Morphology, Size-controlled Synthesis of CoO anostructure and Its Magnetic Property①
JIA Xio YUE Fng YANG Gung PAN Hi-Bo LIU Wen-Ge
Morphology, Size-controlled Synthesis of CoO anostructure and Its Magnetic Property①
JIA XiaoaYUE FangaYANG GuangaPAN Hai-BoaLIU Wen-Geb②
a(350002)b(350001)
CoO nanostructures with tunable morphology and size have been prepared via a simple one-pot solvothermal synthesis. The as-prepared nanoparticles were fully characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), field-emission scanning electron microscope (FESEM), etc. The morphology and size of the product can be easily controlled by adjusting the raw materials added. Reaction time and the solvent ratio also play important roles in the synthesis of octahedral nanostructures. The magnetic property of the as-prepared samples was also investigated.
controlled synthesis, CoO nanostructure, magnetic property
1 INTRODUCTION
Transition metal oxides have a wide variety of applications, including gas sensitivity, photocata- lysis, lithium ion battery, chemical sensors, and so on[1-12]. Metal oxides with various sizes and shapes usually behave unusual physiochemical properties, and continuing to evoke interest. So far, nanoma- terails with special morphology have been conti- nually synthesized[13-22].As the classical antiferro- magnetic transition metal oxide, CoO nanoparticles have been widely studied due to their potential applications based on magnetic[17, 18, 23], elec- tric[24-28], catalytic[29-31], solar[32]properties, etc. Monodisperse CoO nanoparticles have been synthe- sized mainly by thermal decomposition of cobalt surfactant complexes in long-chain hydrocarbon or other organic solvents[33-38]. For example, Yin and Wang have successfully synthesized tetrahedral CoO nanocrystals via the oxidation of Co2(CO)8in toluene in the presence of surfactant Na(AOT)[33]. A series of CoO nanoparticles with tetrahedral, flo- werlike, tetrapod and spherical shapeswere obtained via the decomposition of cobalt(II) oleate or cobalt(II) acetylacetonate complex in long-chain hydrocarbon solvents such as octadecene[36],oleic acid, oleylamine[37], dodecanol[38]and so on. Mean- while, microemulsion method[39], gel method[40]and electrochemical deposition method[41]were also reported for the preparation of CoO products.
Although various methods have been used to synthesize CoO nanoparticles, these methods usually need complex reaction conditions, and the high-cost raw materials often contain some bio- toxic species due to the application of toxic reac- tants. Herein, a simple and environmentally friendly one-pot approach was reported for the controlled synthesis of CoO nanoparticles. Using cost-effec- tive cobalt(II) chloride hexahydrate as the cobalt source, nearly uniform octahedralCoO nanopar- ticles were synthesized. What’s more, the morpho- logies of the products can be easily adjusted by changing the cobalt source added. When cobalt acetate tetrahydrate instead of cobalt(II) chloride hexahydrate was used as the raw material, uniform distorted-octahedralCoO nanoparticles with smaller size would be synthesized.
The magnetic property of the as-prepared samples was also investigated. Both octahedral and distorted-octahedralCoO nanoparticles are paramag- netic at 300 K, and the M. H measurements are linear passing through zero. Octahedral CoO nano- particles showed an increasing slope compared to the distorted-octahedralCoO nanoparticles, which can be attributed to the difference in size and shape between the two samples.
2 EXPERIMENTAL
2.1 Chemicals
The reagents purchased from Sinopharm Che- micalReagent Co. Ltd were all of analytical grade. Anhydroussodium acetate (NaAc),-octanol (C8H17OH), ethanol (CH3CH2OH) and cobalt(II) chloride hexahydrate (CoCl2·6H2O) were used without further purification.Cobalt acetate tetrahy- drate (Co(CH3COO)2·4H2O) and cobalt nitrate hexahydrate (Co(NO3)2·6H2O) were dried in the desiccator for a few days before use.
2.2 Synthesis of octahedral CoO nanoparticles
In a typical experiment, 0.440 g (1.85 mmol) of CoCl2·6H2O and 0.410 g (5 mmol) of NaAc were dissolved in the mixture of-octanol (12 mL) and ethanol (8 mL) under magnetic stirring at 70 ℃for 1 h. Then the precursor solution was placed into a 25 mL autoclave with a Teflon liner. The autoclave was reacted at 230℃ for 4 h. The resultant suspension was collected by centrifugation and washed with deionized water and ethanol for several times.
2.3 Synthesis of distorted-octahedral CoO nanoparticles
Reaction of Co(CH3COO)2·4H2O (0.460 g, 1.85 mmol) with 12 mL of-octanol and 8 mL of ethanol at 220 ℃ for 4 h led to distorted-octahedral CoO nanoparticles.
2.4 Characterization
The nanocrystal structures of the obtained octahe- dral and distorted-octahedral CoO nanoparticles were thoroughly characterized with X-ray powder diffraction (XRD, Rigaku D/Max 2200PC, Curadiation;= 1.5418 Å), scanning electron micro- scopy (SEM, Nova NanoSEM 230), high-resolution TEM (HR-TEM, Tecnai G2 F20 S-TWIN, 200 kV). The magnetic properties of the samples were deter- mined on a SQUID (Quantum Design, MPMSXL-7) magnetometer.
3 RESULTS AND DISCUSSION
The crystal structures of octahedral CoO nano- particles were characterized by XRD, as shown in Fig. 1c. The XRD pattern indicated that all dif- fraction peaks of the as-prepared CoO octahedra were assigned to face-centered cubic CoO structure (JCPDS 43-1004). SEM image in Fig. 1a shows that the synthesized CoO nanocrystals have nearly uniform octahedral morphology with an average diameter of ca. 500 nm. TEM image in Fig. 1b shows the corresponding tetragonal projected shapes of the octahedral product.
The reaction solvent is very important for the structure of the product. In order to investigate the effect of solvent on the crystal morphology, a series of experiments were carried out by using different ratios of solvents with other conditions unchanged. When only-octanol was used as the solvent, octahedral structure of ca. 1 μm in diameter could be obtained, with some irregular column structures around (Fig. 2a). Increasing the volume of ethanol to 5 mL, the product showed mainly octahedral structure of ca. 630 nm but with a wide size distri- bution. The product has optimal narrow range of sizes when the volume ofoctanol and ethanol was 12 and 8 mL respectively, with an average diameter of ca. 500 nm as shown in Fig. 2c. Products with octahedral structure could also be synthesized when adding 10 and 15 mL of ethanol respectively, with decreased sizes form ca. 400 to 270 nm (Fig. 2d and 2e). When the reactants were dissolved in pure ethanol (Fig. 2f), nanoparticles of ca. 100 nm formed instead of octahedral structures. As a result, the solvents play important roles in the synthesis of as-prepared CoO nanoparticles. With the proportion of ethanol increasing, the sizes of octahedral product decreased (from 630 to 270 nm), till the nano- particles (ca. 100 nm) formed instead.
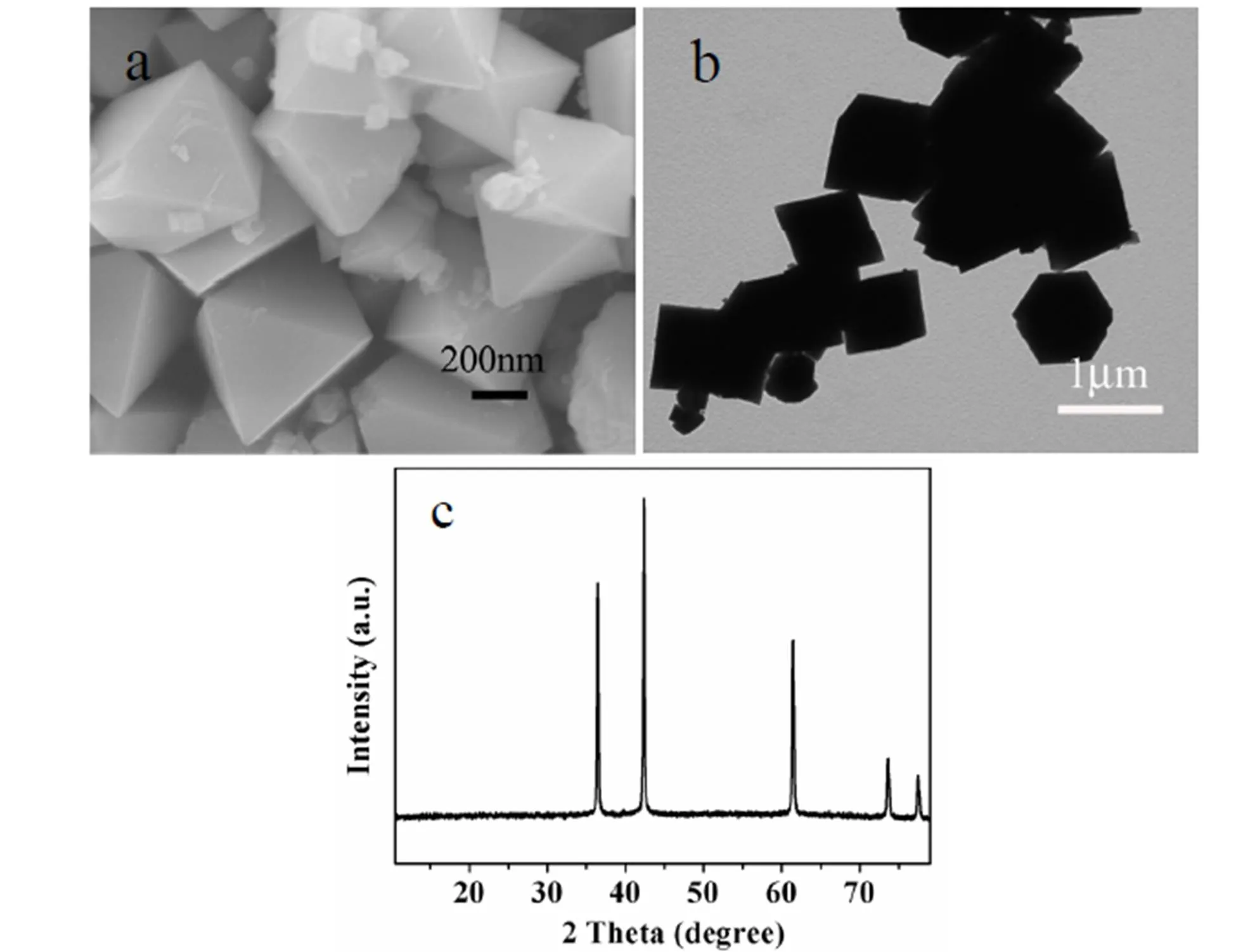
Fig. 1. SEM, TEM images and the corresponding XRD pattern of octahedral CoO nanoparticles in a typical synthesis
Fig. 2. SEM images of the samples synthesized with different solvent ratio.-octanol and ethanol in the ratio of(a)-octanol; (b) 15:5; (c) 12:8; (d) 10:10; (e) 5:15; (f) ethanol
In order to investigate the formation mechanism of as-prepared octahedral CoO nanoparticles, a detailed time-dependent experiment was conducted, and the corresponding XRD patterns are shown in Fig. 3a. When the reaction was conducted for 1 h, the diffraction peaks were weak as a result of the small particle size (ca. 130 nm in SEM image), and the phase is a mixture of face-centered cubic CoO (JCPDS 43-1004) and cobalt oxide chloride hydrate (JCPDS 02-1119, denoted by asterisks). Increasing the reaction time to 2 h, the diffraction peaks assigned to cubic CoO became narrower, while those of cobalt salt precursors weakened. Results in XRD patterns and SEM images (not shown) clearly indicated that pure phase of cubic octahedral CoO nanopartiles began to form at the reaction time of 3 h, and uniform octahedral CoO nanoparticles with size of ca. 500 nm could be prepared at 4 h in a typical synthesis, as shown in Figs. 1 and 2c.
When using CoCl2·6H2O as the raw material, cobalt oxide chloride hydrate was formed at first, and the diffraction peaks of cobalt salt precursors disappeared until 3 h. Thus we believe that the anions were involved in the formation of octahedral CoO nanoparticles. So, different cobalt salt raw materials were also used to investigate the effect of anions on the product. When Co(NO3)2·6H2Oinstead of CoCl2·6H2O was used as the raw material, the phase of product was impure. The upper pattern in Fig. 3b shows CoO (JCPDS 43-1004) and Co3O4(JCPDS 42-1467, denoted by asterisks) were obtained after reaction for 4 h, which may be caused by the oxidizability of NO3-. However, when Co(CH3COO)2·4H2O was used as the raw material, pure phase CoO product would be prepared even after reacting for 1 h, as shown in the other four patterns below in Fig. 3b. The difference in the formation of product may be caused by the lower thermal decomposition temperature of acetate. As shown in Fig. 4a, uniform distorted-octahedral CoO nanoparticles with size of ca. 100 nm were prepared in a typical synthesis. The uniform tetragonal projected shapes further indicated the octahedral structures of the product, and some octahedra even have hollow structures, as shown in Fig. 4b.
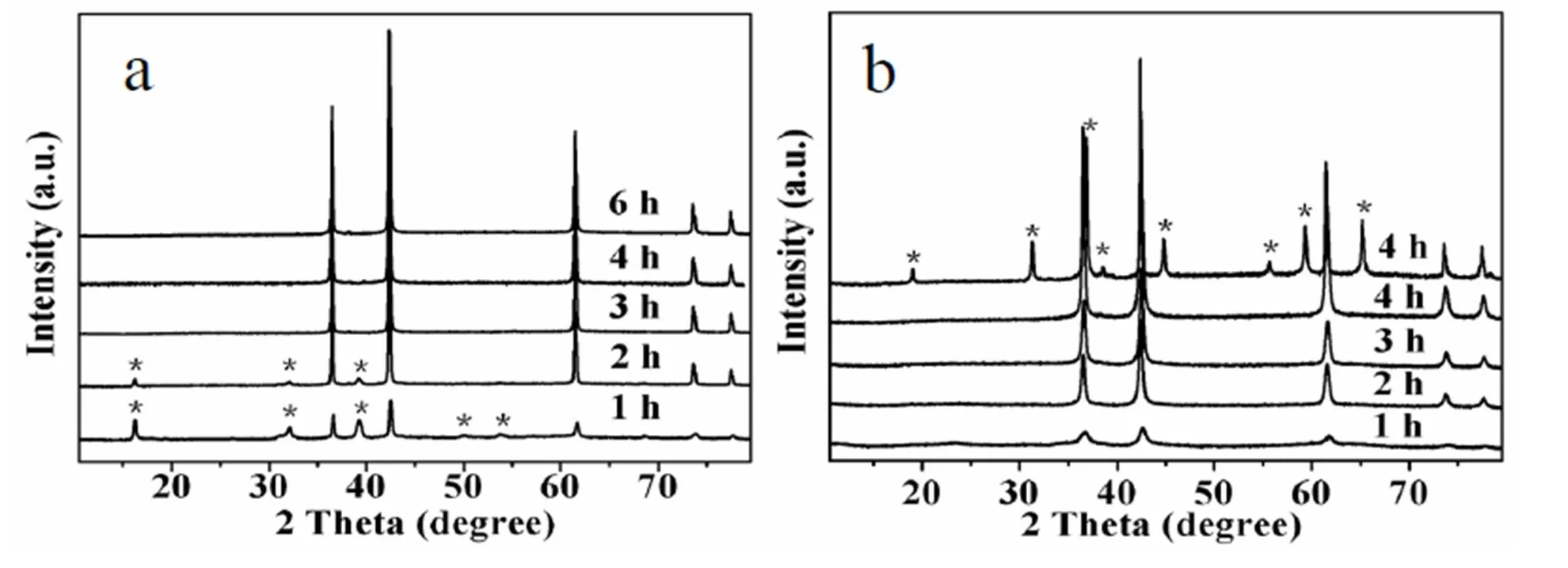
Fig. 3. XRD patterns of the products prepared with different cobalt salts at different reaction time.(a)CoCl2·6H2O (1~6 h); (b) Co(NO3)2·6H2O (denoted by asterisks, 4 h), Co(CH3COO)2·4H2O (1~4 h)
Fig. 4. SEM, TEM images of the as-synthesized distorted-octahedral CoO nanoparticles in a typical synthesis
Furthermore, the magnetic properties of the as- prepared octahedral (S1) and distorted-octahedral (S2) CoO nanoparticles were investigated. As shown in Fig. 5, field-dependent magnetization measure- ments were carried out at 300 K in the applied magnetic field from –80 to 80 kOe. The M. H measurements are linear for both samples S1 and S2, which is consistent with the results that CoO nanoparticles are paramagnetic above Néel tempera- ture (T), and the linear-shape of the hysteresis loop shows the characteristic of antiferromagnetism of CoO nanoparticles[17, 42]. The magnetization of both samples rose rapidly as the applied field increased, and the magnetization of samples S1 and S2 is around 5.6 and 4.9 emu/g at 80 kOe respectively, which is a little higher than the results reported in the literature with similar size[18]. Sample S1 showed a slowly increasing slope compared to sample S2, which may be attributed to the differences in the microstructures of the samples, as different surface environment often shows a great influence on the magnetic properties of nanopartilces[43-47].
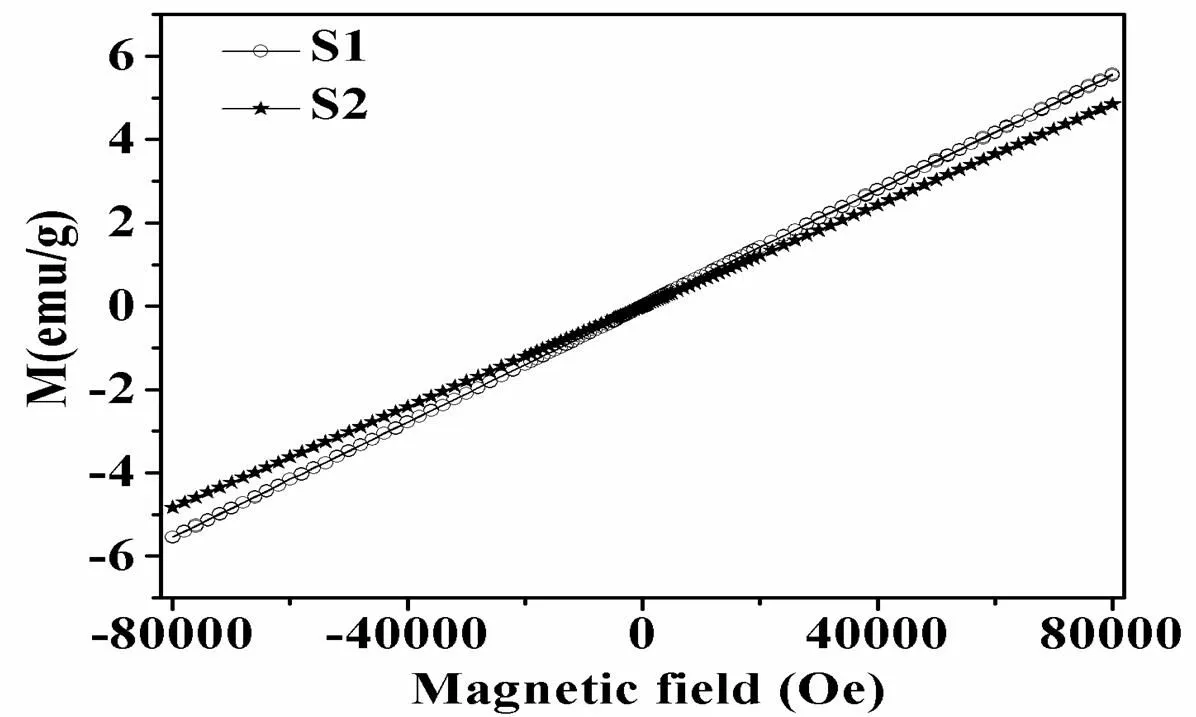
Fig. 5. Field variation of magnetization at 300 K for the as-prepared octahedral and distorted-octahedral CoO nanoparticles
4 CONCLUSION
In summary, one-pot controlled synthesis of octahedral CoO nanoparticles was reported. The morphology and size of the product can be easily controlled by adjusting the reaction parameters, such as solvent ratio, inexpensive inorganic raw materials, etc. Furthermore, the magnetic property shows that both octahedral and distorted-octahedral CoO nano particles were paramagnetic at 300 K, and the mag- netization behavior was related to different mor- phologies and sizes of the samples.
(1) Song, H. J.; Jia, X. H.; Zhang, X. Q. Controllable fabrication, growth mechanism, and gas sensing properties of hollow hematite polyhedra.2012,22, 22699–22705.
(2) Zhao, C.; Zhang, G.; Han, W.; Fu, J.; He, Y.; Zhang, Z.; Xie, E. Electrospun In2O3/-Fe2O3heterostructure nanotubes for highly sensitive gas sensorapplications.2013,15, 6491–6497.
(3) Song, H. J.; Jia, X. H.; Qi, H.; Yang, X. F.; Tang, H.; Min, C. Y. Flexible morphology-controlled synthesis of monodisperse-Fe2O3hierarchical hollow microspheres and their gas-sensing properties.2012,22, 3508–3516.
(4) Kim, J.; Kim, W.; Yong, K. CuO/ZnO heterostructured nanorods: photochemical synthesis and the mechanism of H2S gas sensing.2012, 116, 15682–15691.
(5) Sun, P.; He, X.; Wang, W.; Ma, J.; Sun, Y.; Lu, G. Template-free synthesis of monodisperse-Fe2O3porous ellipsoids and their application to gas sensors.2011, 14, 2229–2234.
(6) Wang, Z.; Zhou, L. Metal oxide hollow nanostructures for lithium-ion batteries.2012,24, 1903–1911.
(7) Guo, Y.; Hu, J.; Wan, L. Nanostructured materials for electrochemical energy conversion and storage devices.2008, 20, 2878–2887.
(8) Wu, H. B.; Chen, J. S.; Hng, H. H.; Lou, X. W. D. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries.2012, 4, 2526–2542.
(9) Litter, M. I. Heterogeneous photocatalysis: transition metal ions in photocatalytic systems.1999, 23, 89–114.
(10) Xu, X.; Randorn, C.; Efstathiou, P.; Irvine, J. T. A red metallic oxide photocatalyst.2012,11, 595–598.
(11) Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and photoinduced hydrophilicity of various metal oxide thin films.2002, 14, 2812–2816.
(12) Chen, Y.; Chen, H.; Zeng, D.; Tian, Y.; Chen, F.; Feng, J.; Shi, J. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery.2010, 4, 6001–6013.
(13) Wei, W.; Wang, Z.; Liu, Z.; Liu, Y.; He, L.; Chen, D.; Umar, A.; Guo, L.; Li, J. Metal oxide hollow nanostructures: fabrication and Li storage performance.2013,238, 376–387.
(14) Wu, Z.; Yu, K.; Zhang, S.; Xie, Y. Hematite hollow spheres with a mesoporous shell: controlled synthesis and applications in gas sensor and lithium ion batteries.2008, 112, 11307–11313.
(15) Liang, H.; Wang, Z. Facile synthesis and photocatalytic activity of cocoon-like hollow hematite nanostructures.2013, 96, 12–15.
(16) Zhu, J.; Yin, Z.; Yang, D.; Sun, T.; Yu, H.; Hoster, H. E.; Hng, H. H.; Zhang, H.; Yan, Q. Hierarchical hollow spheres composed of ultrathin Fe2O3nanosheets for lithium storage and photocatalytic water oxidation.2013, 6, 987–993.
(17) Dai, Q.; Tang, J. Magnetic properties of CoO nanocrystals prepared with a controlled reaction atmosphere.2013,3, 9228–9233.
(18) Dai, Q.; Tang, J. The optical and magnetic properties of CoO and Co nanocrystals prepared by a facile technique.2013,5, 7512–7519.
(19) Wang, X.; Yu, L.; Hu, P.; Yuan, F. Synthesis of single-crystalline hollow octahedral NiO.. 2007, 7, 2415–2418.
(20) Jiang, X.; Herricks, T.; Xia, Y. CuO nanowires can be synthesized by heating copper substrates in air.2002, 2, 1333–1338.
(21) Hu, X.; Zhang, T.; Jin, Z.; Huang, S.; Fang, M.; Wu, Y.; Zhang, L. Single-crystalline anatase TiO2dous assembled micro-sphere and their photocatalytic activity.. 2009, 9, 2324–2328.
(22) Israr-Qadir, M.; Jamil-Rana, S.; Nur, O.; Willander, M.; Larsson, L.; Holtz, P. O. Fabrication of ZnO nanodisks from structural transformation of ZnO nanorods through natural oxidation and their emission characteristics.2014, 40, 2435–2439.
(23) Roth, W. L. Magnetic structures of MnO, FeO, CoO, and NiO.. 1958, 110, 1333–1341.
(24) Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J. M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries.2000, 407, 496−499.
(25) Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Self-assembled mesoporous CoO nanodisks as a long-life anode material for lithium-ion batteries.2012,22, 13826−13831.
(26) Guan, H.; Wang, X.; Li, H.; Zhi, C.; Zhai, T.; Bando, Y.; Golberg, D. CoO octahedral nanocages for high-performance lithium-ion batteries.2012, 48, 4878−4880.
(27) Jiang, J.; Liu, J.; Ding, R.; Ji, X.; Hu, Y.; Li, X.; Hu, A.; Wu, F.; Zhu, Z.; Huang, X. Direct synthesis of CoO porous nanowire arrays on Ti substrate and their application as lithium-ion battery electrodes.2010, 114, 929−932.
(28) Zhou, C.; Zhang, Y.; Li, Y.; Liu, J. Construction of high-capacitance 3D CoO@polypyrrole nanowire array electrode for aqueous asymmetric supercapacitor.2013, 13, 2078–2085.
(29) Lu, A.; Chen, Y.; Zeng, D.; Li, M.; Xie, Q.; Zhang, X.; Peng, D. L. Shape-related optical and catalytic properties of wurtzite-type CoO nanoplates and nanorods.2014,25, 035707.
(30) Liao, L.; Zhang, Q.; Su, Z.; Zhao, Z.; Wang, Y.; Li, Y.; Lu, X.; Wei, D.; Feng, G.; Yu, Q. Efficient solar water-splitting using a nanocrystalline CoO photocatalyst.2014, 9, 69–73.
(31) Lin, H. K.; Chiu, H. C.; Tsai, H. C.; Chien, S. H.; Wang, C. B. Synthesis, characterization and catalytic oxidation of carbon monoxide over cobalt oxide.2003, 88, 169–174.
(32) Barrera, E.; González, I.; Viveros, T. A new cobalt oxide electrodeposit bath for solar absorbers.1998, 51, 69-82.
(33) Yin, J.; Wang, Z. L. Ordered self-assembling of tetrahedral oxide nanocrystals.1997, 79, 2570–2573.
(34) Ghosh, M.; Sampathkumaran, E.; Rao, C. Synthesis and magnetic properties of CoO nanoparticles.2005, 17, 2348–-2352.
(35) Xu, C.; Liu, Y.; Xu, G.; Wang, G. Fabrication of CoO nanorods via thermal decomposition of CoC2O4precursor.2002, 366, 567–571.
(36) Zhang, Y.; Zhu, J.; Song, X.; Zhong, X. Controlling the synthesis of CoO nanocrystals with various morphologies.2008, 112, 5322–5327.
(37) Wang, H.; Si, H.; Zhao, H.; Du, Z.; Li, L. S. Shape-controlled synthesis of cobalt oxide nanocrystals using cobalt acetylacetonate.2010, 64, 408–410.
(38) Zhang, Y.; Zhong, X.; Zhu, J.; Song, X. Alcoholysis route to monodisperse CoO nanotetrapods with tunable size.2007, 18, 195605.
(39) Sun, G.; Zhang, X.; Cao, M.; Wei, B.; Hu, C. Facile synthesis, characterization, and microwave absorbability of CoO nanobelts and submicrometer spheres.2009, 113, 6948–6954.
(40) Ramos, J.; Millan, A.; Palacio, F. Production of magnetic nanoparticles in a polyvinylpyridine matrix.2000, 41, 8461–8464.
(41) Heli, H.; Yadegari, H. Nanoflakes of the cobaltous oxide, CoO: synthesis and characterization.2010, 55, 2139–2148.
(42) Tracy, J. B.; Weiss, D. N.; Dinega, D. P.; Bawendi, M. G. Exchange biasing and magnetic properties of partially and fully oxidized colloidal cobalt nanoparticles.2005,72, 064404.
(43) Jia, X.; Chen, D.; Jiao, X.; He, T.; Wang, H.; Jiang, W. Monodispersed Co, Ni-ferrite nanoparticles with tunable sizes: controlled synthesis, magnetic properties, and surface modification.2008,112, 911–917.
(44) Ghosh, M.; Sampathkumaran, E. V.; Rao, C. N. R. Synthesis and magnetic properties of CoO nanoparticles.2005,17, 2348–2352.
(45) Ambrose, T.; Chien, C. L. Finite-size effects and uncompensated magnetization in thin antiferromagnetic CoO layers.1996, 76, 1743–1746.
(46) Tian, Y.; Yu, B.; Li, X.; Li, K. Facile solvothermal synthesis of monodisperse Fe3O4nanocrystals with precise size control of one nanometre as potential MRI contrast agents.2011, 21, 2476–2481.
(47) Tian, Y.; Yu, B.; Yang, H. Y.; Liao, J. Monodispersed silica nanospheres encapsulating Fe3O4and LaF3: Eu3+nanoparticles for MRI contrast agent and luminescent imaging.2013, 6, 1250052.
14 July 2014;
15 August 2014
the National Natural Science Foundation of China (No. 21201035, No.81371343), the Scientific and Technological
Foundation of Fujian Province (No. JK2013003), and the Natural Science Foundation of Fujian Province (No. 2012J01204)
. Born in 1966, doctor, majoring in the synthesis of materials and biological applications. E-mail: 13705977551@163.com
- 结构化学的其它文章
- Thermoelectric Properties of the CuGaTe2 Crystal from First-principles Calculations: the Role of Doping and Temperature①
- The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
- Synthesis, Crystal Structure and Antiproliferative Activity of 2-(((5-(((5,7-Dimethyl-[1,2,4]triazolo-[1,5-a]pyrimidin-2-yl)thio)methyl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)-4H- chromen-4-one Methanol Solvate①
- Synthesis, Crystal Structure and Luminescent Property of an Eu3+ Complex①
- Synthesis, Dimer Crystal Structure and Herbicidal Activity of 2-(4-Ethoxybenzoyl)cyclopentane-1,3-dione①
- Synthesis, Structureand Characterization of a Biologically Active Compound Based on 5-Nitrosalicylaldehyde Schiff Base①

