Dominant-negative inhibition of glucose-dependent insulinotropic polypeptide impairs function ofβcells in transgenic pigs
Dominant-negative inhibition of glucose-dependent insulinotropic polypeptide impairs function ofβcells in transgenic pigs
Dear Editor:
Glucose-dependent insulinotropic polypeptide(GIP) and proglucagon productglucagon-like peptide-1(GLP-1)and their corresponding receptors promote secretion of glucose-dependent insulin[1]and may be responsible for up to 70%of postprandial insulin secretions[2].A diminished incretin effectis typicalfor type 2 diabetes mellitus(T2DM)patients and theirnormoglycemic firstdegree relatives due to reduced insulinotropic action of GIP[3].Desensitization ofthe GIP/GIPR axis[4],down-regulation of GIP receptor(GIPR)onβcells[5],and variation in the GIPR gene[6]allcontribute to reduced insulinotropic activity of GIP.
GIPRdnencodes human GIPR thatis modified in the region ofthe third intracellularloop by a single mutation (Ala340→Glu340)and deletion ofeightamino acids(residues 319-326)essentialfor signaltransduction[8].In this study,we produced porcine transgenic fibroblasts that overexpressed a GIPRdnunderthe controlofratIns2 promoterin the pancreas.The study protocolwasapproved by the localinstitutionalreview board and animalstudy wasdonein accordancewith established nationaland institutionalguidelines.Oralglucose tolerance tests(OGTTs) in 2-month-old GIPRdnpigs and controls revealed no differences in area under the glucose response curve (AUCglucose)between the two groups(P=0.07, Fig.1A).However,the postprandial blood glucose declined slowly and the area under the insulin response curve(AUCinsulin)in GIPRdnpigsincreased 2.3-fold versus the controls,implying impaired glucose tolerance (P=0.02;Fig.1B).OGTTs in 5-month-old GIPRdnpigs and controls showed no significantdifferencesin glucose levels orinsulin contentbetween the groups(Fig.1C, 1D).Additionally,we observed no significantdifferences in the insulin response profiles between 2-month-old and 5-month-old controls.However,AUCinsulinwas reduced by 44%when the transgenic pigs grew from 2 months to 5 months of ages(Fig.1E,1F).To clarify the effectof GIPRdnexpression on the proliferation ofpancreatic cells, we measured insulin and Ki67 expression by immunohistochemistry in 5-month-old GIPRdnpigsand age-matched controls.Compared with the controls,the numberand volume ofislets in transgenic pigs decreased.Insulin expression in the islets was reduced by 83%(P<0.01) in 5-month-old GIPRdnpigs(Fig.2A-2C).In addition, Ki67 expression in GIPRdnpigs decreased to 36%of controls,suggesting that GIPRdnexpression impaired islet development and reduced insulin secretion (Fig.2D-2F).
Transgenic mice overexpressing a dominant-negative GIPRwere found to develop diabetes accompanied by a marked fasting hypoinsulinemia and severe reduction ofβ-cellmassby 10 daysofage[7].Similarly,transgenic pigs overexpressing a dominant-negative GIPR exhibited a pre-diabetic phenotype of hyperglycemia and hyperinsulinemia in OGTT[8].However,no transgenic pigs had developed fasting hyperglycemia.In this study,2-month-old GIPRdnpigs exhibited a slow decline in postprandial blood glucose and an increase in insulin secretion following an oral glucose load. This increase was approximately 2.3 times higher than in the control,thusimitating insulin secretion in the prodromalstage of T2DM.As the pigs grew up,pancreas developmentwassignificantly impaired due to the compromised GIP/GIPR axis,which resulted in reduced insulin secretion and isletdysfunction.Studies indicated that GIP and GLP-1 induced the transcription of beta cellproliferation,differentiation,survivaland transcription of some genes[9].GIP was believed to be the mitogen of beta cells,resulting in insulin secretion and inhibiting the apoptosis ofbeta cells.In this study, there were no significantdifferences in insulin content between the 5-month-old groups.However,the oralglucose load of 5-month-old transgenic pigs resulted in a 44%decrease in insulin secretion compared with the 2-month-old transgenic pigs.This is consistentwith previous studies an d imp lies progression of T2DM. Defective pancreatic proliferation led to fewer andsmaller islets,which were excessively activated to produ ce more insulin by comp ensatory mechanism. Overload pressure from the compensation mechanism and impaired proliferation for inactivated GIP/GIPR axis further deteriorated islet function.Similar to the previous study[9],the transgenic pigs did not develop diabetes until 2 years of age.Diabetes is caused by many factors;a single gene change would not lead to the occurrence ofthe disease.
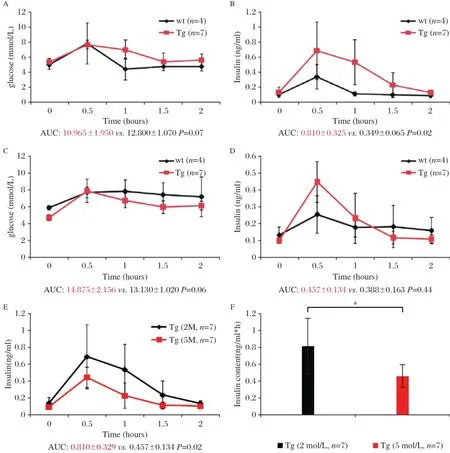
Fig.1Oralglucose tolerance test in GIPRdntransgenic(Tg)and wild type(wt)pigs.(A)Serum glucose,and(B)insulin levels,in 2-monthold pigs(AUC glucose P=0.07,AUC insulin P=0.02).(C)Serum glucose,and(D)insulin levels in 5-month-old pigs(AUC glucose P=0.06,AUC insulin P=0.44).(E)(F)Insulin levels in 2-month-old and 5-month-old GIPRdntransgenic pigs(P=0.02).*P<0.05.
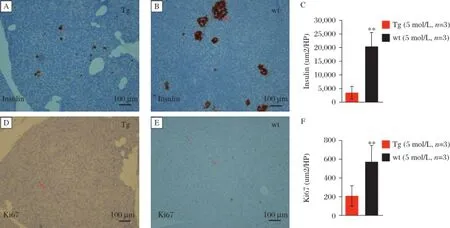
Fig.2Insulin and Ki67 expression in the pancreas.A:transgenic pigs(Tg);B:wild type(wt)pigs.Insulin is expressed in islet.C:transgenic pigs(Tg);B:wild type(wt)pigs.Ki67 expression is decreased in GIPRdntransgenic pigs.(n=3 per group,**P<0.01).
In summary,GIPRdnpigs exhibitimpaired glucose metabolism and beta cellfunction and simulated the phenotype of the prodromalstage in diabetes and progression of the disease.
The study was funded by the National Basic Research Program of China(973 Program,No.2011CBA01003); Natural Science Foundation of JiangSu Province of China,BK2011766,National Natural Science Foundation of China,Young Scholars,81200570,YD, RL and YW are Fellows at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine,Nanjing Medical University.
Yours Sincerely,
JunLin Cheng1,2,△,Ying Wang1,△,Zhengwei Zhang1, Yong Jin1,QianKun Li1,RongGen Want1,Yan Wang1, Xiao Kang Li1,Qiang Xiong1,Man Ling Zhang1, RongFeng Li1,✉,YiFan Dai1,✉
1Jiangsu Key Laboratory of Xenotransplantation, Nanjing Medical University, Nanjing,Jiangsu 210029, China.
2Departmentof Clinical Pharmacology,
Nanjing First Hospital,
Nanjing Medical University,
Nanjing,Jiangsu 210012,
China.
✉Corresponding author:daiyifan@njmu.edu.cn, lirongfeng@njmu.edu.cn.
△These authors contributed equally to this work.
[1]Drucker DJ.The biology of incretin hormones[J].Cell Metab,2006,3(3):153-165.
[2]HolstJJ,Vilsbøll T,Deacon CF.The incretin system and its role in type 2 diabetes mellitus[J].Mol Cell Endocrinol, 2009,297(1-2):127-136.
[3]Nauck MA,Heimesaat MM,Orskov C,et al.Preserved incretin activity of glucagon-like peptide 1[7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus[J].J Clin Invest, 1993,91(1):301-307.
[4]Meier JJ,H u¨cking K,Holst JJ,etal.Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes[J].Diabetes,2001, 50(11):2497-2504.
[5]Vilsbøll T,Krarup T,Madsbad S,etal.Defective amplification of the late phase insulin response to glucose by GIP in obese Type IIdiabetic patients[J].Diabetologia,2002,45(8): 1111-1119.
[6]Saxena R,HivertMF,Langenberg C,etal.Genetic variation in GIPR influences the glucose and insulin responses to an oralglucose challenge[J].Nature Genet,2010,42(2):142-148.
[7]Herbach N,Goeke B,Schneider M,etal.Overexpression of a dominant negative GIP receptor in transgenic mice results in disturbed postnatalpancreatic isletand beta-cell development[J].Regul Pept,2005,125(1-3):103-117.
[8]Renner S,Fehlings C,Herbach N,etal.Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function[J].Diabetes,2010,59(5):1228-1238.
[9]Ehses JA,Pelech SL,Pederson R,et al.Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/ 2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway[J].J Biol Chem, 2002,277(40):37088-37097.
Received 18 March 2015,Accepted 11 May 2015,Epub 20 May 2015
R714.256,Document code:B
The authors reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20150046
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年6期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The evolving concept of physiologicalischemia training vs. ischemia preconditioning
- PCSK9 and triglyceride-rich lipoprotein metabolism
- Generation of monoclonalantibodies against n-3 fatty acid desaturase
- Waldenstrom′s macroglobulinemia associated with Hodgkin′s lymphoma: a case report
- Neuroanatomy and clinicalanalysis of the cervicalsympathetic trunk and longus colli
- A susceptibility locus rs7099208 is associated with non-obstructive azoospermia via reduction in the expression of FAM160B1
