The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
CHEN Wu-Hua ZHANG Zhu-Sen HU Zhi-Biao MI Jin-Xiao
The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
CHEN Wu-Huaa, b, cZHANG Zhu-SencHU Zhi-BiaocMI Jin-Xiaoa, b②
a(361005)b(361005)c(364000)
Polyoxometalates (POMs) with Cd-coordination complexes acting as supporting units are rarely reported. The linkage of the supporting units with inorganic building block (polyanion) is generally established on terminal O-atoms, but scarcely via bridging O-atoms. By introducing liquid small organic molecule (pyridine, C5NH5) as assistant “structure-directing agent”, we obtaineda novel organic-inorganic hybrid polytungstate, (Hpy)4[Cd(phen)2(P2W18O62)]·H2O (1,≈ 3, py = pyridine, phen = 1,10-phenanthroline), under hydrothermal conditions. The single-crystal X-ray diffraction analysis shows that 1 is the first compound containing an asymmetric heteropolyanion, [Cd(phen)2(P2W18O62)]4–, a Wells-Dawson-type polyanion monosupported by Cd-coordination complex via di-bridging O-atoms.
hybrid compound, Wells-Dawson-type, Cd-coordination complex, monosupported structure, bi-bridging O-atom
1 INTRODUCTION
Nowadays, among metal-organic frameworks (MOFs), a kind of supported/capped polyoxometa- lates (POMs) also attracts much interest due to their potential to form similar 0~3 dimensional POM----POM (= transition metal,= organic ligand) frameworks via metal nodes[1–4]. The syner- gistic effect among organic units, inorganic building blocks (polyanions), and metals in the POM----POM framework may provide advanced POM- based materials with additional properties besides the known characteristics of POMs[5–10]. For instan- ces, Wang. reported two Cu-supported hybrid compounds, KH2[(D-C5H8NO2)4(H2O)Cu3][W12O40]·5H2O (D-1) and KH2[(L-C5H8NO2)4(H2O)Cu3][W12O40]·5H2O (L-1)[11], possessing both POM and chiral characteristics. Rodriguez-Albelo. repor- ted a Zn-capped compound, [NBu4]3[PMoV8MoVI4O36(OH)4Zn4(BDC)2]·2H2O[12], possessing both POM and zeolite properties. Therein, the transition metals () played pivotal roles in connecting inorganic building blocks with organic ligands, forming 0~3 dimensional spatial structures. To date, more and more analogous compounds have been synthesized and reported. As for supported POMs, Cu-supported [Cu(I)(BBTZ)][Cu(I)(HBBTZ)2][SiMo12O40]·2.5H2O[13],Ni-supported K[PW12O40[Ni(1,10-phen)2(OH)]2]·2H2O[14],Ag-sup-ported H0.5[{Ag3L2(DMF)3}{Ag2L(DMF)1.5}{Ag0.5(DMF)(H2O)0.25}(P2Mo18O62)]·1.25H2O[15]and Co- supported [Co2(H2O)2(btb)4(HPMoVI10MoV2O40)][16]and [Co2(btx)5(HPMoVI10MoV2O40)][16]have been successfully synthesized and investigated. However, Cd-supported POMs have been rarely reported obviously. To the best of our knowledge, only about twenty-odd Cd-supported POMs have been reported and they can be divided into the following three classes: i) Nine compounds[17-23]derived from the well-known {V18O42} cluster shell, such as [Cd(dien)]2[Cd(dien)(H2O)]2[As4V16O42(H2O)]·2H2O[17], H3[L/D-Cd(Cl)(H2O)(phen)2][{L/D-Cd(H2O)-(phen)2}2{V16O38(Cl)}]·3.5H2O[18], and H2[Cd(bipy)3][Cd(H2O)(bipy)2]{[Cd(H2O)(bipy)2]-(V16O38Cl)}·1.5H2O[19]reported by Yang and co- workers, Peng and co-workers, and Wang and co-workers., respectively. ii) Seven com- pounds derived from another famous sandwich-type [(Mo6P4)2] dimmer (= Cd) like [Cd3(4,4΄- Hbpy)2(4,4΄-bpy)2(H2O)8][Cd(H2PO4)2(HPO4)4-(PO4)2(MoO2)12(OH)6]·7H2O[24], (Hdien)2{[Cd(H2O)- (Hdien)]2Cd[(H2PO4)(HPO4)2(PO4)(MoO2)6(OH)3]2}·8H2O[25], and [Cd2.5(btp)2(H2O)3][Cd0.5(PO4)-(HPO4)3(MoO2)6(OH)3]·2H2O[26]were reported by Wangand co-workers., Xia and co-workers., and Tian and co-workers. correspondingly. iii) Five compounds[27-31]derived from the classical Keggin-type inorganic building block, [(12O42)2] (= Mo/W,= P/Si/B). For example, [(BW12O40)Cd(2,2-bpy)2(H2O)][Cd(2,2-bpy)3]1.5·0.5H2O[27]and [Cd(Htrz)3]2[SiW12O40]·2H2O[28]were reported by Xuand co-workers, and Qin and co-workers, respectively. Among the above- mentioned Cd-supported POMs, only two examples are polytungstate and no Cd-supported Wells- Dawson-type POM has been reported previously. Moreover, all the linkages of polyanions and Cd-coordination complexes in the above-mentioned compounds are established on terminal O-atoms (Ot), i.e., none of them are linked via bridging O-atoms (Ob).
In addition, design and synthesis of a new suppor- ted POM is a challenging task because it is quite difficult to control the metal nodes in connecting both organic ligands and inorganic building blocks. Many subtle and unexpected factors such as tem- perature, pH, solvent and counterion may signifi- cantly affect the self-assembly of these POMs[25]. In particular, the introduction of diverse organic com- pounds in synthesizing POMs may lead to various structural POM-based products[16]. Sometimes, liquid small organic molecules are good “structure- directing agents” for their excellent solubility, less steric hindrance, flexible coordination, good H-bond-forming ability, charge balance,. Based on our ongoing study on related materials[32–35], by introducing liquid small organic molecules (pyridine (py)), tuning pH systematically and optimizing reac- tion conditions, we fortunately obtained 1 (Fig. 1) in a one-pot reaction under mild hydrothermal condi- tions. The single-crystal X-ray diffraction analysis shows that compound 1 is the first Wells-Dawson- type polytungstate monosupported by Cd-coor- dination complex via bi-bridging O atoms. Com- pound 1 is decorated by mixed organic units and represents an interesting latticed steric configuration (Fig. 2). These results were further confirmed by powder X-ray diffraction (PXRD), elemental analy- ses, energy-dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR) spectroscopy and Raman spectroscopy. The thermal properties of compound 1 were also investigated by thermogravimetric analysis (TGA).
2 EXPERIMENTAL
2.1 General procedures
All starting materials were purchased commer- cially and used without further purification. The elemental analyses were carried out using a Perkin- Elmer 2400 elemental analyzer (for C, H and N). The EDS spectrum was recorded using afield-emission scanning electron microscope (FESEM) (Fig. 3a). Powder XRD data were collec- ted using a Philips X’Pert-PRO X-ray diffractometer equipped with Cu-radiation (= 1.54178 Å) (Fig. 3b). The FT-IR spectrum (KBr pellets) was recorded using a Nicolet Impact 410 FTIR in the range of 400~4000 cm–1(Fig. 3c). FT-Raman spectrum was measured with a BRUKER 110·S-1spectrometer (Fig. 3d). XPS analysis was performed using a Physical Electronicsquantum 2000scanning ESCA microprobe equipped with a standard focused mono- chromatic Al(1486.7 eV) X-ray source (Fig. 4a~c).Both IR and Raman spectra were recorded with a spectral resolution of 2 cm–1. TGA was per- formed in N2atmosphere in the temperature range of 30~980 ℃ using a TG 209 F1 thermogravimetric analyzer at a heating rate of 10 ℃·min–1(Fig. 4d).
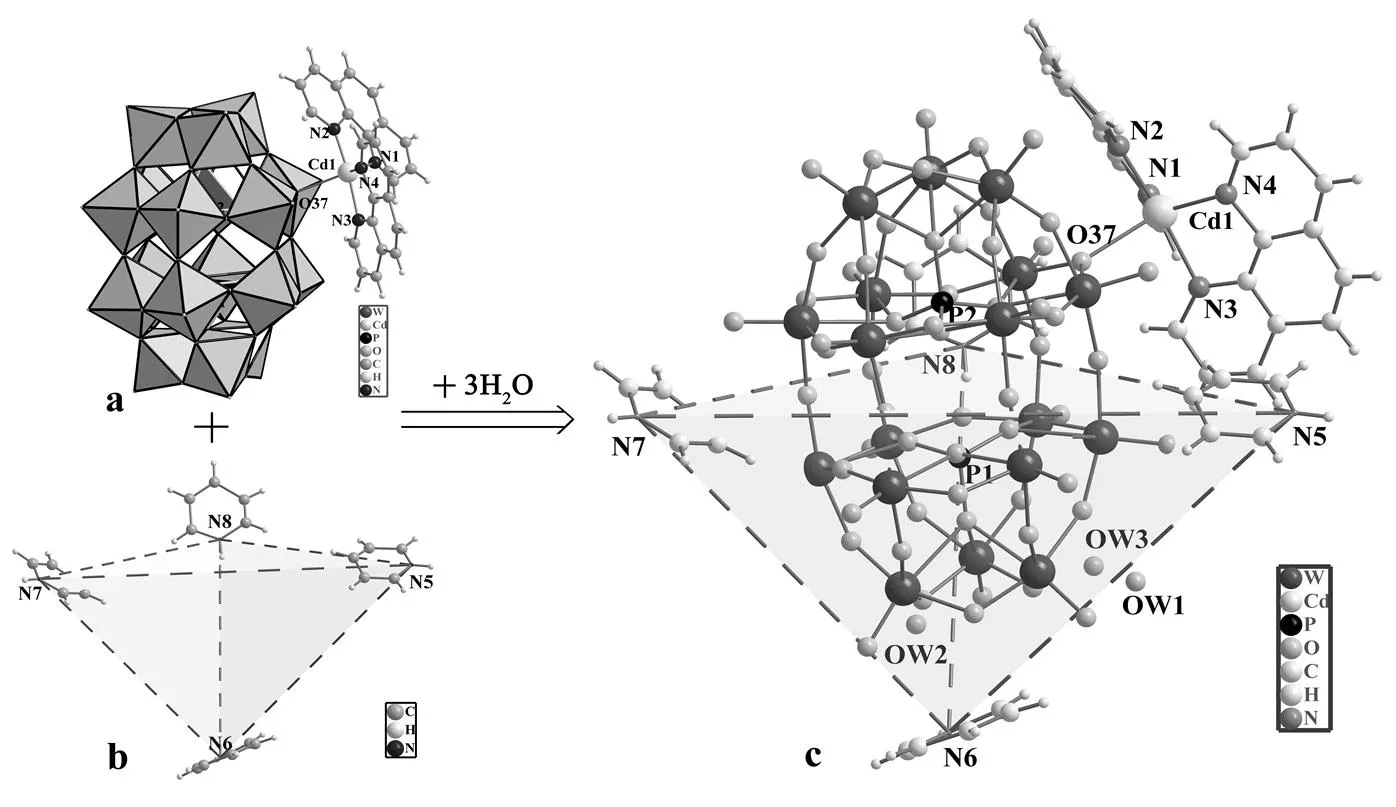
Fig. 1. (a) Asymmetric monosupported [Cd(phen)2(P2W18O62)]4–polyanion; (b) Configuration of four protonated Hpy molecules; (c) View of the fundamental building unitsin the asymmetric unit of (Hpy)4[Cd(phen)2(P2W18O62)]·H2O (n ≈ 3) (1)
Fig. 2. (a) Crystal structure of compound 1viewed along theaxis and the display of H-bonds (dotted lines);(b) a simulated latticed configuration of compound 1 viewed along theaxis;(c) “Chair” configuration composed of six [Cd(phen)2]2+units

Fig. 3. (a) EDS spectrum of compound 1; (b)Experimental and simulated powder X-ray diffraction patterns of compound 1; (c) FTIR spectrum of compound 1; (d) Raman spectrum of compound 1
2.2 Synthesis and characterization
A mixture of Na2WO4·2H2O (1.61 g, 4.88 mmol), Cd(NO3)2·4H2O (0.23 g, 0.93 mmol), H3PO4(1.8 mL, 85 %, 26.31 mmol), 1,10-phenanthroline (0.13 g, 0.72 mmol), pyridine (1 mL, 12.42 mmol), and H2O (15 mL, 833.33 mmol) in a molar ratio of~7:1.3:36:1:18:1200 was prepared and sealed in a 30 mL Teflon-lined stainless steel autoclave with~75% filling. Then, the resulting gel with a pH value of about 5.5 was heated to and held at 160 ℃ in an oven for 4 d. Finally, it was cooled to room temperature at a rate of10 ℃·h–1. The resulting products, containing a mixture of powder and green crystals, were recovered by filtration, washed with distilled water and dried in air at ambient tempera- ture (45% yield based on W). The powder XRD pattern of the manually selected crystals together with the simulated XRD pattern obtained from the single-crystal structure analysis are shown in Fig. 3b. The diffraction peaks fit well with the simulated ones in terms of peak position, indicating the purity of the sample used and a proper crystal-structure analysis given below. Anal. Calcd. (%) for compound 1: W, 51.74; Ag, 6.76; P, 0.97; H, 1.19; N, 3.94; C, 19.89; O, 15.51. Found (%): H, 1.33; N, 3.76; C, 20.01.
It is worth mentioning that the acquirement of compound 1 is asomewhat tricky andlucky event.Above all, the order of adding reactants is a critical factor for synthesizing compound 1. The reactants, Cd(NO3)2·4H2O, phen and distilled water should be firstly mixed under slowly stirring for~120 min at constant temperature of~60 ℃. This key procedure ensures the effective coordination of phen with Cd2+ions. Next, Na2WO4and py were added in by vigorous stirring for 30 min, whereas H3PO4should be added in the last step without stirring. Otherwise, H3PO4would react with phen and py directly, resulting in a large amount of light green gel im- purity instead of compound 1. Note that the reaction is clearly pH-dependent. The pH of the reaction system was adjusted by controlling the amount of H3PO4and nitrogen-containing organic base. The crystals of compound 1 were obtained only at pH = 5~7. Additionally, the pressure and temperature of the reaction system may be the other two critical factors for successfully obtaining compound 1. This experiment was conducted in a 23 mL (instead of 30 mL) Teflon-lined stainless-steel autoclave strictly following the same other conditions, mainly affor- ding grey crystals ((Hpy)6(P2W18O62) (2)). When the reaction temperature was fixed at 190 ℃ (instead of 160 ℃) under similar reaction conditions (namely, 23 mL Teflon-lined stainless-steel auto- clave), mainly colorless crystals of [Cd(phen)3](P2W18O62) (3) were obtained. The structures of compounds 2 and 3, containing only one of the two organic components, were also con- firmed by single-crystal X-ray diffraction analyses.
2.3 Single-crystal structure determination
A suitable single crystal of the synthesized com- pound was carefully selected under an optical micro- scope and glued to a thin glass fiber with epoxy resin. Single-crystal X-ray diffraction data were collected using a Bruker Smart APEX II image-plate area detector equipped with a graphite-mono- chromatic Mo(= 0.71073 Å) radiation in anscan mode (using oscillation frames) in the range of= 3.31~27.52° at room temperature. A total of 149171 independent reflections were collec- ted in the ranges of –14≤≤11, –27≤≤23 and –36≤≤34, of which 12456 observed reflections with2()were used in the structure determina- tion and refinement. The structure was solved by direct methods and refined on2by full-matrix least-squares method using the SHELXS-97 and SHELXL-97 programs[36, 37]. The non-hydrogen atoms (except O(57), C(9), C(22) and C(40)) were refined anisotropically, and the H atoms isotropically. All the H atoms were generated geometrically. The final cycle of refinement shows that compound 1 crystallizesin the orthorhombic system with= 12.116(2),= 23.341(5),= 30.546(2) Å,= 2863.2(12) Å3, space group212121and= 4 at 173(2) K. The final full-matrix least-squares refine- ment converged to= 0.0739,= 0.1348 (= 1/[2(F2) + (0.0426)2+ 83.616], where= (F2+ 2F2)/3),= 1.068, (Δ)max= 3.011, (Δ)mix= –3.593 e/Å3and (Δ)max= 0.006. Selected bond lengths and bond angles of compound 1 are listed in Table 1.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Compound 1
3 RESULTS AND DISCUSSION
3.1 Structure description
The single-crystal X-ray diffraction analysis shows that compound 1 crystallizes in the orthor- hombic space group212121. The asymmetric unit of compound 1 (Fig. 1) consists of one asymmetric monosupported polyanion, [Cd(phen)2(P2W18O62)]4–(Fig. 1a), four discrete protonated pyridine cations ([C5NH6]+) (Fig. 1b) and approximately three H2O molecules.
The classical Wells-Dawson-type polyoxoanion, [P2W18O62]6–, has point group symmetry3hand contains two identical [-PW9O31]3–units, which can be derived from the well-known Keggin-structural [-PW12O40]6–anions by removing a set of three corner-sharing WO6octahedra. All the W centers exhibit a {WO6} octahedral environment. The W–O distances are 1.67(2)~1.80(2) Å for the terminal O atoms (W–Ot), 1.83(2)~2.08(2) Å for the bi-brid- ging O atoms (W–Ob), and 2.32(2)~2.46(2) Å for the center O atoms (i.e., the O atoms bonding to two/three W atoms and one P atom, W–Oc). The P–O bond lengths vary between 1.53(2)and 1.66(2) Å, while the O–P–O bond angles are in the range of 103.7(12)~115.9(13)°. All the above bond lengths and bond angles fall in the normal ranges. The bond valence sum calculations on the tungsten sites affor- ded the values of 5.55~6.16 for all the W atoms with an average value of 5.80[38]. This indicates that the 18 independent W atoms are in the oxidation state of +6.
As shown in Fig. 1a, the asymmetric monosup- ported polyanion, [Cd(phen)2(P2W18O62)]4–, is the most prominent feature of compound 1. This is the first case in which Cd-coordination complexes, [Cd(phen)2]2+, are successfully linked to Wells- Dawson-type polyanions, [P2W18O62]6–, by a bi-bri- dging O atom (i.e., O(37)). The bond distance of W–O(37) at 1.92(2) Å is somewhat longer than those of the other 11 di-bridging “belt” O atoms. This may result from the extra linking of O(37) with the Cd-coordination complex. Two phen ligands crosswise coordinate with a Cd2+ion, and their dihedral angle is 125.6°, with the Cd–O bond almost equally dividing it. The five-coordinated Cd2+ion (connecting with O(37) and four N atoms from two phen ligands) shows a slightly distorted quartet cone. The Cd–O distance at 2.45(2) Å and Cd–N distances from 1.99(3)to2.15(3) Å are within the normal range. Four protonated pyridine molecules play very important roles of organic compensating cations and “structure-directing agents” in forming the asym- metric polyanion, [Cd(phen)2(P2W18O62)]4–. Their tetrahedral configuration (Fig. 1b) surrounds almost half of the Wells-Dawson-type polyanions, [P2W18O62]6–(Fig. 1c). Together by taking the [Cd(phen)2]2+fragment (in the other half of poly- anions) into account, this type of distribution well neutralizes the negative charge of [P2W18O62]6–and stabilizes the compound, well conforming the prin- ciple of the lowest energy.
From a topological point of view, the structure of compound 1 represents a latticed configuration among Cd-coordination complexes and polyanions (Fig. 2a and 2b). Viewed along theaxis, six [Cd(phen)2]2+fragments (roughly surrounding two [P2W18O62]6–) represent a side-viewed “chair-form” configuration (Fig. 2c). The H2O molecules and organic compensating cations (protonated pyridine, [C5NH6]+) also act as important joint parts to stabilize the crystal packing and form an infinite 3D network via electrostatic attractions and abundant H-bonds. Fig. 2a (dotted lines) shows a large num- ber of intra- and intermolecular H-bonds in the crystal packing of compound 1. Various H-bonding parameters with symmetry code are listed in Table 2.

Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°)
Symmetry codes: #1: −+1,+1/2, −+3/2; #2: −+1/2, −+1,−1/2; #3: −,+1/2, −+3/2; #4:−1,,; #5:+1/2, −+3/2, −+2; #6: −+3/2, −+1,+1/2; #7:−1/2, −+1/2, −+2; #8:+1,,
3.2 FTIR and Raman spectra
The FTIR spectrum (Fig. 3c) of compound 1 shows the characteristic bands between 1000 and 700 cm–1, indicating that the polyoxoanion has a Wells-Dawson structure[39]. The band at~958 cm–1can be attributed to the characteristic stretching vibrations of W=Otbond, and those at~910 and 794 cm–1to the asymmetric stretching vibration of W–Ob–W and W–Oc–W. The band at 1087 cm–1results from the vibrations of P–O bond, and those at 1603, 1521, and 1426 cm–1are due to the bending vibrations of 1,10-phenanthroline and pyridine molecules. Moreover, broad bands at~3520 and 3070 cm–1can be assigned to the stretching vibrations of O–H, N–H, and C–H bonds of the two types of protonated organonitrogen units and small inorganic molecule, H2O.
The Raman spectrum (Fig. 3d) of compound 1 shows the presence of a strong peak at 456 cm–1due to the vibration of the terminal W=O groups. The relative weak band at~1640 cm–1can be attributed to the bending vibrations of organic units and H2O molecules.
3.3 XPS spectroscopy
To further demonstrate the ingredient and elemental valence states, the XPS spectrum of compound 1 was also recorded. The two peaks at 35.61 and 37.67 eV shown in Fig. 4a can be attributed to the W4region, W6+(47/2) and W6+(45/2)[40], respectively; those at 404.89 and 411.57 eV (Fig. 4b) are assigned to Cd2+(35/2) and Cd2+(33/2), respectively; while that at 133.57 eV shown in Fig. 4c is owing to P5+(23/2)[40]. The XPS spectral analyses are consistent with the abovemen- tioned composition of compound 1.
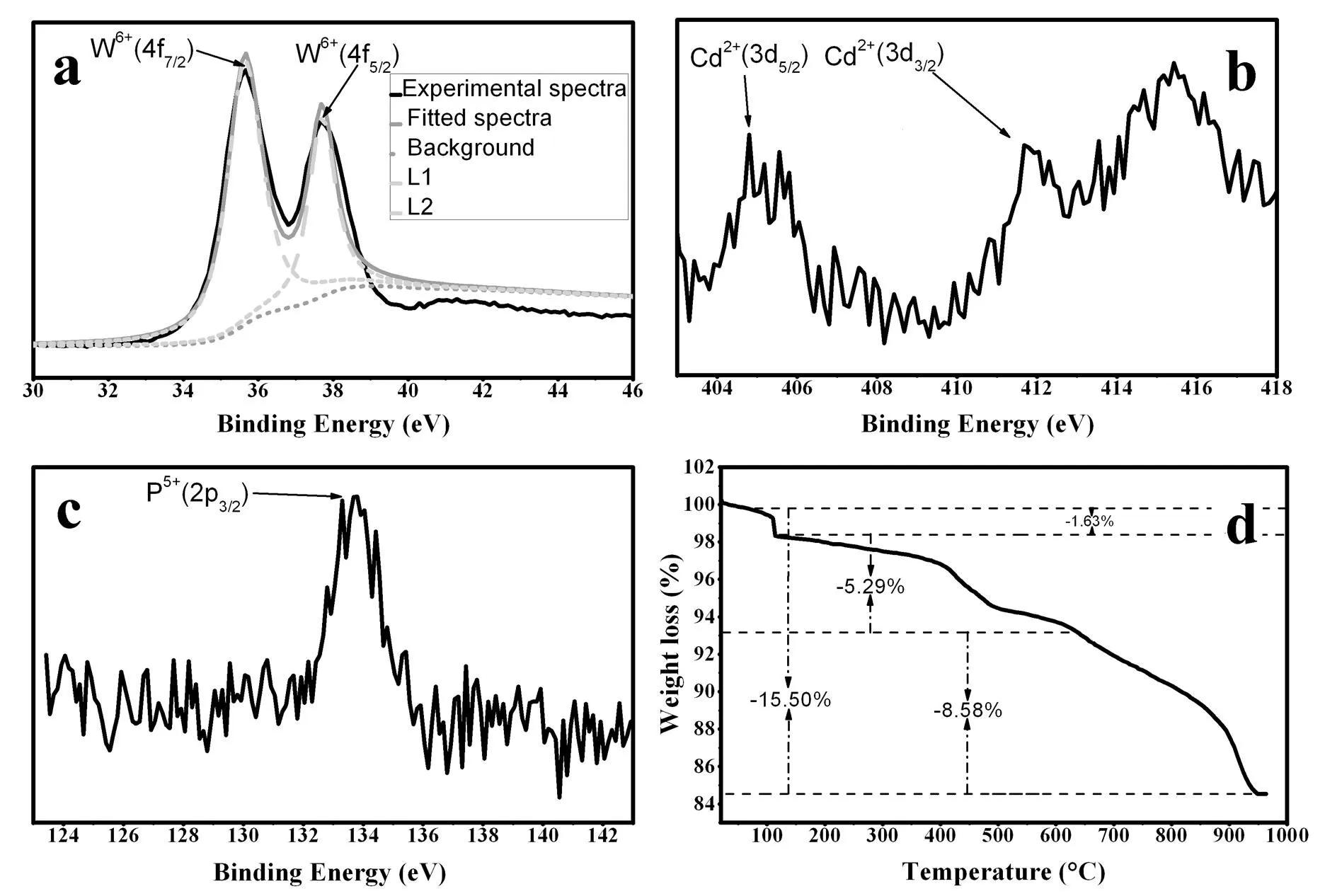
Fig. 4. (a) XPS spectrum for the Mo6+(3d5/2) and Mo6+(3d3/2) states of compound 1; (b) XPS spectrum for the Cd2+(2d3/2) and Cd2+(2d3/2) states of compound 1; (c) XPS spectrum for the P5+(2p3/2) state of compound 1; (d) TGA curve of compound 1 in N2atmosphere
3.4 Thermal properties
The TGA of compound 1 (Hpy)4[Cd(phen)2]-(P2W18O62)·H2O((C5NH6)4[Cd(C12N2H8)2]-(P2W18O62)·H2O,≈ 3) was performed in N2atmosphere in the temperature range of 30~980 ℃. As shown in Fig. 4d, the TGA curves in N2atmos- phere exhibit three steps of weight loss for compound 1. The first weight loss (1.63%) at 115 ℃can be attributed to the release of crystal water and surface-adsorbed H2O molecules. The continued second weight loss (5.29%. Calcd 6.14%) for the sample in the 120~635 ℃ range result from the release of Hpy+([C5NH6]+) cations. The third and last weight loss (8.58%. Calcd. 9.06%) for the sample in the range of 635~949 ℃ is due to the decomposition and release of [Cd(phen)2]2+units. The total weight loss (15.50%) is also consistent with the calculated value (Calcd. 16.23%). The multistep release of organic ingre- dients proves that the phen and py molecules play different roles in compound 1. These results well support the chemical composition of compound 1 from the above crystal structure analyses.
4 CONCLUSION
In summary, we report the synthesis and charac- terization of a novel hybrid POM, (Hpy)4[Cd(phen)2](P2W18O62)·H2O (1,≈ 3), containing Wells- Dawson-type polyanions, [P2W18O62]6–, which asymmetrically monosupports Cd-coordination com- plex by bi-bridging O atoms. The critical issues of the synthetic procedures were elaborated, including the species of reagents, pH, temperature, pressure, and particularly the addition sequence of reactants. Moreover, the liquid small organic molecule, py, plays a crucial role of “structure-directing agent” in affording asymmetric monosupported polyanions, [Cd(phen)2(P2W18O62)]4-. We believe that the pre- sent work will be meaningful in expanding the study of other new POM-based materials.
(1) Lisnard, L.; Dolbecq, A.; Mialane, P.; Marrot, J.; Codjovi, E.;Sécheresse, F. Molecular and multidimensional polyoxotungstates functionalized by {Cu(bpy)}2+groups.. 2005, 3913–3920.
(2) Hou, G. F.; Wang, X. D.; Yu, Y. H. J.; Gao, S.; Wen, B.; Yan, P. F. A new topology constructed from an octamolybdate and metallomacrocycle coordination complex.. 2013, 15, 249–251.
(3) Bakri, R.; Booth, A.; Harle, G.; Middleton, P. S.; Wills, C.; Clegg, W.; Harrington, R. W.; Errington, R. J. Rational addition of capping groups to the phosphomolybdate keggin anion [PMo12O40]3-by mild, non-aqueous reductive aggregation.2012, 48, 2779–2781.
(4) Han, Q.; Ma, P.; Zhao, J.; Wang, J.; Niu, J. A novel 1D tungstoarsenate with mixed organic ligands assembled by hexa-Cu sandwiched keggin units and dinuclear copper-oxalate complexes.. 2011, 14, 767–770.
(5) Yan, D.; Chen, Q.; Xu, Y.; Sun, Q.; Zhu, D.; Song, Y.; Elangovan, S. P. Hydrothermal synthesis, structure characterizations and catalytic property of a zigzag chain structural cluster compound built on the novel tetra-capped and centered by NiII.. 2011, 14, 1314–1317.
(6) Xu, Y.; Xu, J. Q.; Yang, G. Y.; Wang, T. G.; Xing, Y.; Ling, Y. H.; Jia, H. Q. Synthesis and structure of [NH4]4H[PMo8VIV4VV2O42]·24H2O.1998, 17, 2441–2445.
(7) Duan, L. M.; Pan, C. L.; Xu, J. Q.; Cui, X. B.; Xie, F. T.; Wang, T. G. Two- and three-dimensional frameworks constructed from bicapped keggin clusters.. 2003, 14, 2578–2581.
(8) Liu, Y. B.; Cui, X. B.; Xu, J. Q.; Lu, Y. K.; Liu, J.; Zhang, Q. B.; Zhang, T. G. Hydrothermal synthesis and characterization of three one-dimensional chain materials formed by reduced tetra-capped Keggin polyoxoanions and [(en)2]2+(= Cu, Co and Ni) cations.. 2006, 825, 45–52.
(9) Yang, W.; Lu, C.; Zhan, X.; Zhuang, H. Hydrothermal synthesis of the first vanadomolybdenum polyoxocation with a “metal-bonded” spherical framework.. 2002, 41, 4621–4623.
(10) Yu, H. H.; Cui, X. B.; Cui, J. W.; Kong, L.; Duan, W. J.; Xu, J. Q.; Wang, T. G. Hydrothermal synthesis and structural characterization of the first mixed molybdenum-tungsten capped-keggin polyoxometal complex: {[Co(dien)]4[(AsVO4)MoV8WVI4O33(μ2-OH)3]}·2H2O.. 2008, 2, 195–197.
(11) An, H. Y.; Wang, E. B.; Xiao, D. R.; Li, Y. G.; Su, Z. M.; Xu, L. Chiral 3D architectures with helical channels constructed from polyoxometalate clusters and copper-amino acid complexes.. 2006, 45, 904–908.
(12) Dolbecq, A.; Mialane, P.; Lisnard, L.; Marrot, J.; Sécheresse, F. Hybrid organic-inorganic 1D and 2D frameworks with ε-keggin polyoxomolybdates as building blocks.2003, 9, 2914–2920.
(13) Hao, X. L.; Ma, Y. Y.; Zhang, C.; Wang, Q.; Cheng, X.; Wang, Y. H.; Li, Y. G.; Wang, E. B. Assembly of new organic-inorganic hybrids based on copper-bis(trizole) complexes and keggin-type polyoxometalates with different negative charges.. 2012, 14, 6573–6580.
(14) Cui, J. W.; Cui, X. B.; Yu, H. H.; Xu, J. Q.; Yi, Z. H.; Duan, W. J. Hydrothermal syntheses and characterizations of two novel heteropolytungstate supported coordination compounds.. 2008, 361, 2641–2647.
(15) Dang, D. B.; An, B.; Bai, Y.; Niu, J. Y. Assembly of a phospho-molybdic wells-dawson-based silver coordination polymer derived from keggin polyoxoanion cluster.. 2012, 41, 13856–13861.
(16) Wang, X. L.; Zhao, D.; Tian, A. X.; Liu, G. C.; Lin, H. Y.; Wang, Y. F.; Gao, Q.; Liu, X. J.; Li, N. Self-assembly of two keggin-based 3D and 2D complexes with cobalt(II)-bis(trizole) motifs: influenced by the spacer length of the ligands.2012, 388, 114–119.
(17) Zhou, J.; Zheng, S. T.; Fang, W. H.; Yang, G. Y. A new 2-D network containing {As4V16O42(H2O)} cluster units.. 2009, 34, 5075–5078.
(18) Dong, B.; Peng, J.; Tian, A.; Sha, J.; Li, L.; Liu, H. Two new inorganic-organic hybrid single pendant hexadecavanadate derivatives with bifunctional electrocatalytic activities.2007, 52, 3804–3812.
(19) Chen, Y.; Peng, J.; Yu, H.; Han, Z.; Gu, X.; Shi, Z.; Wang, E.; Hu, N. A supramolecular assembly of chiral L/D-[Cd(Cl)(H2O)(phen)2]+and L,L/D,D-dinuclear Cd complex coordinated by phen and {V16O38(Cl)} cluster.2005, 358, 403–408.
(20) Zheng, S. T.; Zhang, J.; Xu, J. Q.; Yang, G. Y. Hybrid inorganic-organic 1-D and 2-D frameworks with {As8V14O42} clusters as buliding blocks.. 2005, 178, 3740–3746.
(21) Zheng, S. T.; Zhang, J.; Yang, G. Y. Hydrothermal syntheses and crystal structures of two novel hybrid materials based on secondary transition-metal-incorporated polyoxovanadate cluster backbones: [Cd(dien)2]2[(dien)CdAs8V13O41(H2O)]·4H2O and [Cd(en)2]2[(en)2Cd2As8V12O40].. 2005, 44, 2426–2430.
(22) Zhou, J.; Zhang, J.; Fang, W. H.; Yang, G. Y. A series of vanadogermanates from 1D chain to 3D framework built by Ge-V-O clusters and transition-metal-complex bridges.. 2010, 16, 13253–13261.
(23) Zhao, D.; Zheng, S. T.; Yang, G. Y. The first di-cadmium-substituted vanadoarsenate derived from-{As8V14O42} shell.2008, 181, 3071–3077.
(24) Ma, Y.; Li, Y.; Wang, E.; Lu, Y.; Wang, X.; Xu, X. Self-assembly of four new extended architectures based on reduced polyoxometalate clusters and cadmium complexes.. 2006, 179, 2367–2375.
(25) Zhang, X.; Yi, Z.; Zhao, L.; Chen, Q.; Wang, X.; Xu, J.; Xia, W.; Yang, C. pH-dependent assembly of a series of inorganic-organic hybrid molybdenum(V) phosphate.. 2010, 12, 595–603.
(26) Wang, X. L.; Cao, J. J.; Liu, G. C.; Lin, H. Y.; Tian, A. X. Ligands directed versatile cadmium-bis(triazole) metal-organic fragments to generate three new two dimensional complexes based on polymolybdenum phosphate.2013, 402, 6–11.
(27) Wang, Y.; Xiao, L. N.; Ding, H.; Wu, F. Q.; Ye, L.; Wang, T. G.; Shi, S. Y.; Cui, X. B.; Xu, J. Q.; Zheng, D. F. Hydrothermal synthesis and crystal structure of the first keggin polyoxometalate supported cadmium coordination complex.. 2010, 13, 1184–1186.
(28) Jiao, Y. Q.; Qin, C.; Sun, C. Y.; Shao, K. Z.; Liu, P. J.; Huang, P.; Zhou, K.; Su, Z. M. Hydrothermal synthesis and structural characterization of a new inorganic-organic hybrid compound with photocatalytic activity based on keggin-type polyanion and cadmium-1,2,4-trizolate units.. 2012, 20, 273–276.
(29) Tian, A.; Ying, J.; Wang, X.; Peng, J. A new two-fold interpenetrating POM-baseeed structure modified by CdIIand flexible bis(trizole) ligand.. 2011, 14, 118–121.
(30) Ying, J.; Liu, X. J.; Tian, A. X.; Wang, X. L. Assembly of a new cadmium(II)-bis(triazole) coordination polymer templated by keggin polyoxometalate.. 2011, 637, 613–617.
(31) Dai, L.; You, W.; Wang, E.; Wang, X.; Han, X.; Li, W.; Fang, Y. 2D rhombus-grid networks constructed from vanadiun-substituted keggin-type polyoxomolybdophosphates and Cd/Zn complex fragments.2009, 362, 4967–4971.
(32) Hu, Z. B.; Qiu, Z. H.; Chen, W. H. Synthesis, crystal structure and characterization of a mixed-valence keggin-structural 12-molybdophosphoric acid compound: NH4(C4N2H12(C2N2H10)2[MoV4MoVI8PO40]·2H2O..2013, 24, 1193–1204.
(33) Chen, W. H.; Hu, Z. B.; Zhang, Z. S.; Qiu, Z. H.; Zhou, Y. L.; Zhao, J. H.; Yuan, Q. L.; Xiang, X. Y.; Wang, X. M. Hydrothermal synthesis and crystal structure of a novel polymolybdate with two kinds of organonitrogen ligands.. 2011, 30, 1178–1182.
(34) Chen, W. H.; Xiang, Y.; Chen, Z. F.; Wu, Q. M.; Zeng, Q. X. Bis(4,4΄-bipyridinium)bis(5-hydrogenphosphate)-pentakis(2-oxido)- decaoxidopentamolybdate dihydrate.2007, 63, M2245–U1960.
(35) Hu, Z. B.; Chen, W. H.; Mi, J. X. A novel mixed-valence and capped-keggin structural polyoxometal complex: {[Co(dien)]4[(PO4)MoV8(WVI0.56MoVI0.44)O33(OH)3]}·H2O (≈ 1).. 2013, 32, 1653–1662.
(36) Sheldrick, G. M.. University of Göttingen, Göttingen, Germany 1990.
(37) Sheldrick, G. M.. University of Göttingen, Göttingen, Germany 1997.
(38) Brown, I. D.; keeffe, M. O .; Navrotsky, A. (eds.)., Vol. 2, Academic Press, New York 1981.
(39) Tian, A.; Han, Z.; Peng, J.; Dong, B.; Sha, J.; Li, B. Two novel hybrid inorganic-organic compounds based on wells-dawson polyanion and transition metal (TM) complex with one-dimensional structure: hydrothermal synthesis and characterization.. 2007, 832, 117–123.
(40) Wagner, C. D.; Riggs, W. M.; Davis, L. E.; Moulder, J. F.; Muilenberg, G. E.. Perkin Elmer Corp. MI.: 1978.
23 May 2014;
27 June 2014 (CCDC 1003304)
① This work was supported by the Foundation of Education Department of Fujian Province (Nos. JB12199 and JA11245), and the National Natural Science Foundation of China (Nos. 21233004 and 40972035)
. Phone: +86-13696905136, Fax: +86-592-2183937, E-mail: jxmi@xmu.edu.cn
- 结构化学的其它文章
- Theoretical Study on the Structures and Properties of Phenobarbital Imprinted Polymers①
- Solvothermal Syntheses, Crystal Structures, Thermal Stability and Quantum Chemistry of Dinuclear Trialkyltin ComplexesConstructed by Camphoric Acid①
- Synthesis, Crystal Structure and Characterization of a Ni(II) Complex of Constructed,6-Bis(benzimidazol-2-yl)pyridine①
- Synthesis, Crystal Structure and Properties of a Copper Complex with the Bicycle[2.2.1]-2-heptene-5,6-dicarboxylic Acid①
- Syntheses, Crystal Structures, and Biological Activities of Two 5-Pyrimidinyl-1,2,4-oxadiazoles
- Synthesis, Structureand Characterization of a Biologically Active Compound Based on 5-Nitrosalicylaldehyde Schiff Base①

