Synthesis, Dimer Crystal Structure and Herbicidal Activity of 2-(4-Ethoxybenzoyl)cyclopentane-1,3-dione①
XU Hai-Zhen XIE Li-Fen ZHANG Hai-Li LI Lei MA Yuan ZHU You-Quan②
Synthesis, Dimer Crystal Structure and Herbicidal Activity of 2-(4-Ethoxybenzoyl)cyclopentane-1,3-dione①
XU Hai-Zhena②XIE Li-Fenb, cZHANG Hai-Lib, cLI Lei MA Yuanb, cZHU You-Quanb, c②
a(300393)b(300071)c(300393)
cyclopentane-1,3-dione, synthesis, crystal structure
1 INTRODUCTION
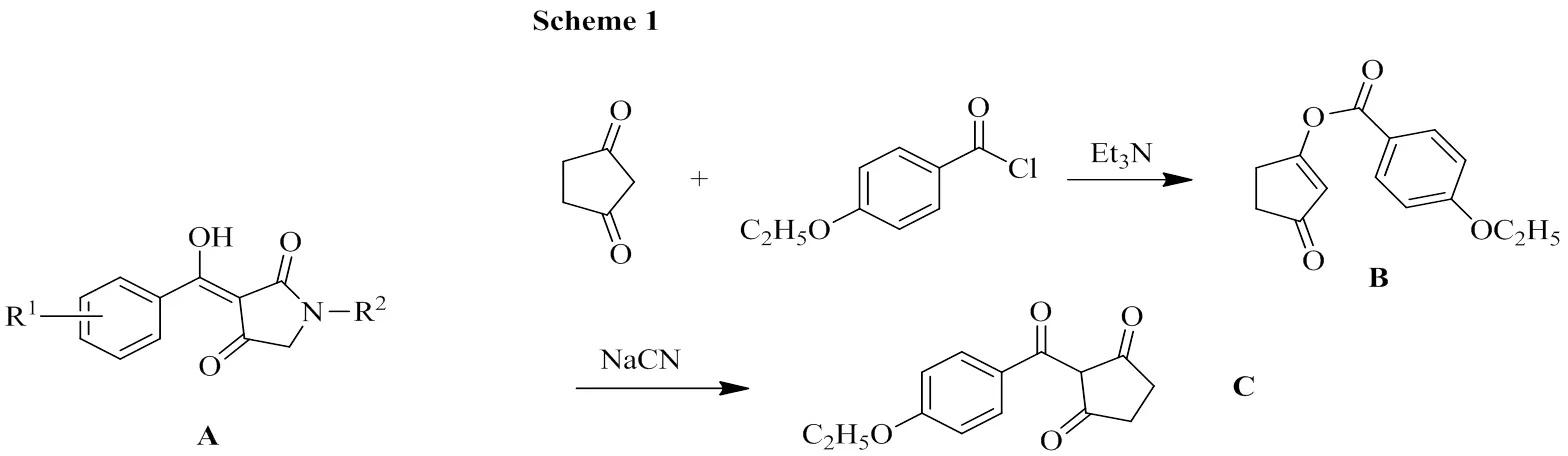
The inhibitors of 4-hydroxyphenylpyruvate dioxygenase (HPPD, EC 1.13.11.27) constitute a new kind of herbicide[1]. A survey of the known 4-HPPD inhibitors revealed that most of them have very similar structural characteristics, i.e., their benzoyl groups are substituted with various electron-withdrawing groups such as chlorine, trifluoro- methyl, nitro, or methanesulfonyl functional groups at ortho- and/or para-positions of the benzene rings[1,2]. Interestingly, a different substitution effect was observed in the series of-hydroxy-substituted 3-benzylidenepyrrolidene-2,4-diones (A) in our previous work[3-5]. The bioassay results showed that when R1was electron-donating, A exhibited better herbicidal activities. In particular, when R1and R2were 2,4-dimethoxyand-propyl respectively, it provided 93% control of.with crop selectivity on corn and soybeans at pre-emergence at 187.5 g/ha. This stimulated us to further modify the central heterocycle to discover new potent herbicides and a series of 2-substituted benzoylcyclopentane- 1,3-dione derivatives were designed and synthesized. Preliminary bioassay results proved that the title compound possessed better herbicidal activities. However, there has been no report on the crystal structure of the title compound (B) so far. For the purpose of exploring the structure-activity relationships for this kind of compounds, compound Cwas synthesized (Scheme 1) and its crystal structure was reported herein for the first time.
2 EXPERIMENTAL
2.1 Reagents and physical measurements
All reagents obtained from commercial sources were of AR grade and treated with standard methods before use. Melting point was determined on a Yanaco P-500 digital melting-point apparatus and uncorrected.1H NMR spectra were recorded on a BRUKER AC-P300 spectrometer in CDCl3with TMS as an internal standard, and chemical shift values () were given in parts per million. Ele- mental analysis was performed on a Yanaco MT-3 CHN elemental analyzer. The diffraction data were collected on a SMART 1000 CCD single-crystal X-ray diffractometer.
2.2 Synthesis of compound B[6]
To the mixture of 2 mmol cyclopentane-1,3-dione, 4.4 mmol (C2H5)3N and 3 mL dry CH3CN, the solution of 4-ethoxybenzoyl chloride (4 mmol) in 3 mL dry CH3CN was dropped. After the reaction mixture was stirred for 1 h, 10 mL water was added, then the mixture was extracted with ethyl acetate (10 mL × 3). The organic layer was dried with sodium sulfate and filtered. The solvent was remo- ved under vacuum and gave a yellow solid (B). Yield: 67.2%, m.p.: 85~87 ℃.1H NMR (300 MHz, CDCl3): 1.46 (t,= 7.0 Hz, 3H, OCH2C3), 2.41~2.58 (m, 2H, C2cycl.), 2.77~3.08 (m, 2H, C2cycl.), 4.13 (q,= 7.0 Hz, 2H, OC2CH3), 6.36 (s, 1H, C=C), 6.96 (d,= 9.0 Hz, 2H, Ar), 8.06 (d,= 9.0 Hz, 2H, Ar).
2.3 Synthesis of compound C[7]
To the mixture of 2 mmol B,3 mL dry CH3CN and 2.2 mmol NaCN was added slowly 2.2 mmol (C2H5)3N. After stirring for 12 h, the reaction mixture was acidified with 10 mL 1 M HCl and extracted with ethyl acetate (10 mL × 3). The organic layer was dried with sodium sulfate follo- wed by filtration. The solvent was removed under vacuum and purified by recrystallization from EA and PE (ethyl acetatepetroleum ether= 1:1) to give the colorless crystals C. Yield: 27%. m.p.: 89~91℃. Anal. Calcd. (%) for C14H14O4: C, 68.28; H, 5.73. Found (%): C, 68.08; H, 5.90. IR (KBr) (trans- mittance (%)): 3453(O–H) (98%), 3006(C–H for benzene ring) (91%), 2990(C–H) (95%), 1695(C=O) (90%), 1602(C=O) (80%), 1423(C=O)(75%) cm-1.1H NMR(CDCl3, 300 MHz)ppm: 1.45 (t,= 6.9 Hz, 3H, OCH2C3), 2.70 (s, 4H, C2C2cycl.), 4.13 (q,= 6.8 Hz, 2H, OC2CH3), 6.95 (d,= 8.7 Hz, 2H, Ar), 8.24 (d,= 8.6 Hz, 2H, Ar).
2.4 Structure determination
A colorless crystal suitable for X-ray diffraction study was cultivated in the test tube from EA and PE (EA:PE= 1:1) by self-volatilization. A crystal with dimensions of 0.22mm × 0.20mm × 0.12mm wasmounted on a SMART 1000 CCD area diffrac- tometer equipped with a graphite-monochromaticMoradiation (= 0.71073 Å). Intensity data were collected at 293(2) K by using a multi-scan mode inthe range of 2.36≤≤27.91° with the following index ranges: –11≤≤11, –11≤≤11 and –19≤≤20. A total of 12246 reflections were collected and 5602 were independent (int= 0.0441), of which 2301 with> 2() were observed. The crystal structure was solved by direct methods with SHELX-97[8]and refined by full-matrix least-squa- res refinements based on2with SHELXL-97. All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were located in the calcu- lated positions and refined with a riding model. The final refinement converged at= 0.0559,= 0.1278 (= 1/[2(F2) + (0.0700)2+ 0.00],= (F2+ 2F2)/3),= 1.035 and (Δ/)max= 0.005.
3 RESULTS AND DISCUSSION
3.1 Crystal structure description
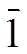
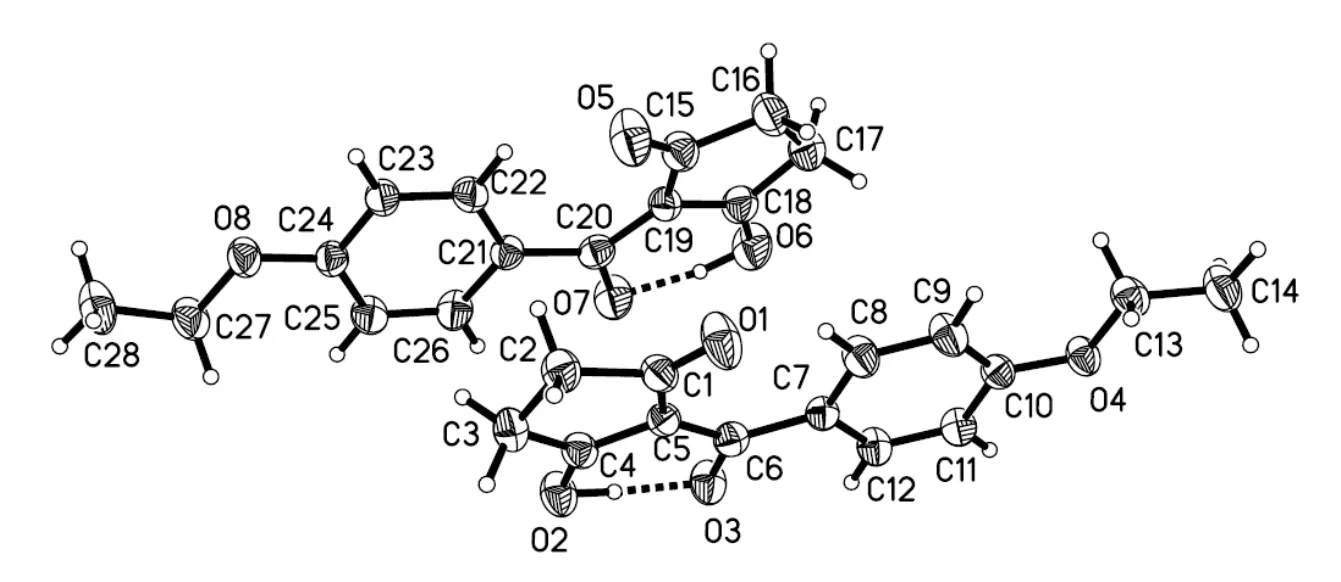
Fig. 1. View of the title compound (C), with displacement ellipsoids drawn at the 30% probability level
Fig. 2. A view of a hydrogen-bonded (dashed lines) chain in (C)
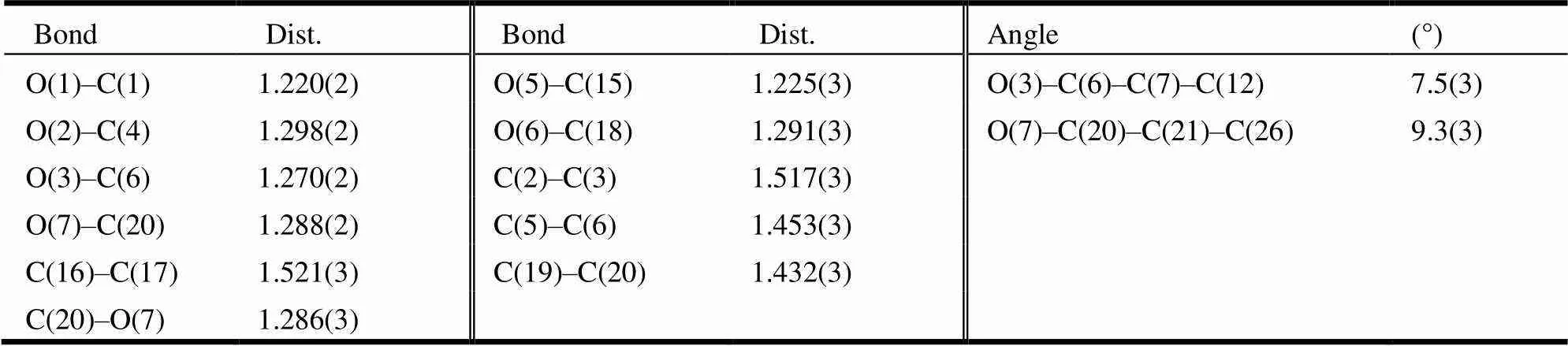
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
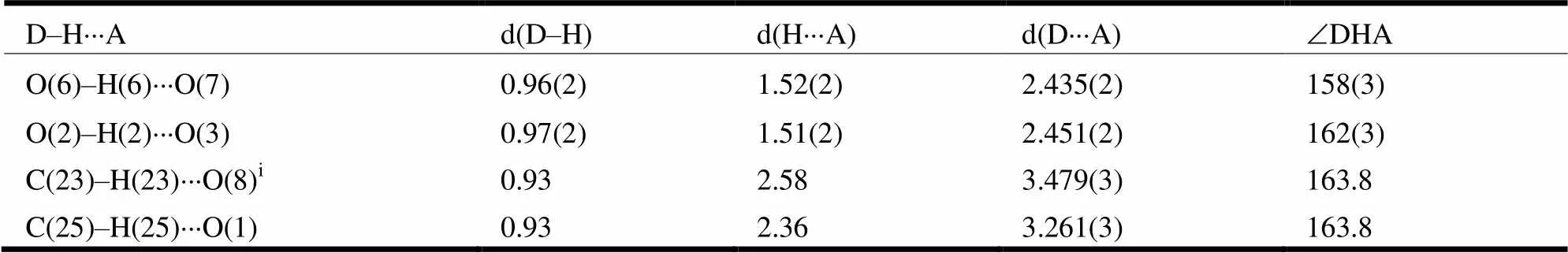
Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°)
Symmetry code: i: 1–, 2–,
The crystal structure of the title compound consists of two molecules with one six-membered ring and one cyclopentane-1,3-dione ring. The two benzene rings (C(21)/C(22)/C(23)/C(24)/C(25)/C(26) and C(7)/C(8)/ C(9)/C(10)/C(11)/C(12)) are copla- nar with carbonyl C(20)=O(7) and C(6)=O(3), and the largest deviations are 0.1089(13) Å for O(7) and 0.0609(12) Å for O(3). The torsional angles for O(7)–C(20)–C(21)–C(26) and O(3)–C(6)–C(7)– C(12) are 9.3(3) and 7.5(3)º. As expected, the O(3)– C(6) (1.270(2) Å) and C(20)–O(7) (1.286(3) Å) bonds are significantly shorter than O(1)–C(1) (1.220(2) Å) or O(5)–C(15) (1.225(2) Å); the O(2)– C(4) (1.298(2) Å) and C(6)–O(18) (1.288(3) Å) bonds are significantly longer than O(1)–C(1) (1.220(2) Å) or O(5)–C(15) (1.225(2) Å); the C(5)– C(6) (1.453(3) Å) and C(19)–C(20) (1.432(3) Å) are significantly longer than C(2)–C(3) (1.517(3) Å) or C(16)–C(17) (1.521(3) Å). The bond lengths within this part of the molecule lie between the classical single- and double-bond lengths, indicating extensive conjugation. Two strong intramolecular O(6)–H(6)···O(7) and O(2)–H(2)···O(3) hydrogen bonds (Table 2) are observed. The crystal structure includes intermolecular C–H···O hydrogen bonds (Fig. 2). There is an intermolecularstacking interaction between the two neighbouring parallel phenyl rings in the title compound (C(7)/C(8)/C 9)/C(10)/C(11)/C(12)), phenyl ring, symmetry code: 2–,1–,1–). The centroid-to- centroid distance, plane-plane distance and displa- cement distance are 3.861, 3.453 and 1.726 Å respectively.
3.2 Evaluation of the bioactivity
The biological activities for the title compound were also investigated. As a result, this compound provided 93.8% and 87.2% control ofandrespectively at post-emergence at 375 g/hm2.
(1) Hirai, K.; Uchida, A.; Ohno, R. Major synthetic routes for modern herbicide classes and agrochemical characteristics.v2002, 179–289.
(2) Lee, D. L.; Knudsen, C. G.; Michaely, W. J.; Chin, H.L.;Nguyen, N. H.; Carter, C. G.; Cromartie, T. H.; Lake, B. H.;Shribbs, J. M.; Fraser, T. The structure-activity relationships ofthe triketone class of HPPD herbicides.1998, 54,377–384.
(3) Zhu, Y.Q.; Hu, F.Z.; Zou, X.M.; Yao, C.S.; Liu, B.; Li, Y.H.; Yang, H.Z. Synthesis of 1-benzyl-3-(alpha-hydroxy-(un)substitutedbenzylidene)pyrrolidine-2,4-diones and biological activity.2005, 25, 419–423 (in Chinese).
(4) Zhu, Y.Q.; Yao, C.S.; Zou, X.M.; Hu, F.Z.; Liu, B.; Li, Y.H.; Yang, H.Z. The synthesis and herbicidal activity of 1-alkyl-3-(alpha-hydroxy-substituted benzylidene) pyrrolidine-2,4-diones.2005, 10, 427–434.
(5) Zhu, Y.Q.; Zou, X.M.; Hu, F.Z.; Yao, C.S.; Liu, B.; Li, Y.H.; Yang, H.Z. Synthesis and herbicidal evaluation of novel 3-(R-hydroxy-substitutedbenzylidine)pyrrolidine-2,4-diones.2005, 53, 9566–9570.
(7) Kim, T. H.; Oh, D. R.; Na, H. S.; Lee, H. C. Synthesis and immunosuppressive activity of novel succinylacetone analogues.2003, 26, 192–196.
(8) Sheldrick, G. M.. Göttingen University, Germany 1997; Sheldrick, G. M., Göttingen University, Germany 1997.
28 March 2014;
18 June 2014 (CCDC 993828)
① We are grateful for financial support by the National Basic Research Program of China (973 Program, No. 2010CB126103), the National Natural Science Foundation of China (No. 21372134, No. 21072108), NFFTBS (No. J1103306), the National Key Technologies R&D Program (2011BAE06B05-3) and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin)
. E-mail: zyq8165@nankai.edu.cn and hxxyxhz@mail.tjnu.edu.cn
- 结构化学的其它文章
- Thermoelectric Properties of the CuGaTe2 Crystal from First-principles Calculations: the Role of Doping and Temperature①
- Morphology, Size-controlled Synthesis of CoO anostructure and Its Magnetic Property①
- The First Hybrid Wells-Dawson-type Polytungstate Monosupported by Cd-coordination Complex via Di-bridging O-atom①
- Synthesis, Crystal Structure and Antiproliferative Activity of 2-(((5-(((5,7-Dimethyl-[1,2,4]triazolo-[1,5-a]pyrimidin-2-yl)thio)methyl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)-4H- chromen-4-one Methanol Solvate①
- Synthesis, Crystal Structure and Luminescent Property of an Eu3+ Complex①
- Synthesis, Structureand Characterization of a Biologically Active Compound Based on 5-Nitrosalicylaldehyde Schiff Base①

