Synthesis, Crystal Structure and Luminescent Property of an Eu3+ Complex①
LI Dong-Ping LIU Qi LIAN Qing-Yun JIAO Xiao-Yan LI Yong-Xiu
Synthesis, Crystal Structure and Luminescent Property of an Eu3+Complex①
LI Dong-Ping②LIU Qi LIAN Qing-Yun JIAO Xiao-Yan LI Yong-Xiu②
(330031)
A new Eu(Ⅲ) complex, {[Eu(tta)2(C2H5OH)(NO3)]2(bmp)}·bmp (tta =2-thenoyltri- fluoro- acetonate, bmp =2,2΄-bipyrimidine), has been synthesized and characterized by elemental analysis, IR, luminescence and single-crystal X-ray diffraction analysis. The complex crystallizes in the monoclinic21/space group, with= 11.894(6),14.235(8),= 19.078(10) Å,= 103.423(9)º,= 3142(3) Å3,= 2,M= 1721.10,D= 1.819 g/cm3,(000) = 1696, the final= 0.0452 and= 0.1398 for 5375 observed reflections with> 2(). In the complex, each Eu(Ⅲ) ion is coordinated with four oxygen atoms from two tta ligands and two nitrogen atoms from a bmp ligand, three oxygen atoms from one NO3-anion and one ethanol molecule, forming a distorted tricapped trigonal prism geometry. Luminescence measurement indicates that the europium complex is a strong red emitter in the solid state at room temperature.
Eu(Ⅲ) complex, luminescence,-diketone
1 INTRODUCTION
Recently, lanthanide complexes are very attractive for the development of new materials with potential applications in molecular magnetism, organic light- emitting diodes, sensors and optoelectronic applica- tions[1-4]. Owing to the significant magnetic aniso- tropy arising from large unquenched orbital angular momentum, lanthanide compounds are good candi- dates for functional materials with intriguing ma- gnetic properties[5]. On the other hand, lanthanide complexes with electronic transition within the 4subshells result in unique optical properties, such as long luminescence lifetimes, sharp emission bands and large stokes shifts[6]. However, thetransitions in lanthanide ions are Laporte forbidden, and then the molar absorptivity of lanthanide complexes is very low. Therefore, synthesis and design of lanthanide complexes with suitable ligands acting as “antenna” are crucial to obtain excellent luminescent materials[7].
-diketone and 2,2΄-bipyrimidine ligands have been used successfully in many lanthanide com- plexes to bridge metal ions or function as channel for energy and electron transfer[8]. For instance, solid-state luminescence measurements on complexes, [Ln(NO3)3(bmp)2], [Ln(NO3)3(bmp)2]·THF and [Ln(NO3)3(bmp)(MeOH)2]·MeOH, have revealed that 2,2΄-bipyrimidine ligand is an efficient sensitizer of the luminescence for lanthanide com- plexes[9a]. The energy transfer via bmp channel in Eu(Ⅲ) complex is quite efficient with high quantum yield about 80.0%. Inspired by this, homodinuclear or homotrinuclear lanthanide complexes bridged bybmp ligands are expected to be synthesized by using [Ln(NO3)3(bmp)2] and Ln(-diketone)3·2H2O as raw materials. Such lanthanide complexes may be ex- cellent photoluminescent materials. Furthermore, the two building blocks, [Ln(NO3)3(bmp)2] and Ln(- diketone)3·2H2O, with different lanthanide anions can be utilized to construct multifunctional hetero- dinuclear or heterotrinuclear complexes.
However, after a treatment of [Eu(NO3)3(bmp)2] with Eu(tta)3·2H2O in methanol at room temperature, an unexpected new Eu(Ⅲ) complex was obtained owing to the ligand-exchange reaction occurring in the self-assembly process. Herein, we report the synthesis, crystal structure and luminescence of the complex with the formula of {[Eu(tta)2(C2H5OH)- (NO3)]2(bmp)}·bmp. This work also revealed that compounds [Eu(NO3)3(bmp)2] and Eu(tta)3·2H2O may be instable in ethanol solution.
2 EXPERIMENTAL
2.1 General methods
Eu(NO3)3(bmp)2[9a]and Eu(tta)3·2H2O[9b]were synthesized according to the literature method. Other reagents were obtained from commercial suppliers and used as received. FT-IR spectra were recorded from KBr pellets in the range of 400~4000 cm-1on a vector 22 Bruker spectrophotometer. Elemental analyses of C, H, and N were performed on a Per- kin-Elmer 240C analyzer. Luminescence measure- ment was performed on an Edinburgh Analytical Instrument FLS920 fluorescence spectrometer at ambient temperature. Crystal structure determination was carried out on a Bruker SMART APEXCCD diffractometer.
2.2 Preparation
A mixture of Eu(NO3)3(bpm)2(0.05 mmol) and Eu(tta)3·2H2O (0.05 mmol) was dissolved in 10 mL ethanol, and then stirred at room temperature for 4 hours. The resulting solution was filtered and kept untouched at room temperature for several days. Pure colorless flake crystals were obtained, washed with ethanol and dried in air. Yield: 40% based on Eu(NO3)2(bpm)2.Anal. Calcd. forC52H40Eu2-F12N10O16S4: C, 36.29; H, 2.34; N, 8.14%. Found: C, 36.28; H, 2.36; N, 8.13%. IR: (cm-1): 3436 (w), 1601 (s), 1583 (s), 1543 (s), 1507 (m), 1465 (m), 1409 (s), 1383 (s), 1351 (m), 1308 (s), 1250 (m), 1230 (m), 1191 (m), 1143 (m), 1063 (w), 934 (w), 862 (w), 761 (w), 719 (w), 682 (w), 641 (w), 581 (w).
2.3 Crystal structure determination
A suitable single crystal for X-ray single-crystal diffraction with dimensions of 0.26mm × 0.22mm × 0.20mm was selected and mounted on a glass fiber. The diffraction data were collected on a Bruker SMART APEXCCD diffractometer equipped with a graphite-monochromatic Moradiation (= 0.71073 Å) at 291(2) K. A total of 28520 reflections were collected in the range of 1.80<<27.50° (2max= 51.4, –15≤≤15, –17≤≤18, –21≤≤24) by using a-scan mode. 7160 reflections were unique (int= 0.0695) and 5375 observed reflections were used in the succeeding structure calculations. The final refinement converged at= 0.0578,= 0.1398 (= 1/[2(F2) + (0.0835)2+ 0.00], where= (F2+ 2F2)/3),= 1.017, (Δ)max= 0.977 and (Δ)min= –0.911 e/Å3. The data integration and empirical absorption corrections were carried out by SAINT program[10a]. Absorption corrections were made using SADABS program. The structure was solved directly using the SHELXS-97[10b], and re- fined on2by full-matrix least-squares methods using the SHELXL-97 program package[10c]. All non-hydrogen atoms were refined first isotropically, and then anisotropically. Hydrogen atoms bonded to carbon and oxygen atoms were generated geome- trically and refined isotropically with the riding mode.
3 RESULTS AND DISCUSSION
3.1 IR analyses
IR spectra of free tta ligand show strong band at 1658 cm-1, which are attributable to stretching vibrations of theC=O group of the ligand. In the complex, the strong bands have shifted to 1601 and 1465 cm-1, indicating that the tta ligand is coor- dinated with the metal center. The strong bands at 1583 and 1543 cm-1are identified as the C=N and C=C stretching vibrations of bmp ligand. The free NO3-anion shows absorption peaks at 1390, 881 and 790 cm-1[11]. Compared with the free NO3-ion, the absorption peaks at 1507, 1250, 1063 and 761 may be ascribed to the stretching vibrations of coor- dinated NO3-anion in the complex. These are in accordance with the results of crystal structure analysis.
3.2 Crystal structure of the complex
Single-crystal X-ray diffraction analyses reveal that the title complex crystallizes in monoclinic space group21/. The selected bond distances and bond angles of the compoundare shown in Table 1. The hydrogen bonding interactions of the compound are shown in Table 2.As shown in Fig. 1, the title complex consists of a guest bmp ligand and a centrosymmetric bimetallic unit, which contains two Eu(Ⅲ) ions, fourttaligands, two NO3-ions, onecoordinated bmp ligandand two ethanol molecules. It is worth to note that the title complex was obtained by the reaction of Eu(NO3)2(bpm)2and Eu(tta)3·2H2O in ethanol solution at room tempe- rature. Under the self-assembly process, the complex Eu(NO3)3(bpm)2may be decomposed partially in solution owing to the ligand-exchange reaction with water molecules[9a]. However, according to the crystal structure, the tta ligand of Eu(tta)3·2H2O was also partially displaced by NO3-ion and ethanol molecule in complex during the self-assembly process. To the best of our knowledge, such ligand- exchange reactions in-diketone complexes are rarely reported.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for the Title Complex
Symmetry code: (a) 1–, 1–, –

Table 2. Hydrogen-Bonding Geometry Parameters (Å, °) for the Title Complex
(a)–+1, –+1, –; (b),+1,; (c) –+1.5, –0.5+, –+0.5; (d) –+2, –+1, –
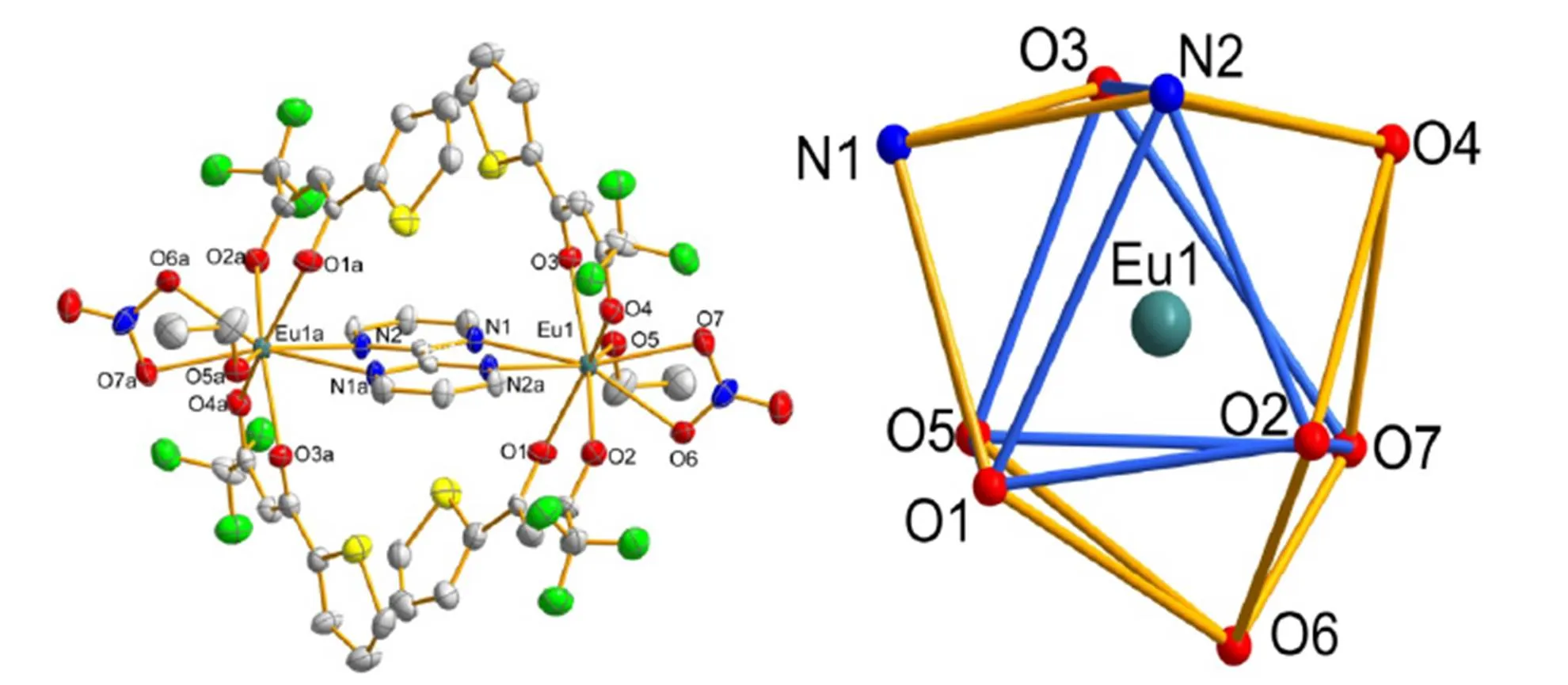
Fig. 1. (Left) Coordination environment of the Eu(Ⅲ) atom in the title complex. Hydrogen atoms and the free bmp ligand are omitted for clarity. Symmetry codes: (a)–+1, –+1, –. (Right) Distorted tricapped trigonal prism coordination environment of the Eu(Ⅲ) ion in the title complex
In the complex, each Eu(Ⅲ) ion is coordinated to seven oxygen and two nitrogen atoms from two distinct tta ligands, one NO3-ion, one ethanol molecule and one bmp ligand, respectively. In the complex, the average bond lengthof Eu−O (ethanol) (2.428 Å) is shorter than that of Eu−O (NO3-) (2.514 Å) but longer than that of Eu−O (tta) (2.347 Å). Moreover, the average Eu−O (tta) bond length is comparable to the value of reported Eu/tta com- plex[9c]. The Dy−N bond lengths are 2.626(2) and 2.670(2)Å, respectively.The shortest intramolecular Eu−Eu distance in the compound is 6.948 Å, which is longer than that of [Eu(tta)3]2bmp[9c]. Both the coordinated and free bmp ligands in the complex are almost planar, with the mean deviations of 0.05 and 0.08 Å for planes N(1)−C(19)−C(20)−C(21)− N(2)− C(22) and N(3)−C(23)−C(24)−C(25)−N(4)−C(26), respectively. The coordination sphere around the Eu(Ⅲ) atom can be described as a distorted tricap- ped trigonal prism polyhedron (Fig. 1). The dihedral angles of O(1), O(5), O(3), N(2a); O(1), O(5), O(2), O(7) and O(2), O(7), O(3), N(2a) in the complex are 3.38, 7.59 and 12.05º correspondingly, indicating the planes of trigonal prism are distorted. The peaks of the tricapped trigonal prism polyhedron are held by O(4), O(6) and N(1) atoms.
Notably, there are abundant hydrogen bonding interactions among the centrosymmetric Eu(Ⅲ) bimetallic units and uncoordinated bmp ligands in the complex. For instance, the free bmp ligands in the complex are linked with adjacent binuclear Eu(Ⅲ) units by O–H···N typical hydrogen bonding interactions to form a 1D chain in theplane (Fig. 2). The d(D···A) distance of hydrogen bonds is 2.795 Å. And then, the 1D chains are further connected by atypical hydrogen bonding interactions, such as C–H···N, C–H···O and C–H···F, to form a 3D supra- molecule. In the open supramolecular 3D framework, each Eu(Ⅲ) bimetallic unit is surrounded by four free bmp ligands (Fig. 3).Undoubtedly, such typical and atypical hydrogen bonding interactions are beneficial to the stabilization of the complex.
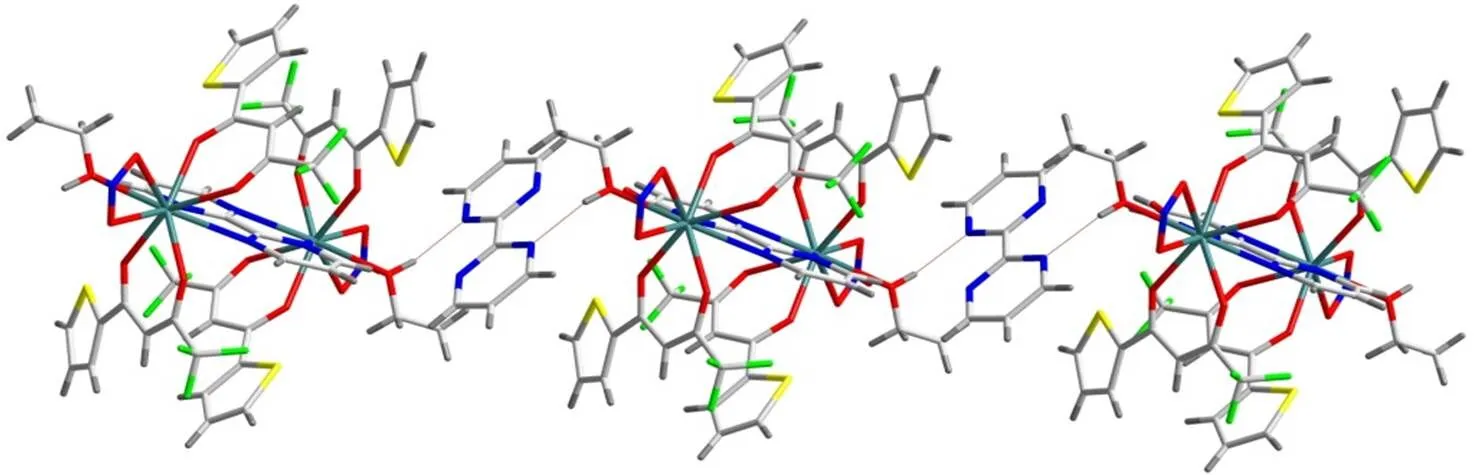
Fig. 2. 1d chain constructed by hydrogen bonds among the binuclear Eu(Ⅲ) units and the uncoordinated bmp ligands
Fig. 3. Packing diagram of the title complex. Hydrogen atoms are omitted for clarity
3.3 Powder X-ray diffraction and luminescence property
In the title complex, ligand-exchange reactions occurred among tta ligand, bmp ligand and NO3-anions. However, such ligand-exchange reactions in-diketone complexes are rarely reported. To confirm the phase purity and the exactness of crystal structure of the complex, X-ray powder diffraction was performed on single-crystal samples. As shown in Fig. 4, the powder X-ray diffraction of single- crystal samples is in agreement with the simulated one from the crystal data. It suggests that the crystal structure is representative of the bulk material.
The luminescent property of Eu(Ⅲ) complex was investigated at room temperature in the solid state. As elucidated in Fig. 5, the solid state sample shows a broad absorption excitation peak between 330 and 500 nm with the maximum excitation wavelength at 394 nm. The emission spectrum of the title complex exhibits the characteristic emission of Eu(Ⅲ) ion. In the emission spectrum, the band at 536 nm is assigned to the5D0→7F0transition, while those at 591, 619, 651 and 698 nm are corresponding to the5D0→7F1,5D0→7F2,5D0→7F3and5D0→7F4transi- tions, respectively. The narrow and strong fluore- scence of the title complex at 619 nm could be considered as a good candidate for red fluorescent materials.
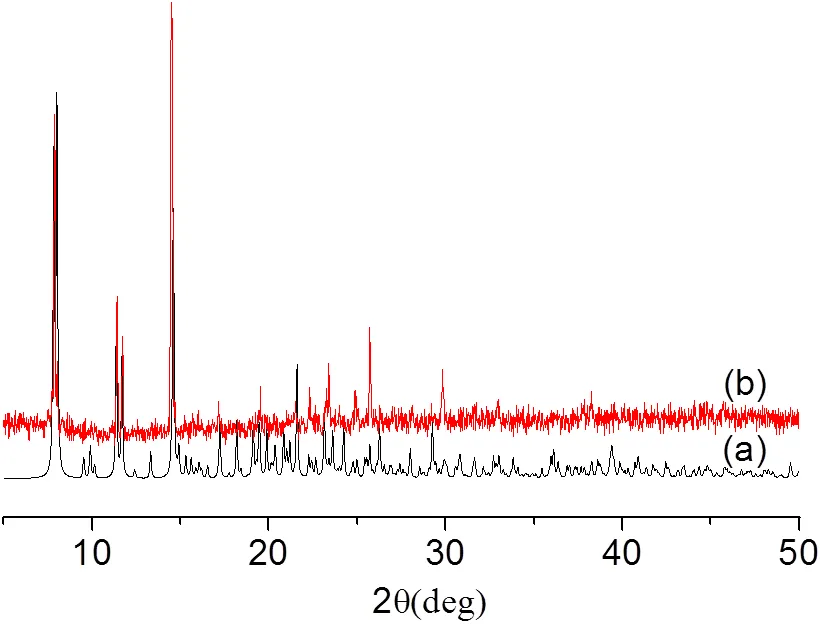
Fig. 4. Powder X-ray diffraction patterns for the title complex: (a) simulated patterns; (b) as-synthesized products
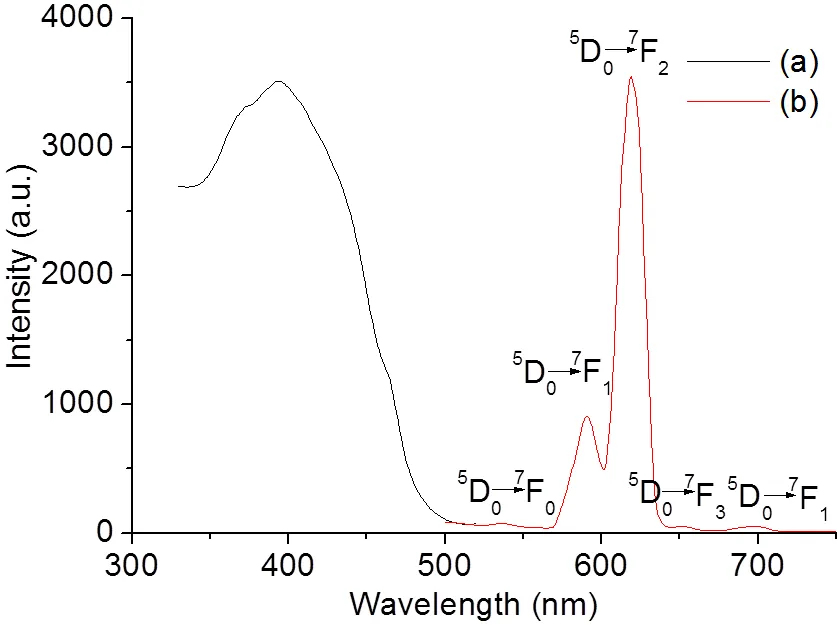
Fig. 5. Solid state excitation (a) and emission (b) spectra of the title complex
4 CONCLUSION
The novel title Eu(Ⅲ) complex, {[Eu(tta)2-(C2H5OH)(NO3)]2(bmp)}·bmp, has been synthesized unexpectedly by ligand-exchange reaction of [Eu(NO3)3(bmp)2] and Eu(tta)3·2H2O during the self-assembly process. The complex shows intense characteristic emission of Eu(Ⅲ) ion and may be a good red emitter with potential applications in photoluminescent materials.
(1) (a) Zhang, P.; Zhang, L.; Wang, C.; Xue, S. F.; Lin, S. Y.; Tang, J. K. Equatorially coordinated lanthanide single ion magnets.. 2014, 136, 4484–4487. (b) Wei, X. H.; Yang, L. Y.; Liao, S. Y.; Zhang, M.; Tian, J. L.; Du, P. Y.; Gu, W.; Liu, X. A series of rare earth complexes with novel non-interpenetrating 3D networks: synthesis, structures, magnetic and optical properties.2014, 43, 5793–5800. (c) Zucchi, G.; Thuery, P.; Riviere, E.; Ephritikhine, M. Europium(II) compounds: simple synthesis of a molecular complex in water and coordination polymers with 2,2΄-bipyrimidine-mediated ferromagnetic interactions.. 2010, 46, 9143–9145.
(2) Lima, P. P.; Paz, F. A. A.; Brites, C. D. S.; Quirino, W. G.; Legnani, C.; Costa e Silva, M.; Ferreira, R. A. S.; Junior, S. A.; Malta, O. L.; Cremona, M. White OLED based on a temperature sensitive Eu3+/Tb3+beta-diketonate complex.. 2014, 15, 798–808.
(3) Tang, K.; Zheng, Y.; Zhang, W.; Wang, Q. Determination of nitrite in water by an optical and electrochemical lanthanide sensor.. 2014, 6, 612–616.
(4) Algar, W. R.; Kim, H.; Medintz, I. L.; Hildebrandt, N. Emerging non-traditional Forster resonance energy transfer configurations with semiconductor quantum dots: investigations and applications.2014, 263, 65–85.
(5) (a) Edelmann, F. T. Lanthanides and actinides: annual survey of their organometallic chemistry covering the year 2012.2014, 261, 73–155. (b) Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets.. 2013, 42, 3278–3288.
(6) (a) Irfanullah, M.; Iftikhar, K. Synthesis and spectroscopic analysis of an extended series of hetero dinuclear complexes containing two different lanthanides in 1:1 stoichiometry.2013, 394, 373–384. (b) Madelyn, H. B.; Jason, D. D.; Amanda, N. L.; Robert, D. P.; Steven, M. B. Structure and emission spectra of dinuclear lanthanide(Ⅲ)-diketonate complexes with a bridging 2,2΄-bipyrimidine ligand.2009, 28, 188–194. (c) Cao, Y. Y.; Chen, Y. M.; Liu, W.; Li, Y. H. Syntheses and crystal structures of five SrⅡ-LnⅢ(Ln = Nd, Pr, Er) heterometallic coordination polymers.2013, 32, 1859–1867.
(7) (a) Li, H. Y.; Wu, J.; Huang, W.; Zhou, Y. H.; Li, H. R.; Zheng, Y. X.; Zuo, J. L. Synthesis and photoluminescent properties of five homodinuclear lanthanide (Ln3+= Eu3+, Sm3+, Er3+, Yb3+, Pr3+) complexes.2009, 208, 110–116. (b) Albert, F.; Shawn, S. Luminescent and structural properties of a Eu(Ⅲ) complex: formation of a one-dimensional array bridged by 2,2΄-bipyrimidine.. 2007, 10, 636–638. (c) Wang, X. L.; Bao, X.; Wei, Y. J.; Wang, F. W.; Chen, Y. H.; Xu, P. P. Two tri-spin complexes based on lanthanide (LnⅢ= DyⅢand TbⅢ) and nitronyl nitroxide radicals: syntheses, structures and properties.. 2013, 32, 805–810. (d) Sun, C. Y.;Lv, Q.; Zhang, H. Y.; Li, W. J.; Chang, Z. D.; Zheng, X. J. Syntheses, structures and properties of lanthanide coordination polymers constructed from mixed acid.. 2012, 31, 27–32.
(8) Deepika, D.; Daniel, C.; Gregory, R.; Gilford, S. V.; Shawn, S. Dinuclear lanthanide(Ⅲ) complexes containing-diketonate terminal ligands bridged by 2,2΄-bipyrimidine.2006, 9, 979–981.
(9) (a) Zucchi, G.; Maury, O.; Thury, P.; Gumy, F.; Bnzli, J. C. G.; Ephritikhine, M. 2,2΄-bipyrimidine as efficient sensitizer of the solid-state luminescence of lanthanide and uranyl ions from visible to near-infrared.2009, 15, 9686–9696. (b) Melby, L. R.; Rose, N. J.; Abramson, E.; Caris, J. C. Synthesis and fluorescence of some trivalent lanthanide complexes.1964, 86, 5117–5125. (c) Swavey, S.; Krause, J. A.; Collins, D.; D’Cunha, D.; Fratini, A. X-ray structure and temperature dependent luminescent properties of two bimetallic europium complexes.2008, 27, 1061–1069.
(10) (a) SAINT.. Bruker AXS, Madison Inc: WI 2001. (b) Sheldrick, G. M.. University of Göttingen, Germany 1997. (c) Sheldrick, G. M.University of Gottingen, Germany 1997.
(11) Lin, M.; Yang, Y.; Xian, C. Y. Synthesis and crystal structure analysis of lanthanide complexes Ln(NO3)2(phen)2(CH3COCHCOCH3).. 2010, 26, 120–125.
14 April 2014;
12 June 2014(CCDC996426)
the National Natural Science Foundation of China (21101090),the Natural Science Foundation of Jiangxi Province (20114BAB213001) and the Education Department of Jiangxi Province (GJJ12041)
. E-mail: nculdp@126.com and yxli@ncu.edu.com
- 结构化学的其它文章
- Thermoelectric Properties of the CuGaTe2 Crystal from First-principles Calculations: the Role of Doping and Temperature①
- Morphology, Size-controlled Synthesis of CoO anostructure and Its Magnetic Property①
- The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
- Synthesis, Crystal Structure and Antiproliferative Activity of 2-(((5-(((5,7-Dimethyl-[1,2,4]triazolo-[1,5-a]pyrimidin-2-yl)thio)methyl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)-4H- chromen-4-one Methanol Solvate①
- Synthesis, Dimer Crystal Structure and Herbicidal Activity of 2-(4-Ethoxybenzoyl)cyclopentane-1,3-dione①
- Synthesis, Structureand Characterization of a Biologically Active Compound Based on 5-Nitrosalicylaldehyde Schiff Base①

