Syntheses, Crystal Structures, and Biological Activities of Two 5-Pyrimidinyl-1,2,4-oxadiazoles
HUANG Tong-Hui CHEN Hu CHEN Jie ZHANG Ai-Dong
Syntheses, Crystal Structures, and Biological Activities of Two 5-Pyrimidinyl-1,2,4-oxadiazoles
HUANG Tong-Huia, bCHEN HuaaCHEN JieaZHANG Ai-Dongb
a(221004)b(430079)
Two 5-pyrimidinyl-1,2,4-oxadiazoles were synthesized through two different routes and their structures were characterized by single-crystal X-ray diffraction, NMR and MS. Compound 3, 5-(2-chloro-4-methyl-6-phenylpyrimidin-5-yl)-3-phenyl-1,2,4-oxadiazole, crystalli- zes in orthorhombic, space groupwith= 19.1575(11),= 8.2115(5),= 21.2035(12) Å,= 3335.6(3) Å3and= 4. Compound 6, 5-(2,6-dichloropyrimidin-4-yl)-3-phenyl-1,2,4-oxadiazole, crystallizes in monoclinic space groupwith= 8.4275(13),= 5.4088(8),= 13.493(2) Å,= 99.768(3)º,= 4658.6(6) Å3and= 8. Preliminary bioassay indicated that the two title compounds had good herbicidal activities.
synthesis, crystal structure, herbicidal activity, 5-pyrimidinyl-1,2,4-oxadiazole
1 INTRODUCTION
The pyrimidine rings represent a class of versatile building blocks and have widespread applications in pharmaceuticals and agrochemical industry. A num- ber of synthetic pharmacophores with anti-inflam- matory[1], antifungal[2]and anticancer[3]activities are based on the pyrimidine motif. Many pyrimidine derivatives, such as diclosulam[4]and flupyrsul- furon-methyl[5], are commercially available herbici- des. On the other hand, substances containing a substituted 1,2,4-oxadiazole fragment are frequently used in drug discovery with versatile biological activities such as antiviral[6], antimycobacterial[7]and anti-cancer[8]activities.
In our previous study, we developed an efficient approach for the synthesis of novel 5-(pyrimidin-5- yl)-1,2,4-oxadiazoles with a wide diversity in subs- tituents[9]and found that the 5-pyrimidinyl-1,2,4- oxadiazoles possessed potential herbicidal activities against(rape) and(barnyard grass)[10]. In order to investi- gate their properties and expand their applications, we prepared two 5-pyrimidinyl-1,2,4-oxadiazole derivatives, discussed the crystal structures and evaluated the herbicidal activity.
2 EXPERIMENTAL
2.1 General instruments
The two title compounds were prepared by the method outlined in Scheme1. All melting points were measured with a Buchi B-545 melting point apparatus and uncorrected. MS spectra were performed with a Finnigan Trace MS 2000 organic mass spectrometer using the electron ionization (EI) method. IR spectra were recorded on a PE-983 infrared spectrometer as KBr pellets with absorption in cm-1. NMR spectra were recorded in CDCl3on a Varian Mercury 600 spectrometer and resonances are reported relative to TMS. Elementary analyses were taken on a Vario EL III elementary analysis instrument. X-ray single-crystal diffraction analysis was performed on a Bruker SMART-APEX equip- ment.
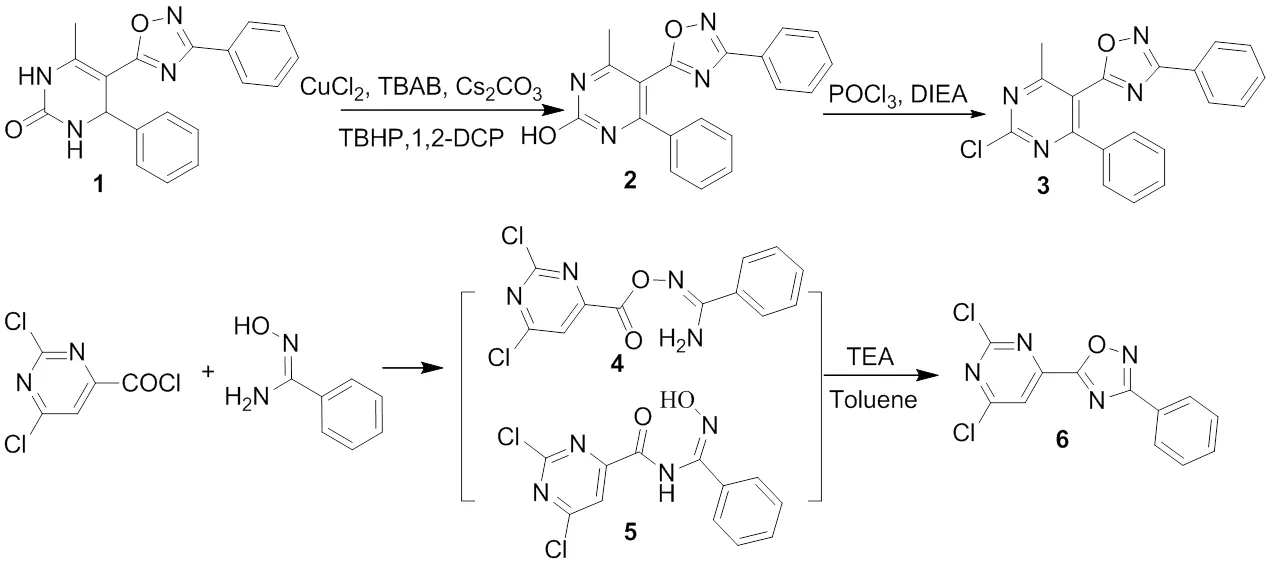
Scheme 1. Synthesis of 5-pyrimidinyl-1,2,4-oxadiazoles 3 and 6
2.1.1 Synthesis of 5-(2-chloro-4-methyl-6-phenylpyrimidin-5-yl)-3-phenyl-1,2,4-oxadiazole (3)
The procedures for the syntheses of 5-(6-methyl- 4-phenyl-3,4-dihydro-2(1)-pyrimidinon-5-yl)-3- phenyl-1,2,4-oxadiazole (1) and 5-(4-methyl-6- phe-nyl-2-pyrimidinol-5-yl)-3-phenyl-1,2,4-oxadia-zole (2) were performed according to the reported method[9]. Under a nitrogen atmosphere, a mixture of 4-methyl-6-phenyl-5-(3-phenyl-1,2,4-oxadiazol-5-yl)pyrimidin-2-ol(3.3 g, 20 mmol) and phos- phorus oxychloride (25 mL) in the presence of diisopropylethylamine (DIPEA) (1.5 g, 12 mmol) was heated at 95~100 ℃ for 3 h. The volatiles were evaporated under reduced pressure and the residue was poured into ice water and extracted with ethyl acetate. The organic phase was washed with aqueous NaHCO3and water successively, and dried over Na2SO4. Then the solvent was eva- porated and the residue was purified by column chromatography on silica gel eluted with petroleum ether/EtOAc (10:1, V/V) to give compound 3.
Compound 3:white solid; yield: 86%; m.p.: 69~70 ℃. IR (KBr): 3433, 1604, 1540, 1297 cm-1.1H- NMR (600 MHz, CDCl3):= 0.96 (t,= 7.2 Hz, 3H, Pr-CH3), 1.79~1.75 (m, 2H, CH2), 2.62 (s, 3H, 6-CH3), 2.81 (t,= 7.2 Hz, 2H, CH2), 7.58~7.53 (m, 3H,-H +-H), 8.16 (d,= 7.2 Hz, 2H,-H).13C NMR (150 MHz, CDCl3):= 22.1, 23.3, 23.3, 37.8, 117.0, 125.8, 127.3, 128.8, 131.5, 162.0, 168.6, 170.1, 172.5, 173.4. MS (EI, 70 eV): m/z (%) = 315 (M++ 1, 24), 279 (100), 237 (36), 197 (45). Anal. Calcd. for C16H15ClN4O (%): C, 61.05; H, 4.80; N, 17.80. Found (%): C, 61.42; H, 4.98; N, 18.22.
2.1.2 Synthesis of 5-(2,6-dichloropyrimidin-4-yl)-3-phenyl-1,2,4-oxadiazole (6)
To a stirred solution of N΄-hydroxybenzimidamide (1.5 g, 11 mmol) and TEA (triethylamine) (1.21 g, 10 mmol) in toluene (25 mL) at 0 ℃ was added 2,6-dichloropyrimidine-4-carbonyl chloride (2.11 g, 10 mmol, in a solution in 20 mL of toluene) dropwise. The reaction mixture was stirred at 0 ℃. Two intermediates 4 and 5, which were respectively formed by-acylation and-acylation, were observed by TLC analysis within 30 min. And then, the mixture was heated at reflux for another 2 h. After cooling to room temperature, the volatiles were removed under reduced pressure and the residue was extracted with ethyl acetate, washed with water and dried over MgSO4. The solvent was evaporated and the product was isolated by column chromatography on silica gel eluted with petroleum ether/EtOAc (10:1, V/V) to give compound 6.
Compound 6: Colorless acicular crystal, yield 73%, m.p.: 176~177 ℃. IR (KBr): 3389, 1651, 1498, 1324 cm-1.1H NMR (CDCl3, 600 MHz):7.52~7.57 (m, 3H, PhH), 8.17~8.18 (m, 2H, PhH), 8.21 (s, 1H, pyrimidine-H); MS (70 eV)/(%): 293 (M+, 5), 292 (9), 119 (100). Anal. Calcd. (%) for C12H6Cl2N4O: C, 49.17; H, 2.06; N, 19.11. Found (%): C, 49.42; H, 2.12; N, 18.82.
2.2 Structure determination
Suitable single crystals of compound 3 (0.16mm × 0.12mm × 0.10mm) and 6 (0.20mm × 0.10mm × 0.10mm) were mounted on a Bruker SMART CCD diffractometer equipped with a graphite-mono- chromatic Mo(= 0.71073 Å) radiation at 298(2) K for data collection using an-collection with an-2scan mode. The crystal structure was solved by direct methods using the SHELXS-97 program[11]and refined with SHELXL-97[12]by full-matrix least-squares techniques on2. All the non-hydro- gen atoms were refined anisotropically and the hydrogen atoms were determined with theoretical calculations and refined isotropically. For com- pound 3, a total of 19364 reflections including 3642 independent ones (int= 0.0348) were collected in the range of 1.92<<27.00º, of which 3245 obser- ved reflections with> 2() were used in structure determination and refinements. A full-matrix least- squares refinement gave the final= 0.0552,= 0.1374 (= 1/[2(F2) + (0.0654)2+ 1.2328], where= (F2+ 2F2)/3),= 1.127, (Δ/)max= 0.001, (Δ)max= 0.335 and (Δ)min= –0.224 e/Å3. For compound 6, a total of 4103 reflections including 1743 independent ones (int= 0.0851) were collected in the range of 2.66<<25.99º, of which 1858 observed reflections with> 2() were used in structure determination and refinements. A full-matrix least-squares refine- ment gave the final= 0.0444,= 0.1073 (= 1/[2(F2) + (0.0492)2+ 0.0000], where= (F2+ 2F2)/3),= 1.053, (Δ/)max= 0.011, (Δ)max= 0.273 and (Δ)min= –0.261 e/Å3.
3 RESULTS AND DISCUSSION
Two new 5-pyrimidinyl-1,2,4-oxadiazoles 3 and 6were synthesized through two different routes, respectively. The two compounds were successfully crystallized and their structures were determined by single-crystal X-ray diffraction analysis. The NMR, ESI-MS and elemental analyses were in accordance with the proposed structures.
Crystals of compounds 3 and 6 were obtained from methanol/dichloromethane and methanol solution, respectively. The perspective views of compounds 3 and 6 with atomic labeling scheme are shown in Fig. 1 and the selected bond lengths and bond angles are listed in Table 1. Each of the two molecules contains benzene, pyrimidine and oxadia- zole rings with the bond lengths and bond angles falling in normal ranges as compared to those observed in a similar structure[13]. The bonds of C(4)–C(6), C(3)–C(12) and C(13)–C(14) in com- pound 3 and C(4)–C(5) and C(6)–C(7) in compound 6 are shorter than the normal C–C single bond (1.524 Å) and longer than the C–C double bond (1.317 Å), which suggest these rings are delocalized. The bond lengths of C(12)–N(3) (1.284(2) Å), C(13)–N(3) (1.378(2) Å) in compound 3 and C(5)–N(4) (1.304(4) Å), C(6)–N(4) (1.375(5) Å) in compound 6are located in a scope between the C–N single bond (1.4710 Å) and C–N double bond (1.2730 Å), implying a conjugated system operation in the oxadiazoles.

Table 1. Herbicidal Activities of the Title Compounds
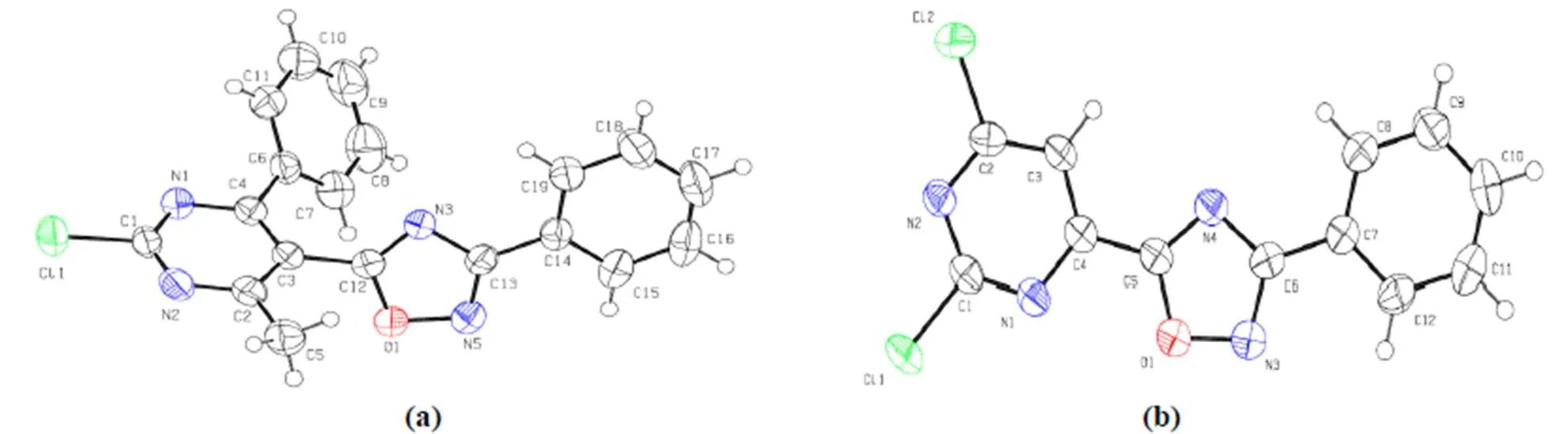
Fig. 1. (a) Molecular structure of 3; (b) Molecular structure of 6
Fig. 2. (a) Crystal packing of compound 3; (b) Crystal packing of compound 6
Compared to the structure of 6, the difference of 3 is the oxadiazole substitution position and subs- tituent on the pyrimidine ring. In the two molecules, the oxadiazole and attached phenyl rings are almost coplanar with the dihedral angles to be 6.22° and 4.31°, respectively. For the crystal structure of compound 3, the dihedral angle between the oxa- diazole and pyrimidine rings is 66.02° due to the steric hindrance effect of the phenyl group attached to the pyrimidine ring. In contrast, the dihedral angle between the oxadiazole ring (O(1)/C(5)/N(4)/C(6)/N(3)) and pyrimidine ring (C(1)–C(4)/N(1)–N(2)) is 7.84° in 6. As shown in Fig. 2a, the molecules of 3 are stacked along the-axis to form one-dimensional chains via-inte- ractions of pyrimidine rings (centroid-centroid distance: 3.350 Å). As shown in Fig. 2b, the mole- cules of 6 are stacked along the diagonals ofplane via-interactions of benzene, pyrimidine and oxadiazole rings (the centroid-centroid distan- ces are 3.349, 3.304 and 3.362 Å, respectively) to form a one-dimensional chain. Several parallel one- dimensional chains form a layer, and the extending direction of such chains in the neighboring layers is almost vertical.
4 BIOLOGICAL ACTIVITIES
The herbicidal activities of the title compounds against(rape) and(barnyard grass) were evaluated in preli- minary assays at dosages of 100, 10 and 1 mg/L according to a reported procedure[9], and compared with distilled water as the control. As shown in Table 2, both of the compounds possessed potential herbicidal activities by inhibiting the root and stalk growth of not only monocotyledonous barnyard grass, but also dicotyledonous rape. For instance, both of the compounds showed inhibitory rates of >70% to the root growth and >60% to the stalk growth of barnyard grass at dosage of 100 mg/L. However, both of the compounds showed low inhibitory control to grass with the reduction of dosage to 10 and 1 mg/L. The compounds possess more potent inhibitory activity against the root/stalk growth of monocots (exhibit a relative selectivity. Further investigation on the biological activity is still in progress in our laboratory.
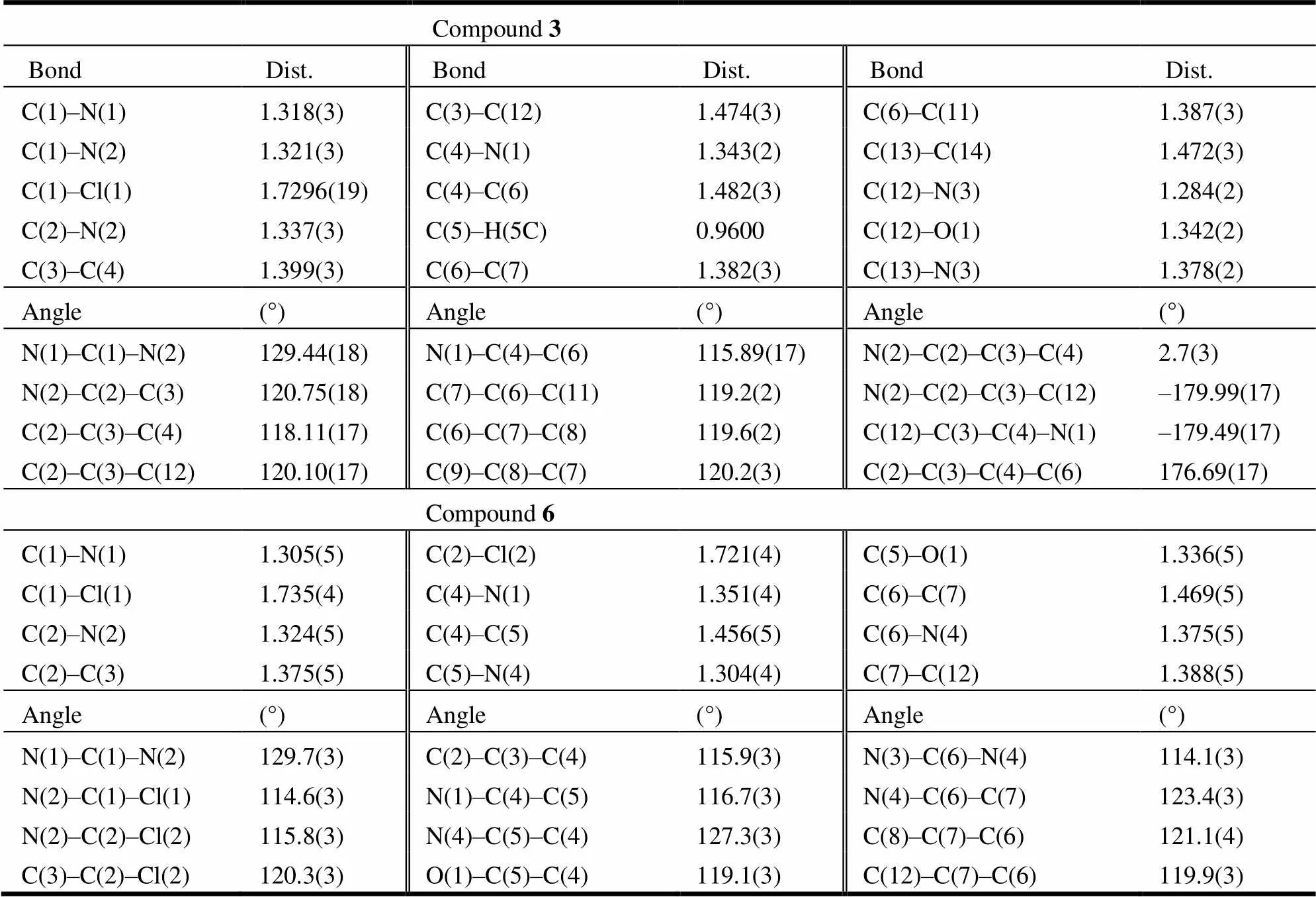
Table 2. Selected Bond Lengths (Å) and Bond Angles (°) of 3 and 6
Symmetry transformation: a:,+1,; b: –,, –+1/2; c: –, –+1, –
(1) Keche, A. P.; Hatnapure, G. D.; Tale, R. H.; Rodge, A. H.; Birajdar, S. S.; Kamble, V. M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation.. 2012, 22, 3445–3448.
(2) Hanafy, F. I. Synthesis and antifungal activity of some new pyrido[2,3-d]pyrimidines.2011, 2, 65–69.
(3) Shao, H.; Shi, S. H.; Huang, S. L.; Hole, A. J.; Wang, S. D. Substituted 4-(thiazol-5-yl)-2-(phenylamino)pyrimidines are highly active CDK9 inhibitors: synthesis, X-ray crystal structures, structure-activity relationship, and anticancer activities.2013, 56, 640–659.
(4) Anjan, B.; Pritam, G.; Suhrid, R. B.; Chiranjit, K.Studies on the persistence of diclosulam in soybean crop.2012, 18, 29–37.
(5) Singles, S. K.; Dean, G. M.; Kirkpatrick, D. M.; Mayo, B. C.; Langford-Pollard, A. D.; Barefoot, A. C.; Bramble, F. Q. Fate and behaviour of flupyrsulfuron-methyl in soil and aquatic systems.1999, 55, 288–300.
(6) Marechal, X.; Genin, E.; Qin, L.; Sperandio, O.; Montes, M.; Basse, N.; Vidal, J.; Villoutreix, B. O. 1,2,4-Oxadiazoles identified by virtual screening and their non-covalent inhibition of the human 20S proteasome.2013, 20, 2351–2362.
(7) Almansour, A. I.; Suresh Kumar, R.; Arumugam, N.; Sriram, D. A solvent free, four-component synthesis and 1,3-dipolar cycloaddition of 4(H)-pyrans with nitrile oxides: synthesis and discovery of antimycobacterial activity of enantiomerically pure 1,2,4-oxadiazoles.2012, 53, 416–423.
(8) Khatik, G. L.; Kaur, J.; Kumar, V.; Tikoo, K.; Nair, V. A. 1,2,4-Oxadiazoles: a new class of anti-prostate cancer agents.2012, 22, 1912–1916.
(9) Huang, T. H.; Tu, H. Y.; Liu, M.; Hou, C. J.; Zhang, A. D.Synthesis and biological activity of 5-pyrimidinyl-1,2,4-oxadiazole derivatives.2011, 31, 891–896.
(10) Huang, T. H.; Tu, H. Y.; Liu, M.; Wang, Y. F.; Zhang, A. D. Synthesis of novel 5-(pyrimidin-5-yl)-1,2,4-oxadiazole derivatives via the Biginelli reaction and subsequent oxidative dehydrogenation.2011, 83, 93–97.
(11) Sheldrick, G. M.,. University of Göttingen, Germany 1997.
(12) Sheldrick, G. M.,. University of Göttingen, Germany 1997.
(13) Fun, H. K.; Mohd, M. R.; Rai, S.; Arun, M. I.; Shetty, P. 5-(2,4-Dichlorophenyl)-3-(4-nitrophenyl)-1,2,4-oxadiazole.2010,E66, o1196–o1197.
28 March 2014;
10 July 2014 (CCDC 773291 for 3 and 973037 for 6)
① This work was supported bythe National Natural Science Foundation of China (Nos. 81302629, 21172088)
. Born in 1982, Ph. D, majoring in organic synthesis and medicinal chemistry. E-mail: tonghhuang@gmail.com
- 结构化学的其它文章
- Thermoelectric Properties of the CuGaTe2 Crystal from First-principles Calculations: the Role of Doping and Temperature①
- Morphology, Size-controlled Synthesis of CoO anostructure and Its Magnetic Property①
- The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
- Synthesis, Crystal Structure and Antiproliferative Activity of 2-(((5-(((5,7-Dimethyl-[1,2,4]triazolo-[1,5-a]pyrimidin-2-yl)thio)methyl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)-4H- chromen-4-one Methanol Solvate①
- Synthesis, Crystal Structure and Luminescent Property of an Eu3+ Complex①
- Synthesis, Dimer Crystal Structure and Herbicidal Activity of 2-(4-Ethoxybenzoyl)cyclopentane-1,3-dione①

