黔产毛萼香茶菜二萜成分
陶 晨,李齐激,叶林虎,张建新,杨小生*
1贵州省中国科学院天然产物化学重点实验室,贵阳 550002;2 黔南民族医学高等专科学校,都匀 558003
毛萼香茶菜Isodon erioealyx (Dunn)Hara.为唇形科香茶菜属为多年生草本植物,主要分布在我国西南的贵州、云南、四川及广西等省,为民间常用草药,具有清热利痰、凉血散瘀的功效,主要用于治疗感冒、风湿关节炎、癌症、急性黄疸性肝炎、烂脚丫等[1,2]。植物化学研究发现其主要含有二萜、三萜、黄酮类成分,尤其富含贝壳杉烷二萜[3-6]。药理研究显示该植物提取物能显著拮抗平滑肌收缩[7],抗菌和抗炎作用[8],毛萼乙素对人脐静脉内皮细胞系的增殖、迁移、小管形成具有不同程度的抑制作用[9],还发现其可以靶向AML1-ETO 原癌蛋白激活细胞凋亡途经治疗白血病[10]。目前,已有毛萼香茶菜清热利咽片上市销售,效果较好。笔者在对该植物成分进行结构修饰研究过程中,从中分离、鉴定了10个二萜化合物:6-乙酰基-毛萼晶B(1)、毛萼晶O(2)、12-hydroxydehydro-abietic acid(3)、neorabdosin(4)、毛萼晶D(5)、毛萼晶E(6)、毛萼晶B(7)、毛萼晶N(8)、coetsoidin A(9)、毛萼晶L(10);其中化合物1 为新天然产物,3 为首次从该植物中分离。
1 仪器与材料
Varian INOVA-400 核磁共振仪测定(TMS 为内标);HP-5973 型质谱分析仪;API QSTAR Pulsar 质谱仪;Pol I 自动旋光仪;XT-4 显微熔点仪(温度未校正);Agilent 1100 型液相色谱仪;柱层析硅胶(200~300 目)和薄层层析硅胶板(GF254)均为青岛海洋化工厂生产;柱层析RP-C18硅胶为Merck 公司生产;Sephadex LH-20 为Amersham Pharmacia Biotech AB公司生产;除高效液相色谱用溶剂为色谱纯外,其余试剂均由工业纯重蒸所得。
实验药材于2012年7 月采自贵州省贵阳市,经贵阳中医学院陈德媛教授鉴定为毛萼香茶菜Isodon eriocalyx (Dunn)Haral 植物的嫩枝和叶,凭证标本保存于贵州省中国科学院天然产物化学重点实验室。
2 提取与分离
经自然干燥的毛萼香茶菜嫩枝叶(20 kg)粉碎后,用75%乙醇加热回流提取3 次(3 h/次),减压回收溶剂得浸膏1.5 kg。将浸膏加水完全溶解后,分别用石油醚、乙酸乙酯、正丁醇各萃取3 次,合并、浓缩萃取液得石油醚萃取物0.3 kg,乙酸乙酯萃取物0.8 kg,正丁醇萃取物0.3 kg,水萃取物0.1 kg。乙酸乙酯萃取物经硅胶柱层析石油醚-乙酸乙酯梯度洗脱(20∶1→0∶1)得到5 个组分(Fr 1~Fr 5)。Fr 2(10∶1,87 g)经MCI 柱层析除掉色素后,再用硅胶柱层析石油醚-丙酮梯度洗脱(10∶1→0∶1)得到3 个组分(Fr 2-1、Fr 2-2、Fr 2-3);Fr 2-1(8∶1)经凝胶柱色谱洗脱(氯仿-甲醇1∶1)化合物3(27mg);Fr 2-2经氯仿-甲醇1∶1 重结晶得化合物2(63 mg)。Fr 3(7∶1,151 g)经MCI 柱层析除掉色素后,再用硅胶柱层析石油醚-丙酮梯度洗脱(10∶1→0∶1)得到7 个组分(Fr 3-1→Fr 3-7);Fr 3-2(8∶1)经硅胶柱层析石油醚-丙酮等度洗脱(10∶1)得化合物4(273 mg);Fr 3-5(6∶1)经硅胶柱层析石油醚-丙酮等度洗脱(8∶1)得化合物5(993mg),化合物7(93 mg);Fr 3-5(5∶1)经硅胶柱层析石油醚-丙酮等度洗脱(7∶1)得化合物9(870 mg);Fr 4(5∶1,137g)经MCI 柱层析除掉色素后,再用硅胶柱层析石油醚-丙酮梯度洗脱(8∶1→0∶1)得到4 个组分(Fr 4-1→Fr 4-4);Fr 4-1(8∶1)经凝胶柱色谱洗脱(甲醇)得化合物1(226 mg);Fr 4-2(5∶1)经制备HPLC 甲醇-水(20-80)洗脱得化合物8(61 mg)、10(114 mg);Fr 4-3(3∶1)经凝胶柱色谱洗脱(甲醇)后丙酮重结晶得6(319 mg)。
3 结构鉴定
化合物1 白色粉末(氯仿),mp.183~184.5℃,[-186.9° (c=0.63,MeOH);根据EI-MS m/z 386[M]+,HR-ESI-MS m/z 409.1638 (calcd for C22H26O6Na,409.1627)和NMR 数据推算其该化合物分子式为C22H26O6,不饱和度为10。从1H NMR、13C NMR 和HMQC 谱(见表1)可知,该化合物含有3 个甲基[含1 个乙酰甲基δH2.11(3H,s),δC24.6(-OAc)]、5 个亚甲基[含1 个环外双键信号δH5.08(1H,s,H-17a),4.84 (1H,s,H-17b),δC157.2 (C-16),109.4 (C-17);1 个含氧取代亚甲基信号δH4.32 (1H,d,J=9.6 Hz,H-20a),3.98 (1H,d,J=9.6 Hz,H-20b),δC65.5 (C-20)]、6 次甲基[含1 个环内双键信号δH6.77 (1H,d,J=10.0 Hz,H-3),5.90 (1H,d,J=10.0 Hz,H-2),δC127.5 (C-2),160.7 (C-3),1 个含氧取代次甲基δH5.62 (1H,br s,H-6),δC74.4 (C-6)]和8 季碳[含1 个含氧取代季碳信号δC96.8 (C-7),2 个羰基信号δC198.2 (C-1),213.6 (C-15)]。结合植物化学分类学知识和上述光谱信息可初步推测该化合物为香茶菜属植物中较为典型的对映贝壳杉烷型二萜。
在HMBC 谱(见表1、图1)中,低场质子信号δH5.08 (H-17a),4.84 (H-17b)与羰基碳δC213.6(C-15)/35.2 (C-13)远程相关,提示含有对映贝壳杉烷型二萜中典型的α,β-不饱和酮;δH6.77 (H-3)与δC198.2 (C-1)/ 30.0 (C-18)/21.9 (C-19)远程相关,5.90 (H-2)与δC198.2 (C-1)远程相关提示环内双键位于A 环C-1、C-2 位;δH4.32 (H-20a),3.98 (H-20b)与δC198.2 (C-1)/ 52.1 (C-5)/96.8 (C-7)远程相关,δH5.62 (H-6)与δC52.1 (C-5)/ 96.8 (C-7)/ 171.8 (-OAc)远程相关提示乙酰基位于C-6 位,C-20 位含氧基团与C-7 位形成氧环。经综合分析1D NMR、2D NMR 光谱数据及其理化性质,并与文献[11]报道毛萼晶B 6-OH 经乙酰化反应所得衍生物[7]进行比对,相关数据基本一致,但本文为首次从植物中分离得到,故鉴定化合物1为6-乙酰基-毛萼晶B(6-acetyl-Maoecrystal B)。
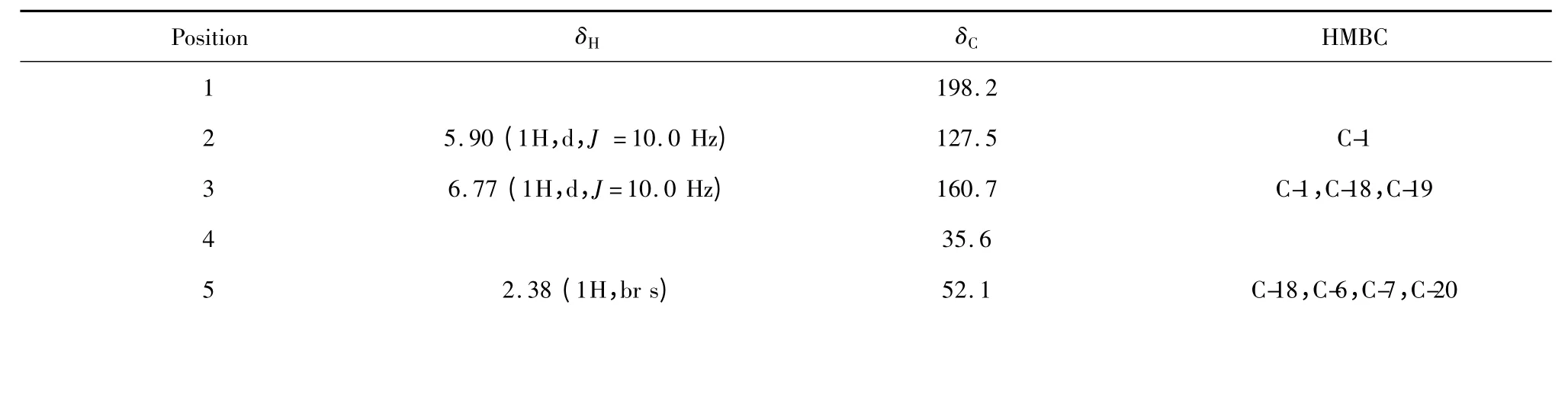
表1 化合物1 的1H NMR、13C NMR 和HMBC 数据(CD3OD)Table 1 1H NMR,13C NMR and HMBC data of compound 1(CD3OD)
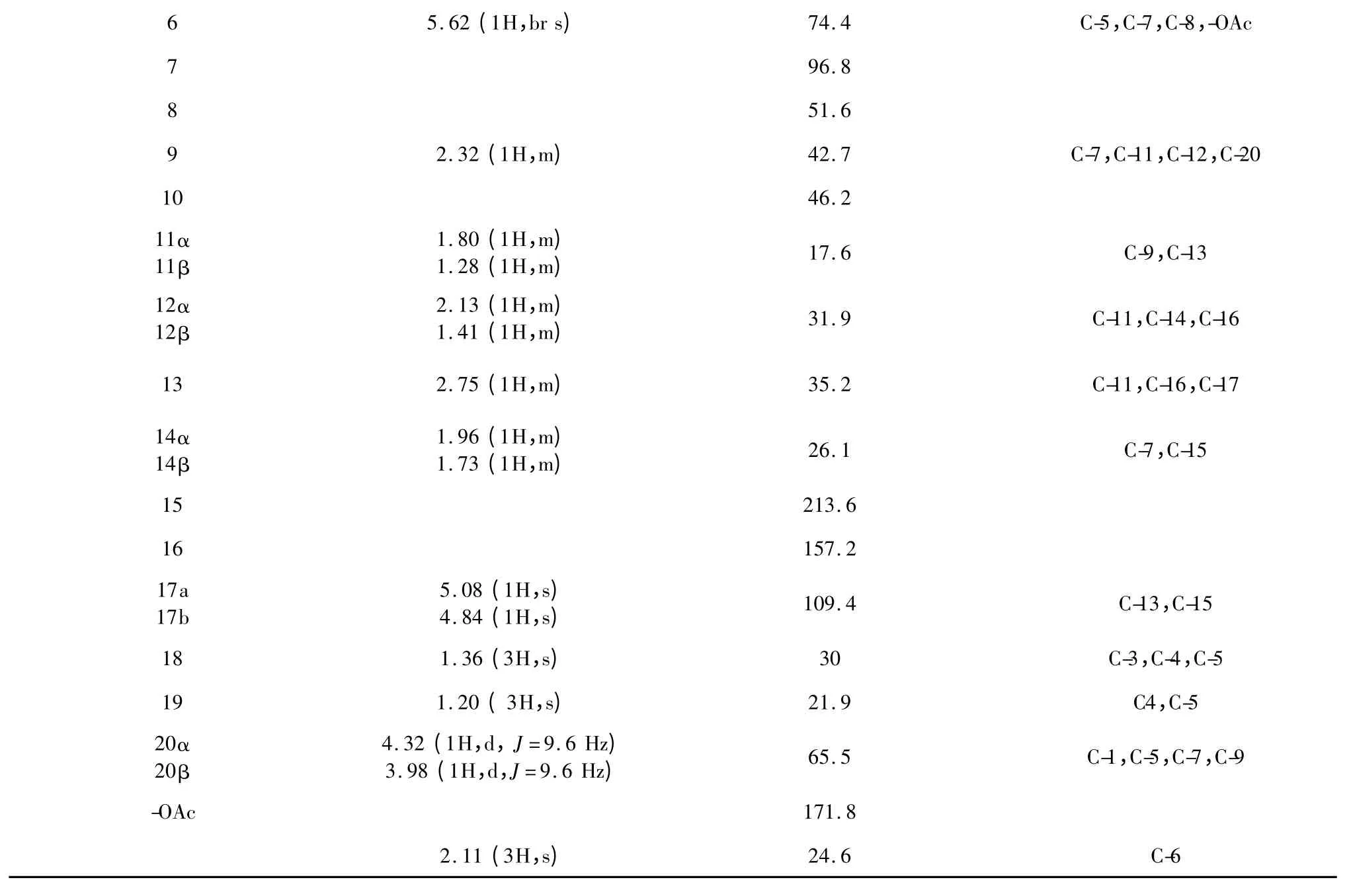
图1 化合物1 的结构和HMBC(→)远程相关Fig.1 Chemical structure and key HMBC (→)correlation of compound 1
化合物2 无色针晶(氯仿-甲醇1∶1),EI-MS m/z 406[M]+;1H NMR (C5D5N,400 MHz)δ:5.51(1H,d,J=12.8 Hz,H-20a),5.38 (1H,d,J=12.8 Hz,H-20b),5.13 (1H,dd,J=11.4,3.4 Hz,H-1),2.16 (3H,s,-OAc),1.07 (3H,s,H-19),1.24 (3H,s,H-18),0.90 (1H,s,J=3.2 Hz,H-16);13C NMR (C5D5N,100MHz),δ:75.9 (C-1),24.9 (C-2),39.5 (C-3),34.1 (C-4),58.5 (C-5),174.2(C-6),170.7 (C-7),59.1 (C-8),45.5 (C-9),43.0(C-10),17.3 (C-11),31.5 (C-12),32.6 (C-13),24.9 (C-14),216.9 (C-15),48.8 (C-16),11.5 (C-17),33.6 (C-18),23.4 (C-19),69.2 (C-20),170.5,21.7 (-OAc)。以上数据与文献[12]报道一致,故鉴定化合物2 为毛萼晶O(Maoecrystal O)。
化合物3 黄色粉末(氯仿),EI-MS m/z:316(M+);1H NMR (CDCl3,400 MHz),δ:6.83 (1H,s,H-14),6.62 (1H,s,H-11),1.22 (3H,d,J=5.8 Hz,H-17),1.23 (3H,d,J=5.8 Hz,H-16),1.27(3H,s,H-19),1.20 (3H,s,H-20);13C NMR (CDCl3,100 MHz)δ:37.8 (C-1),18.5 (C-2),36.6(C-3),47.4 (C-4),44.5 (C-5),21.9 (C-6),29.2(C-7),127.0 (C-8),147.7 (C-9),36.8 (C-10),110.8 (C-11),150.7 (C-12),131.7 (C-13),126.7(C-14),26.7 (C-15),22.5 (C-16),22.7 (C-17),184.8 (C-18),16.2 (C-19),25.0 (C-20)。以上数据与文献[13]报道一致,故鉴定化合物3 为(12-hydroxydehydro-abietic acid)。
化合物4 无色结晶(氯仿-甲醇1:1),EI-MS m/z 430([M]+);1H NMR (CDCl3,400 MHz),δ:6.12 (1H,t,J=5.2 Hz,H-15),5.61 (1H,d,J=8.4 Hz,H-6),5.05 (1H,d,J=2.0 Hz,H-17a),5.03(1H,d,J=2.0Hz,H-17),4.37 (1H,d,J=10.0 Hz,H-20a),4.15 (1H,dd,J=1.6 Hz,H-20),3.71(1H,brs,H-3),2.20,2.13 (each 3H,s,2 ×-OAc),1.28 (3H,s,H-18),1.08 (3H,s,H-19);13C NMR(CDCl3,100 MHz)δ:202.5 (C-1),41.7 (C-2),77.0 (C-3),37.5 (C-4),47.6 (C-5),73.9 (C-6),207.9 (C-7),56.5 (C-8),39.5 (C-9),51.2 (C-10),20.6 (C-11),32.7 (C-12),34.4 (C-13),35.2(C-14),73.3 (C-15),149.7 (C-16),108.7 (C-17),29.1 (C-18),22.9 (C-19),62.0 (C-20),170.1,169.4,21.0,20.5 (OAc):以上数据与文献[14,15]报道一致,故鉴定化合物4 为Neorabdosin。
化合物5 无色针晶(氯仿-丙酮),EI-MS m/z:432,C24H32O7;1H NMR (CDCl3,400 MHz)δ:5.67(1H,t,br s,H-15),5.13 (1H,s,H-17a),5.11 (1H,s,H-17b),4.89 (1H,t,J=3.6 Hz,H-6),4.37 (1H,dd,J=10.0,1.2 Hz,H2-20a),3.96 (1H,dd,J=10.0,1.6 Hz,H-20b),2.24,2.08 (each 3H,s,2 ×-OAc),0.98 (3H,s,H-18),0.92 (3H,s,H-19);13C NMR (CDCl3,100 MHz)δ:212.2 (C-1),35.2 (C-2),38.0 (C-3),32.5 (C-4),53.2 (C-5),74.5 (C-6),96.5 (C-7),51.0 (C-8),42.3 (C-9),48.6 (C-10),17.2 (C-11),31.8 (C-12),35.3 (C-13),25.9(C-14),74.5 (C-15),157.0 (C-16),109.8 (C-17),29.7 (C-18),23.0 (C-19),64.6 (C-20),173.6,170.1,21.9,21.0 (2 ×-OAc)。以上数据与文献[16]报道一致,故鉴定化合物5 为毛萼晶D(Maoecrystal D)。
化合物6 无色结晶(丙酮),EI-MS m/z 392[M]+;1H NMR (CDCl3-CD3OD,400 MHz)δ:5.07(1H,s,H-17a),5.05 (1H,s,H-17b),4.70 (1H,br s,H-15),4.31 (1H,s,H-1),4.24 (1H,d,J=9.6 Hz,H-20a),4.07 (1H,d,J=8.8 Hz,H-20b),3.72(1H,m,H-6),1.98 (3H,s,-OAc),1.11 (3H,s,H-18),1.02 (3H,s,H-19);13C NMR (C5D5N,100 MHz)δ:74.6 (C-1),25.6 (C-2),38.5 (C-3),33.7(C-4),58.4 (C-5),76.8 (C-6),97.2 (C-7),52.6(C-8),42.9 (C-9),39.9 (C-10),17.3 (C-11),32.7 (C-12),37.0 (C-13),26.8 (C-14),75.1 (C-15),162.1 (C-16),107.1 (C-17),32.8 (C-18),22.0 (C-19),63.0 (C-20),170.0,21.5 (-OAc)。以上数据与文献[16]报道一致,故鉴定化合物6 为毛萼晶E(Maoecrystal E)。
化合物7 无色粉末(氯仿),EI-MS m/z 388[M]+;1H NMR (CDCl3,400 MHz)δ:6.76 (1H,d,J=10.0 Hz,H-3),5.89 (1H,d,J=10.0 Hz,H-2),5.64 (1H,brs,H-6),5.05 (1H,brs,H-17a),4.82(1H,s,H-17b),4.31 (1H,d,J=10.8 Hz,H-20a),3.98 (1H,J=10.8 Hz,H-20b),3.96 (1H,d,J=2.0 Hz,H-7),2.12 (3H,s,-OAc),1.28 (3H,s,H-18),1.20 (3H,s,H-19);13C NMR (CDCl3,100 MHz)δ:198.0 (C-1),127.5 (C-2),160.5 (C-3),35.5 (C-4),52.2 (C-5),72.7 (C-6),96.8 (C-7),51.6 (C-8),42.5 (C-9),46.2 (C-10),17.6 (C-11),32.5 (C-12),35.6 (C-13),26.7 (C-14),74.4(C-15),157.2 (C-16),108.9 (C-17),29.2 (C-18),27.1 (C-19),64.8 (C-20),171.7,21.1(Oac)。以上数据与文献[17]报道一致,故鉴定化合物7 为毛萼晶B(Maoecrystal B)。
化合物8 白色粉末(氯仿),EI-MS m/z 360[M]+;1H NMR (CDCl3,400 MHz)δ:6.57 (1H,d,J=10.4 Hz,H-3),5.92 (1H,d,J=10.4 Hz,H-2),4.90 (1H,d,J=11.2 Hz,H-20a),4.12 (1H,d,J=11.2 Hz,H-20),1.30 (3H,s,H-18),1.28 (3H,s,H-19),1.18 (3H,d,J=6.8 Hz,H-17);13C NMR(CDCl3,100 MHz)δ:196.2 (C-1),125.0 (C-2),156.4 (C-3),36.0 (C-4),53.1 (C-5),170.2 (C-6),169.3 (C-7),59.0 (C-8),43.2 (C-9),49.8(C-10),18.1 (C-11),29.7 (C-12),35.3 (C-13),29.5 (C-14),216.4 (C-15),51.1 (C-16),16.8 (C-17),31.8 (C-18),24.7 (C-19),69.0 (C-20)。以上数据与文献[12]报道一致,故鉴定化合物8 为毛萼晶N(Maoecrystal N)。
化合物9 无色粉末(氯仿),EI-MS m/z 386[M]+;1H NMR (CDCl3,400 MHz)δ:6.34 (1H,t,J=2.4 Hz,H-15),5.10 (1H,d,J=1.2 Hz,H-17a),5.07 (1H,d,J=2.4 Hz,H-17b),4.61 (1H,d,J=8.8 Hz,H-20a),4.27 (1H,d,J=8.8 Hz,H-20b),3.78 (1H,t,J=2.8 Hz,H-3),3.25 (1H,d,J=7.6 Hz,H-9),2.14 (3H,s,-OAc),1.55 (3H,s,H-18),1.22 (3H,s,H-19);13C NMR (CDCl3,100 MHz)δ:205.8 (C-1),41.5 (C-2),77.4 (C-3),40.2 (C-4),132.5 (C-5),143.5 (C-6),193.9 (C-7),53.7(C-8),27.3 (C-9),52.5 (C-10),19.2 (C-11),31.9 (C-12),40.9 (C-13),38.4 (C-14),75.9 (C-15),150.7 (C-16),108.9 (C-17),23.1 (C-18),21.7 (C-19),67.0 (C-20),170.9,21.0 (-OAc)。以上数据与文献[18]报道一致,故鉴定化合物9 为Coetsoidin A。
化合物10 白色粉末(氯仿),EI-MS m/z 390[M]+;1H NMR (CDCl3,400 MHz)δ:9.80 (1H,d,J=4.8 Hz,H-7),5.20 (1H,d,J=12.0 Hz,H-20),4.70 (1H,d,J=12.0 Hz,H-20),4.75 (1H,m,H-1),2.04 (3H,s,-OAc),1.21 (3H,s,H-18),1.17(3H,d,J=7.6 Hz,H-17),1.00 (3H,s,H-19);13C NMR (CDCl3,100 MHz)δ:74.8 (C-1),24.0 (C-2),39.8 (C-3),34.0 (C-4),61.7 (C-5),202.3(C-6),169.7 (C-7),58.7 (C-8),43.4 (C-9),43.2(C-10),17.1 (C-11),29.1 (C-12),35.0 (C-13),28.3 (C-14),215.5 (C-16),50.9 (C-16),16.7 (C-17),32.9 (C-18),24.0 (C-19),67.6 (C-20),170.3,21.1 (-OAc)。以上光谱数据与文献[19]报道一致,故鉴定化合物10 为毛萼晶L(Maoecrstal L)。
1 The Editorial Committee of Flora of Guizhou (贵州植物志编辑委员会).Flora of Guizhou (Vol.8)(贵州植物志 第八卷).Chengdu:Sichuan Ethnic Publishing House,1989.489.
2 Sun HD,Huang SX,Han QB.Diterpenoids from Isodon species and their biological activities.Nat Prod Rep,2006,23:673-698.
3 Sun HD (孙汉董),Xu YL (许云龙),Jiang B (姜北).Diterpenoids from Isodon Species (香茶菜属植物二萜化合物).Beijing:Science Press,2001.113-114.
4 Xu MJ (许美娟),Cheng PY (程培元),Lin YL (林永乐),et al.Studies on chemical constituents of Rabdosia eriocalyx.J Chin Pharm Univ (中国药科大学学报),1987,18:113-116.
5 Wang J,Lin ZW,Sun HD.Flavonoids form Isodon eriocalyx.Nat Prod Sci,1998,4:38-41.
6 Wang WG,Du X,Li XN,et al.New bicyclo[3.1.0]hexane unit ent-kaurane diterpene and its seco-derivative from Isodon eriocalyx var.laxiflora.Org Lett,2012,14:302-305.
7 Li HL (李惠兰),Su C (苏沧),Wang MD (王懋德),et al.Study on Isodon eriocalyx against the spasmolysi of intestinal smooth muscle of guinea pig.J Yunnan Univ Tradit Chin Med (云南中医学院学报),1995,18:17-23.
8 Su H (舒晔),Wang MD (王懋德),Song YP (宋骥鹏),et al.Anti-inflammatory and bacteriostatic action of extract fromRabdosia eriocalyx (Dunn)Hara.J Yunnan Univ Tradit Chin Med (云南中医学院学报),1995,18(4):9-12.
9 Li SW (李思维),Li ML (李玛琳),Han QB (韩全斌),et al.Effect of oridonin,xindongnin A,ponicidin,eriocalyx in Bon human angiogenesis in vitro.Chin Pharm J (中国药学杂志),2007,42:1063-1066.
10 Wang L,Zhao WL,Yan JS,et al.Eriocalyxin B induces apoptosis of t(8;21)leukemia cells through NF-κB and MAPK signaling pathways and triggers degradation of AML1-ETO oncoprotein in a caspase-3-dependent manner.Cell Death Differ,2007,14:306-317.
11 Yu Z,Niu XM,Qian LP,et al.Synthesis and cytotoxicity of some new eriocalyxin B derivatives.Eur J Med Chem,2007,42:494-502.
12 Wang J (王佳),Zhao QS (赵勤实),Lin ZW (林中文),et al.Three new 6,7-seco-ent-kaurane diterpenoids from Isodon eriocalyx.Acta Botanica Yunnanica (云南植物研究),1997,19:191-193.
13 Yoshitaka K,Hironori O,Harukuni T,et al.Potential antitumor-promoting diterpenoids from the stem bark of Picea glehni.J Nat Prod,2000,63:817-820.
14 Wang XR (王先荣),Wang SQ (王素卿),Hu HP(胡惠平),et al.Studies on the chemical components of Veined Rabdosia.Chin Tradit Herb Drugs (中草药),1994,25:115-119.
15 Sun HD (孙汉董),Lin ZW (林中文),Wang DZ (王德祖),et al.The structure of neorabdosin.Acta Chim Sin (化学学报),1985,43:481-483.
16 Li CB (李春葆),Sun HD (孙汉董),Zhou J (周俊).The structures of new diterpenoids.maoecrystal A-E from Rabdosia Eeriocalyx.Acta Chim Sinca (化学学报),1988,46:657-662.
17 Wang ZY (王宗玉),Xu YL (许云龙).New diterpenoid constituents of rabdosia eriocalyx.Acta Botanica Yunnanica(云南植物研究),1982,4:407-411.
18 Wang J (王佳),Lin ZW (林中文),Sun HD (孙汉董).A novel diterpenoid from Isodon Eriocalyx.Acta Botanica Yunnanica (云南植物研究),1997,19:438-440.
19 Shen XY,Isogai A,Furiha K,et al.6,7-Seco-ent-kaurane diterpenoid from Rabdosia eriocalyx.Phytochemistry,1994,35:820-821.