acknowledgements
Thiswork wassupported by grantsfrom the National Natural Science Foundation of China(No.81270255 to L-SW).
[1]KotwaniP,Kwarisiima D,Clark TD,etal.Epidemiology and awareness ofhypertension in a rural Ugandan community:a cross-sectional study[J].BMC Public Health,2013,13(1): 1151.
[2]Krahn AD,Manfreda J,Tate RB,etal.The naturalhistory of atrial fibrillation:incidence,risk factors,and prognosis in the Manitoba Follow-Up Study[J].Am J Med 1995, 98(5):476-484.
[3]New approaches to antiarrhythmic therapy,part II:emerging therapeutic applications of the cellbiology of cardiac arrhythmias[J].Circulation,2001,104(24):2990-2994.
[4]Makkar KM,Sanoski CA,Spinler SA.Role of angiotensin-converting enzyme inhibitors,angiotensin II receptor blockers,and aldosterone antagonists in the prevention of atrial and ventricular arrhythmias[J].Pharmacotherapy,2009,29(1):31-48.
[5]Jibrini MB,Molnar J,Arora RR.Prevention of atrial fibrillation by way of abrogation of the renin-angiotensin system:a systematic review and meta-analysis[J].Am J Therb,2008,15(1):36-43.
[6]Anand K,Mooss AN,Hee TT,et al.Meta-analysis:inhibition of renin-angiotensin system prevents new-onset atrial fibrillation[J].Am Heart J,2006,152(2):217-222.
[7]Healey JS,Baranchuk A,CrystalE,etal.Prevention ofatrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers:a meta-analysis[J].J Am Coll Cardiol,2005,45(11):1832-1839.
[8]Higgins JP,Thompson SG,Deeks JJ,etal.Measuring inconsistency in meta-analyses[J].BMJ,2003,327(7414):557-560.
[9]Armitage P,Berry G,Matthews JNS.Analysing Means and Proportions.Statistical Methods in Medical Research[J]. Blackwell Science Ltd 2008:83-146.
[10]WachtellK,Lehto M,Gerdts E,etal.Angiotensin IIreceptor blockade reducesnew-onsetatrialfibrillation and subsequent stroke compared to atenolol:the Losartan Intervention For End Point Reduction in Hypertension(LIFE)study[J]. J Am Coll Cardiol,2005,45(5):712-719.
[11]Yamashita T,Inoue H,Okumura K,et al.Randomized trial of angiotensin II-receptor blocker vs.dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension(J-RHYTHM II study)[J].Europace,2011,13(4):473-479.
[12]Du H,Fan J,Ling Z,et al.Effect of nifedipine versus telmisartan on prevention of atrial fibrillation recurrence in hypertensive patients[J].Hypertension,2013,61(4): 786-792.
[13]Fogari R,Mugellini A,Destro M,etal.Losartan and prevention of atrial fibrillation recurrence in hypertensive patients[J].J Cardiovasc Pharmacol,2006,47(1):46-50.
[14]Fogari R,Derosa G,Ferrari I,etal.Effectof valsartan and ramiprilon atrialfibrillation recurrence and P-wave dispersion in hypertensive patients with recurrent symptomatic lone atrial fibrillation[J].Am J Hypertens,2008,21(9): 1034-1039.
[15]Fogari R,Zoppi A,Maffioli P,et al.Effectof telmisartan on paroxysmal atrial fibrillation recurrence in hypertensive patients with normal or increased left atrial size[J]. Clin Cardiol,2012,35(6):359-364.
[16]Galzerano D,Di Michele S,Paolisso G,etal.A multicentre,randomized study of telmisartan versus carvedilolfor prevention of atrial fibrillation recurrence in hypertensive patients[J].J Renin Angiotensin Aldosterone Syst,2012, 13(4):496-503.
[17]Hansson L,Lindholm LH,Ekbom T,etal.Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study[J].Lancet, 1999,354(9192):1751-1756.
[18]Julius S,Kjeldsen SE,Weber M,etal.Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine:the VALUE randomised trial[J].Lancet,2004,363(9426):2022-2031.
[19]Hansson L,Lindholm LH,Niskanen L,etal.Effectofangiotensin-converting-enzyme inhibition compared with conventionaltherapy on cardiovascular morbidity and mortality in hypertension:the CaptoprilPrevention Project(CAPPP)randomised trial[J].Lancet,1999,353(9153):611-616.
[20]Schmieder RE,Kjeldsen SE,Julius S,et al.Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade:the VALUE trial[J].J Hypertens,2008, 26(3):403-411.
[21]Huang G,Xu JB,Liu JX,et al.Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers decrease the incidence of atrial fibrillation:a metaanalysis[J].Eur J Clin Invest,2011,41(7):719-733.
[22]Han M,Zhang Y,Sun S,etal.Renin-Angiotensin System Inhibitors Prevent the Recurrence of Atrial Fibrillation: A Meta-Analysis of Randomized Controlled Trials[J]. J Cardiovasc Pharmacol,2013,62(4):405-415.
✉Corresponding author:Lian-Sheng Wang,MD,PhD,Department of Cardiology,The First Affiliated Hospitalof Nanjing MedicalUniversity, 300 Guangzhou Road,Nanjing,Jiangsu 210029,China.Tel/fax:+86-25-83724440,E-mail:drlswang@njmu.edu.cn.
Received 07 November 2014,Accepted 05 July 2015,Epub 28 October 2015
R544.1,Document code:A
The authors reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140149
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The evolving concept of physiologicalischemia training vs. ischemia preconditioning
- PCSK9 and triglyceride-rich lipoprotein metabolism
- Generation of monoclonalantibodies against n-3 fatty acid desaturase
- Dominant-negative inhibition of glucose-dependent insulinotropic polypeptide impairs function ofβcells in transgenic pigs
- Waldenstrom′s macroglobulinemia associated with Hodgkin′s lymphoma: a case report
- Neuroanatomy and clinicalanalysis of the cervicalsympathetic trunk and longus colli
