Solvothermal Syntheses, Crystal Structures, Thermal Stability and Quantum Chemistry of Dinuclear Trialkyltin ComplexesConstructed by Camphoric Acid①
YU Jiang-Xi KUANG Dai-Zhi FENG Yong-Lan ZHU Xiao-Ming JIANG Wu-Jiu ZHANG Fu-Xing
Solvothermal Syntheses, Crystal Structures, Thermal Stability and Quantum Chemistry of Dinuclear Trialkyltin ComplexesConstructed by Camphoric Acid①
YU Jiang-Xi KUANG Dai-Zhi②FENG Yong-Lan ZHU Xiao-Ming JIANG Wu-Jiu ZHANG Fu-Xing
(421008)
Two dinuclear organotin complexes C8H14(CO2SnCy3)2(1) (Cy = cyclohexyl group) and C8H14[CO2Sn(CH2CMe2Ph)3]2(2) were synthesized by the reactions of camphoric acid with tricyclohexyltin hydroxide and bis[tri(2-methyl-2-phenyl)propyltin] oxide under solvothermal conditions, and these complexes were characterized by infrared spectra, elemental analyses, and H NMR spectra. The crystal of 1 belongs to the monoclinic system, space group21/with1.83478(19),= 1.52707(18),1.9849(2) nm,= 122.515(7)°,= 4,= 4.6896(9) nm3,D= 1.324 g/cm3,(Mo) = 1.103 mm-1,(000) = 1952,= 0.0697 and= 0.2040. In addition, thermal stability and quantum chemical calculation of 1 were also studied.
trialkyltin complex, camphoric acid, solvothermal synthesis, structure, quantum chemistry
1 INTRODUCTION
Organotin has attracted intensive attention with its bioactivity and wide applications in industry and agriculture. For example, organotin has been made successfully into the preservation of woods, fungi- cides, germicides, insecticides, and additives of marine antifouling coat[1-4]. Some organotin prepara- tions may inhibit the activity of tumor cells and therefore are potential anticancer agents[5], and some others may be used also as catalysts in some organic reactions, such as reduction, coupling[6, 7], esterifica- tion and interesterification[8, 9], and used as thermal stabilizer of PVC[10]. Organotin may form multiple configurations such as ladder-type, drum-type, cap- type, cage-type, ring, 1D, 2D and 3D configura- tions[11-17]through different alkyl groups and ligands connected with tin atom by Sn–C (O, N, S), and therefore organotin may have multiple performances due to their multiple configurations. Constituting new functional materials with special performances based on the assembly of polybasic carboxylic acid with metals has become a field of active researches. Camphoric acid attracted wide attention with its excellent constitutional function[18, 19]. Ma Chunlinsynthesized a series of trialkyltin camphoric carboxylates with innovative configuration and good thermal stability through the reaction of camphoric acid with R3SnCl (R = Me, Bu, Bz, Ph)[17]. Zhang Rufenobtained a 3D supramolecular organotin complex through the reaction of camphoric acid with Me3SnCl under hydrothermal conditions[20]. Recent- ly, we have prepared two dinuclear trialkyltin com- plexes, 1 and 2,reacting camphoric acid with Cy3SnOH, [(PhCMe2CH2)3Sn]2O under solvother- mal conditions, and report here their synthesis and characterization.
2 EXPERIMENTAL
2.1 Instruments and reagents
Tricyclohexyltin hydroxide and bis[tri(2-methyl- 2-phenyl)propyltin] oxide purchased from Zhejiang Huaxing Chemical & Pesticides Co., Ltd were chemically pure. DL-camphoric acid of chemical reagent grade was obtained from Shanghai Joint State-Private Xinzhong Chemical Factory. Methanol was analytically pure, purchased from Sino-Tianjin BASF Chemical Co., Ltd., and used directly without further purification.
Melting point was measured by a X4 binocular stereo microscopic melting point meter (Beijing Tektronix Instrument Co. Ltd.) without correction. The contents of C and H were determined by PE- 2400 (II) elemental analyzer (US PE Corporation). Infrared spectrum was recorded by an IR Prestige-21 infrared spectrometer (Japan Shimadzu Corporation, 4000~400 cm-1), and NMR spectrum by Bruker Avance 500 and Bruker Avance 400 NMR Spectrometer (Swiss Bruker Corporation, with TMS as internal standard). Thermogravimetric analyses were executed on a TG 209 F3 thermogravimetric analyzer (Germany Netzsch Corporation).
2.2 Syntheses of the complexes
As shown in Fig. 1, a mixture of DL-camphoric acid (1 mmol), tricyclohexyltin hydroxide (2 mmol) or bis[tri(2-methyl-2-phenyl)propyltin] oxide (1 mmol), CH3OH (10 mL) was stirred, and then placed in a 25 mL Teflon-lined stainless-steel vessel which was heated to 130 ℃ for 4 d and finally cooled to room temperature at a rate of 5 ℃·h-1to obtain complex 1 or 2.
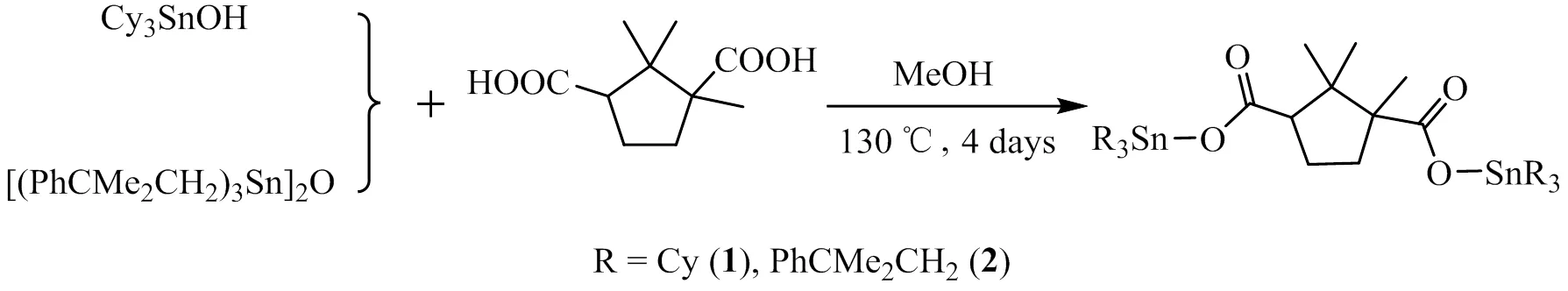
Fig. 1. Synthesis reactions of 1 and 2
Complex (1): 0.773 g, colorless crystal, yield 82.7%, m.p: 206~207 ℃. Elemental composition: (C46H80O4Sn2); theoretical value (%): C, 59.12; H, 8.63. Measured value (%): C, 59.20; H, 8.59. IR (KBr, cm-1): 2918 (m), 2845 (m), 1653 (vs), 1614(vs), 1450 (m), 1402(m), 1283 (m), 1246 (m), 1213 (w), 1167 (m), 1124 (m), 1084 (m), 991 (m), 937 (w), 878 (w), 841 (w), 802(w), 783 (w), 577 (w), 523 (w), 440 (w).1H NMR (CDCl3, 500MHz),(ppm): 2.80 (t,= 9.5 Hz, 1H, CC(CH2)CO2), 0.86~2.53 (m, 79H, -CH2-, -CH3).
Complex (2): 0.922 g, colorless crystal, yield 74.7%. m.p: 106~107 ℃. Elemental composition: (C70H92O4Sn2); theoretical value (%): C, 68.09; H, 7.50. Measured value (%): C, 68.09; H, 7.50. IR (KBr, cm-1): 3059 (w), 3026 (w), 2961 (m), 2922 (m), 1647 (vs), 1495 (w), 1443 (m), 1366(m), 1304 (s), 1285 (m), 1244 (w), 1192 (w), 1076 (m), 1030 (w), 999 (w), 768 (m), 700 (s), 615 (w), 554 (w).1H NMR (CDCl3, 400MHz),(ppm): 7.10~7.28 (m, 30H, Ph-H), 2.72 (t,= 9.2 Hz, 1H, CC(CH2)CO2), 0.89~2.59 (m, 61H, -CH2-, -CH3).
2.3 Crystal structure determination of complex 1
A crystal of complex 1 (0.25mm × 0.17mm × 0.13mm) was chosen for data collection which was performed on a Bruker Smart Apex II CCD diffrac- tometer equipped with a graphite-monochromated Moradiation (= 0.071073 nm) using a-scan mode at 296(2) K. A total of 26,124 reflections were collected in the range of 1.81≤≤25.05°, of whcih 8,280 were independent (int= 0.0251) and 5,580 were observed (> 2()). All the data were corrected byfactors and multi-scan absorbance. The crystal structure was solved by direct methods. The non-hydrogen atoms, H21X and H21Y were located in successive difference Fourier syntheses, and other hydrogen atoms were placed in the calculated positions. All hydrogen and non-hy- drogen atoms were refined by isotropic and anisotro- pic thermal parameters through full-matrix least- squares method.The final0.0697,0.2040, (Δ)max= 1554 and (Δ)min= –1650 e·nm-3. All cal- culations were completed by SHELXTL-97 program. Selected bond lengths and bond angles of complex 1 are given in Table 1.
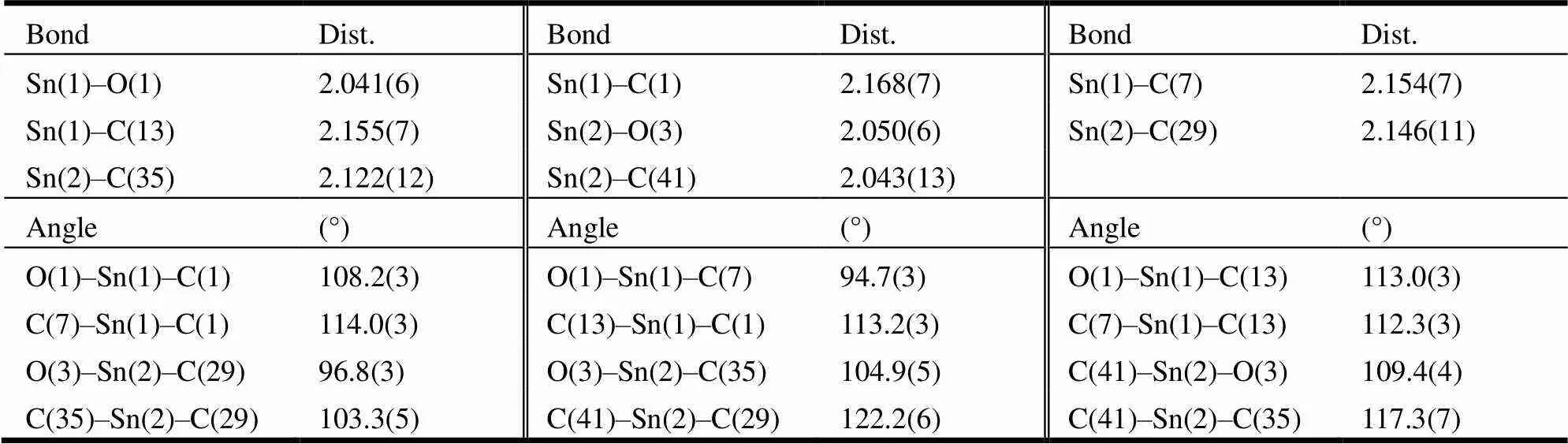
Table 1. Bond Length (Å) and Bond Angle (°)of Complex 1
3 RESULTS AND DISCUSSION
3.1 IR and 1H NMR
The absorption caused by carboxyl-hydroxyl association appearing at 2500~3300 cm-1in the ligand disappeared in the complex, indicating that the carboxyl group was deprotonated and coor- dinated with the tin atom; carboxyl unsymmetric and symmetric stretching vibrations occurring at 1700 and 1283 cm-1in the ligand shifted to 1653, 1614, 1450 and 1402 cm-1in complex 1 respectively, with the difference to be 203and 212 cm-1, showing that the carboxyl group was coordinated in a mono- dentate manner in 1[21], which agreed with the result of X-ray single-crystal diffraction. In complex 2, carboxyl symmetric and unsymmetric stretching vibration appeared at 1647and 1304 cm-1, sugges- ting that the carboxyl group was coordinated still in a monodentate manner in 2. For complex 1, the stretching vibration of Sn–Cand Sn–O bonds appeared at 577and 440 cm-1, while at 615and 554 cm-1in complex 2[22].
In the1H NMR spectra, the total numbers of protons calculated by integration are in agreement with the expected molecular composition of the complexes. Carboxyl-hydroxyl proton peak appea- ring at 12.10 ppm in the ligand disappeared in the complexes, indicating the formation of complexes 1 and 2. Triple absorption peaks at 2.80 ppm (1) and 2.72 ppm (2) in the ligand correspond to the methyne H peak of camphoric acid; multiple absorp- tion appearing within 0.86~2.53 ppm in 1and 0.89~2.59 ppm in 2 was attributed to the H peaks of methyl and methylene groups (cyclohexyl methyne for 1).
3.2 Crystal structure analysis of complex 1
The molecular structure of complex 1 is shown in Fig. 2 (ellipsoids probability: 10%). From this figure, we can see that a molecule of camphoric acid was connected with 2 tricyclohexyltins to form a dinu- clear configuration. The tin atom was coordinated with methyne carbons of 3 tricyclohexyl groups and a carboxyl oxygen atom of camphoric acid to generate a tetrahedral configuration with tin atom as the center. Three groups of Sn–C bonds in each tin atom had different bond lengths, and 3 groups of angle ∠C–Sn–O all deviated from 109.5° for a regular tetrahedron, showing that the tin atom and coordinated atoms formed a distorted tetrahedron. The distances between the tin atom and carboxyl oxygen atoms are as follows: Sn(1)–O(1) = 2.041(6) Å, and Sn(2)–O(3) = 2.050(6) Å, smaller than the sum of covalent radii of 2 atoms (2.160 Å), suggesting that stable coordination bonds are formed between Sn(1) and O(1) as well as between Sn(2) and O(3). The distances between the tin atom and other carboxyl oxygen atoms are as below: Sn(1)···O(2) = 2.8836(74) Å and Sn(2)···O(4) = 2.9689(78) Å. The interactions were weak, and therefore carboxyl group was coordinated with a tin atom in a monodentate manner in complex 1.
In the crystal of complex 1, a 1D ribbon-like structure is formed through the actions of C–H···O hydrogen bond (H(11B)···O(2)i2.5507(93) Å,∠C(11)–H(11B)···O(2)i132.856(904)°, i: –, –1/2+, –1/2–) between cyclohexyl hydrogen of one molecule and carbonyl oxygen of the adjacent other molecule (Fig. 3).
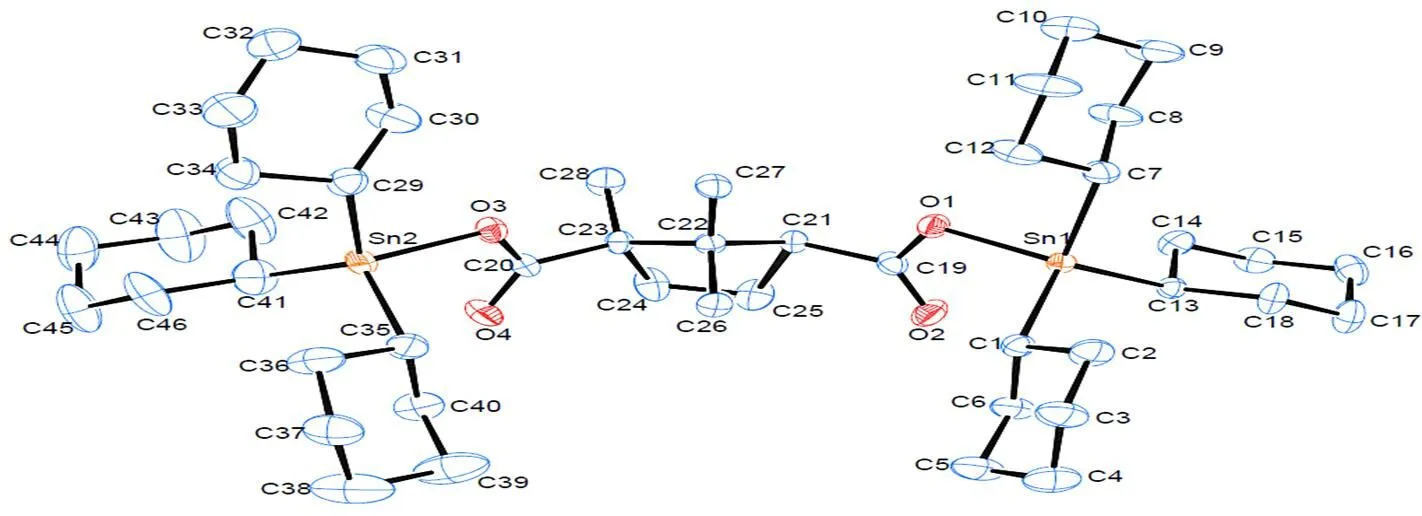
Fig. 2. Molecular structure of 1(Hydrogen atoms and the second component of disordered parts were omitted for clarity)
Fig. 3. 1D ribbon-like structure of 1 (i: –, –1/2 +, –1/2 –) constituted by hydrogen bonds (Some H atoms and cyclohexyl groups are omitted for clarity)
3.3 Thermal stability
Thermogravimetric analysis was carried out for complex 1 to investigate its thermal stability (Fig. 4). The thermogravimetric curve shows that 1 is stable up to 182 ℃, and then a noticeable weight loss up to 395 ℃appears (nearly 75.2 %). After that, no weight loss occurs. Assuming that the residue cor- responds to SnO2, the observed weight is basically in agreement with the calculated value (calcd. 32.3%). Thus, complex1shows good thermal stability.
3.4 Quantum chemical study
Quantum chemical calculation of complex 1 was performed with Gaussian03W program at the B3LYP/LANL2DZ level on a P4 personal computer. Molecular configuration parameters used in calcu- lation were obtained from the X-ray experimental data. 132 atoms, 626 basis functions, 1,594 primitive gaussians, 198electrons and 198electrons were involved in the calculation.
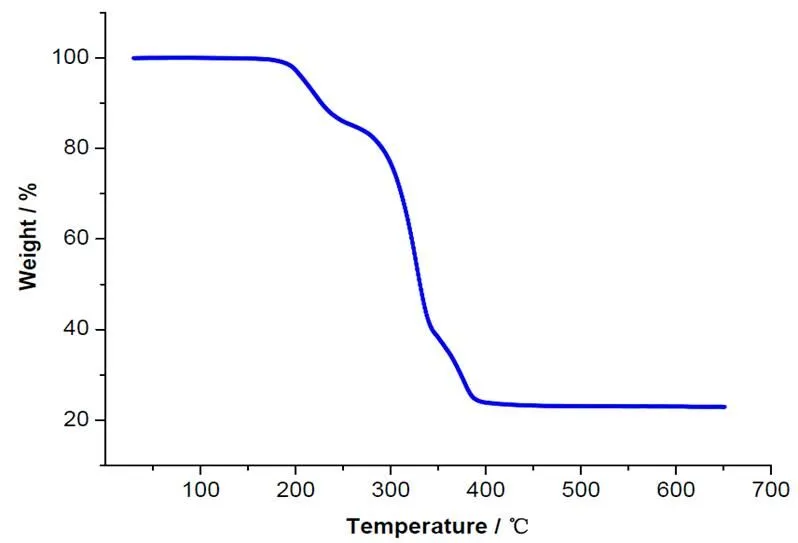
Fig. 4. Thermogravimetric curve of 1
3.4.1 Frontier molecular orbital energy
The overall stability of the structure correlates closely with the total energy of the system as well as the frontier molecular orbital energy. Based on calculation, for complex 1, the total energy was –2108.08855 a.u., the energy of the highest occupied molecular orbital (HOMO)HOMO= –0.21995 a.u., and that of the lowest unoccupied molecular orbital (LUMO)LUMO= –0.10980 a.u.,showing that the total energy and energy level of the occupied molecular orbital were both lower relatively, and the energy gap between the highest and lowest unoccupied molecular orbitals Δ= 0.11015 a.u.. It proves that the structure of complex 1 is stable. From the point of oxidation-reduction and charge transfer, the absolute value of the highest occupied molecular orbital energy |HOMO| was a great minus value, suggesting that electrons were difficult to ionize from HOMO, and therefore complex 1 was not liable to lose its electrons and its ground state was stable relatively.
3.4.2 Molecular orbital composition
In order to explore the bonding characteristics of the complex, the molecular orbitals were investigated systematically, and the calculation result is shown in Table 2 and Fig. 5.
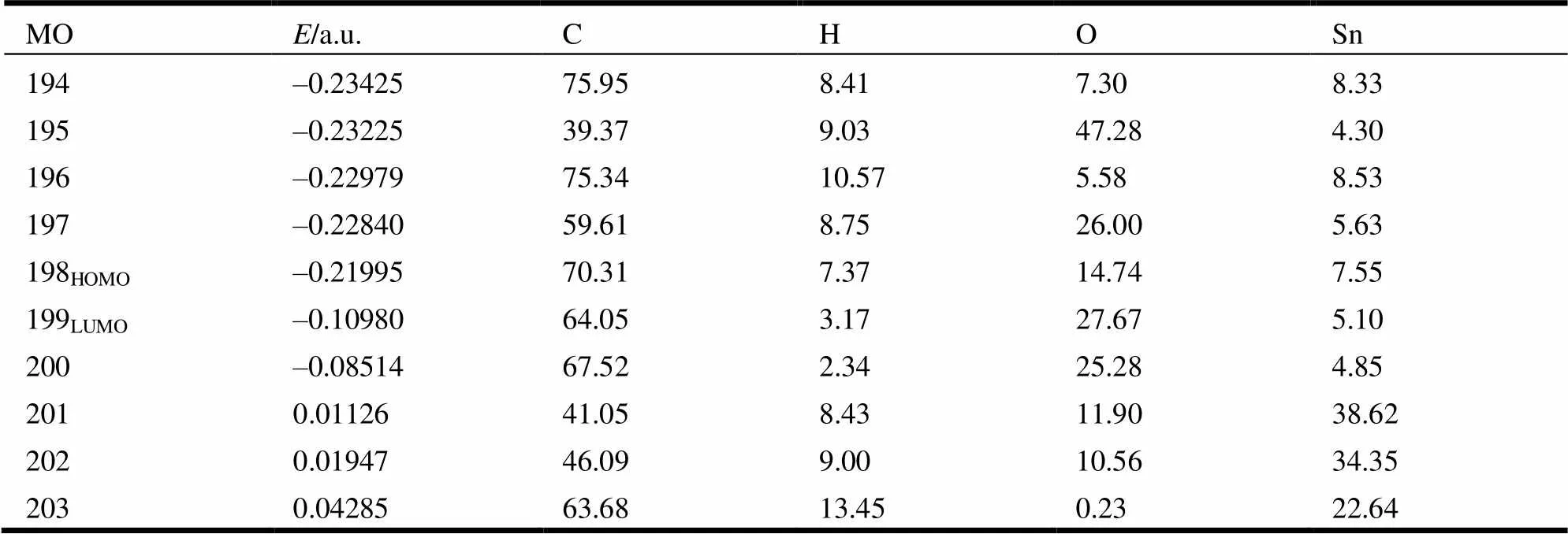
Table 2. Composition of Molecular Orbitals of Complex 1 (%) (B3lyp/Lanl2dz)
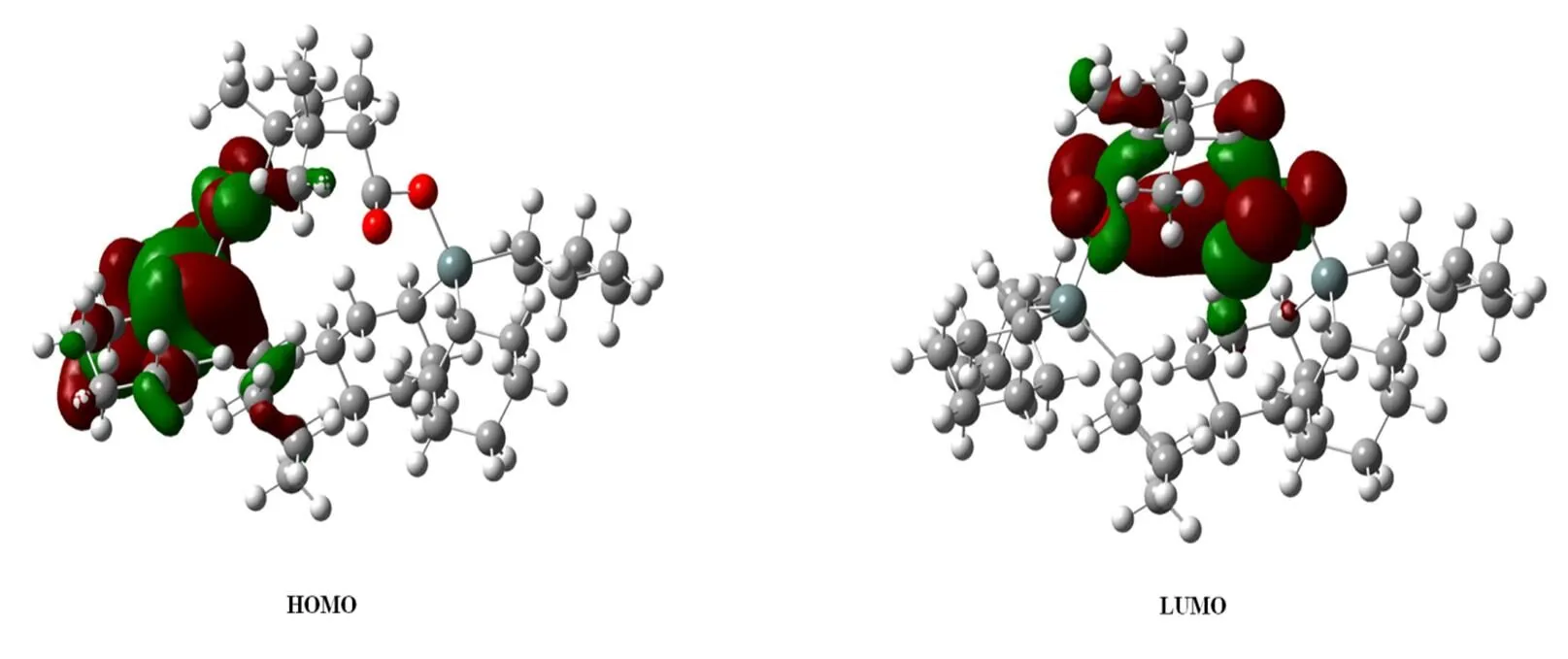
Fig. 5. Schematic diagram of the frontier molecular orbitals of 1
As to the composition of HOMO, carbon atoms made the greatest contribution to HOMO (70.31%), followed by the oxygen atoms (14.74%). The contribution of other atoms to HOMO was less, for example, the contribution of tin atom was 7.55%, and that of hydrogen atom was 7.37%. As regards the composition of LUMO, the contribution of various atoms changed noticeably. The contribution of carbon atoms to LUMO was up to 64.05%, oxygen atoms 27.67%, tin atoms 5.10%; and that of all hydrogen atoms was 3.17%.
The comparison of orbital components between HOMO and LUMO demonstrated that when the complex transferred to the excited state from its ground state, electrons transferred mainly from the orbital of carbon atom to that of oxygen atom to form the electron transfer complex.
(1) Rahman, M. M.; Jusoh, I.; Affan, M. A.; Husaini, A.; Hamdan, S. Efficacy of novel organotin(IV) complexes on non-durable tropicalwood against decay fungi.2013, 71, 463–471.
(2) Salam, M. A.; Affan, M. A.; Arafat, M. A.; Saha, R.; Nasrin, R. Synthesis, characterization, and antibacterial activities of organotin(IV) complexes with 2-acetylpyridine-N(4)-cyclohexylthiosemicarbazone (HAPCT).2013, 24, 43–52.
(3) Knowles, C. O. Chemistry and toxicology of quinoxa-line, organotin, organofluorine, and formamidine acaricides.. 1976, 14, 93–102
(4) Omae, L.Organotin antifouling paints and their alternatives.2003, 17, 81–105.
(5) Hussain, M.; Rehman, Z.; Hanif, M.; Altaf, M.; Rehman, A.; Ali, S.; Cavell, K. J. Structural studies of diethyltin(IV) derivatives and their biological aspects as potential antitumor agents against agrobacterium tumefacien cells.2011, 25, 412–419.
(6) Dumartin, G.; Pourcel, M.; Delmond, B.; Donard, O.; Pereyre, M.In situ generated, polymer-supported organotin hydrides as clean reducing agents.. 1998, 39, 4663–4666.
(7) Yabe, Y.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Palladium on charcoal-catalyzed ligand-free Stille coupling.2010, 66, 8654–8660.
(8) Jousseaume, B.; Guillou, V.; Noiret, N.; Pereyre, M.; Francés, J. M. Synthesis and thermal decomposition of substituted [2-(acyloxy)alkyl]diorganotin compounds, Bu2Sn(X)CH2CHR1OCOR2(X halogen 2,4-pentanedionate or OCOR), potential sources of organotin catalysts.1993, 450, 97–102.
(9) He, X.; Li, Z.; Su, K.; Cheng, B.; Ming, J. Study on the reaction between bisphenol A and dimethyl carbonate over organotin oxide.2013, 33, 20–23.
(10) Allen, D. W.; Brooks, J. S.; Unwin, J.; McGuinness, J. D.Studies of the degradation of organotin stabilizers in poly(vinyl chloride) during gamma irradiation.1987, 1, 311–317.
(11) Chandrasekhar, V.; Schmid, C. G.; Burton, S. D.; Holmes, J. M.; Day, R. O.; Holmes, R. R. New drum and ladder organooxotin carboxylates.. 1987, 26, 1050–1056.
(12) Chen, M. S.; Kuang, D. Z.; Zhang, C. H.; Deng, Y. F.; Li, W.; Yang, Y. Q. Synthesis and crystal structure of a hexameric organooxotin cluster from benzilic acid.2005, 24, 1249–1253.
(13) Swamy, K. C. K.; Day, R. O.; Holmes, R. R. Organotin clusters. 7. polynuclear chlorine containing organooxotin clusters.. 1992,31, 4184–4193.
(14) Chandrasekhar, V.; Thilagar, P.; Bickley, J. F.; Steiner, A. Alternating hydrophilic and hydrophobic pockets in the channel structures of organostannoxane prismanes: preferential confinement of guest molecules.. 2005, 127, 11556–11557.
(15) Prabusankar, G.; Murugavel, R. Hexameric organotin carboxylates with cyclic and drum Structures.2004, 23, 5644–5647.
(16) Yin, H. D.; Li, F. H.; Li, L. W.; Li, G. Self-assembly of triorganotin(IV) or diorganotin(IV) moieties and 2-methylpyrazine-5-acid: syntheses, characterizations and crystal structures of monomeric, polymeric or tetranuclear macrocyclic compounds.2007, 692, 1010–1019
(17) Ma, C.; Wang, Q.; Zhang, R.Self-assembly of triorganotin complexes: syntheses, characterization, and crystal structures of dinuclear, 1D polymeric chain, and 2D network polymers containing chiral (+)-(1R,3S)-camphoric acid and meso-cis-4-cyclohexene-1,2-dicarboxylic acid ligands.2008, 2008, 1926–1934.
(18) Zhang, L. P.; Ma, J. F.; Yang, J.; Liu, Y. Y.; Wei, G. H. 1D, 2D, and 3D metal-organic frameworks based on bis(imidazole) ligands and polycarboxylates: syntheses, structures, and photoluminescent properties.. 2009, 9, 4660–4673.
(19) Zhang, M. D.; Di, C. M.; Qin, L.; Yao, X. Q.; Li, Y. Z.; Guo, Z. J.; Zheng, H. G. Diverse structures of metal-organic frameworks based on a new star-like tri(4-pyridylphenyl)amine ligand.. 2012, 12, 3957–3963.
(20) Zhang, R. F.; Wang, Q. F.; Yang, M. Q.; Wang, Y. R.; Ma, C. L. Five new dicarboxylate organotin complexes under hydrothermal condition: syntheses, characterization and crystal structures of 1D, 2D and 3D polymers constructed from dimeric tetraorganodistannoxane units.2008, 27, 3123–3131.
(21) Xie, Q. L.; Xu, X. H.; Zhang, D. K. Synthesis and structure analyais of tribenzyltin carboxylate.1992, 50, 508–514
(22) Ke, Y. K.; Dong, H. R.; Chemical Industry Press: Beijing 1998, 932–935
5 August 2013;
17 July 2014 (CCDC 951496)
Natural Science Foundation of Hunan Province (No. 13JJ3112), Scientific & Technological Projects of Hunan Province (No. 2013TZ2025, 2014NK3086), Open Foundation of Innovation Platform of Hunan Provincial University (No. 13K105, 14K014), Scientific & Technological Projects of Hengyang City (No. 2012KJ30), Cultivation projects Based on Collaborative Innovation Center of Hengyang Normal University (No.12XT02) and the Youth Backbone Teacher Training Program of Hengyang Normal University (2012)
. Kuang Dai-Zhi, (1955-), male, professor, majoring in the research of organometallic chemistry. E-mail: hnkcq@qq.com
- 结构化学的其它文章
- Thermoelectric Properties of the CuGaTe2 Crystal from First-principles Calculations: the Role of Doping and Temperature①
- Morphology, Size-controlled Synthesis of CoO anostructure and Its Magnetic Property①
- The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
- Synthesis, Crystal Structure and Antiproliferative Activity of 2-(((5-(((5,7-Dimethyl-[1,2,4]triazolo-[1,5-a]pyrimidin-2-yl)thio)methyl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)-4H- chromen-4-one Methanol Solvate①
- Synthesis, Crystal Structure and Luminescent Property of an Eu3+ Complex①
- Synthesis, Dimer Crystal Structure and Herbicidal Activity of 2-(4-Ethoxybenzoyl)cyclopentane-1,3-dione①

