对映选择性羟化乙基苯的碳氢键合成 (R)-和 (S)-1-苯基乙醇
陈永正,李 珂,单 静
(遵义医学院 药学院,贵州 遵义 563099)
Chiralsec-alcohols have broad utility for the synthesis of various of fine chemicals, pharmaceuticals, and agrochemicals[1]. Numerous syntheses of chiralsec-alcohols have been studied for many years, such as reduction, hydrolysis, and kenetic resolution[2]. Though regio- and stereoselective hydroxylations provided a direct access to synthesize chiral alcohols, chemical hydroxylations often suffer from poor chemo-, regio-, and enantioselectivity[3]. Thus, biohydroxylation reaction can be employed as a useful tool for this type of transformation[4]. Recent years, some purified enzymes and microbial whole-cell catalysts have been developed for hydroxylation of EB. For example,Mortierellaisabellina,Helminthosporiumspecies,Cunninghamellaechinulatawere capable of performing hydroxylation of EB to produce (R)-PE with 9%-10% yield in 33%-55%ee, respectively[5]. Resting cells ofFusariummoniliformeMS31 converted EB into (R)-PE with 98%ee[6]. Adamet.al. isolated aBacillusmegateriumstrain from topsoil by a selective screening procedure, this strain performed the hydroxylation of EB to form (R)-PE with 19%ee[7]. Martin Kluge group reported that the peroxygenase ofAgrocybeaegeritacatalyzed EB to (R)-PE with 99% conversion and 99%eeat 1 mM substrate concentration[8]. Moreover, Yadav et. al. reported that the mycelia ofAspergillusnigerMTCC-404 have been shown to act as a biocatalyst for the transformation of EB to (R)-PE in 99%eeand 72% yield[9]. Previously, we discoveredPseudomonasputidaZMUT03 andIsoptericolajiangsuensisZMUT11 as a hydroxylation catalyst with unique benzylic substrate specificity as well asR- andS-selectivity and high enantioselectivity[10-13].
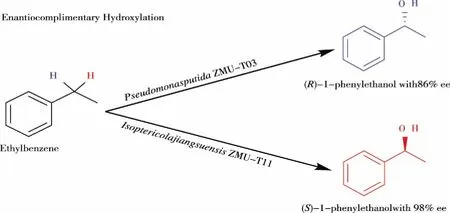
Fig 1 Enantioselective hydroxylation of EB to produce (R)-PE and (S)-PE
However, there only are a few enzymes and whole-cell biocatalyst to showS-selectivity for hydroxylation of EB. For examples, Gibsonet.al. purified a naphthalene dioxygenase fromPseudomonassp. Strain NCIB 9816-4 to transform EB to (S)-PE with 61% yield and 77%ee[14]. Recently, Heider and coworkers tested EB dehydrogenase from the denitrifying bacterium Azoarcus sp. strain EbN1, which catalyzes the oxygen-independent, stereospecific hydroxylation of EB to (S)-PE with unknowneeat 60 μM substrate concentration[15]. Herein we reported two newly discovered biocatalystPseudomonasputidaZMUT03 andIsoptericolajiangsuensisZMUT11, which catalyzed the biotransformation of EB to (R)-PE and (S)-PE in 86%eeand 98%ee, respectively (Fig 1). Especially,IsoptericolajiangsuensisZMUT11 showedS-selcetive for hydroxylation of EB. It provides an oxidation access for the efficient synthesis (R) and (S)-PE with cleaner and greener access.
1 Experimental
Soil samples used for screening were collected from different areas of Guizhou Province (China). The enrichment medium was M9 medium. In a 250 ml Erlenmeyer flask, 50 ml of M9 medium was inoculated with 1.0 g of soil sample, and 0.1% (v/v) toluene was added as the sole carbon source. After shaking at 200 rpm at 30 ℃ for a week, 0.5 ml cell supernatant was taken from the Erlenmeyer flask and added in another 50 ml of M9 medium, then repeated this procedure again. Cell supernatant was diluted and then plated onto M9 agar plates, which were further incubated at 30 oC for 4-5 days until colonies appeared. New single colony was obtained.
New single colony was identified with followed procedure: Universal primers of bacteria (27F: 5’-AGAGTTTGATCCTGGCTCA-3’ and 1492R: 5’-AGAGTTTGATCCTGGCTCA-3’) were used to amplify the 16S rRNA gene sequence. DNA analysis was completed using BLAST network services provided by the National Centre for Biotechnology Information (NCBI, United States). The sequence of ZMU-T03 and ZMU-T11 was 99% identical with the 16S rRNA sequence ofPseudomonasputidaJX908720 andIsoptericolajiangsuensis. Therefore, the strain ZMU-T03 and ZMU-T11 was identified and subsequently marked asPseudomonasputidaZMUT03 andIsoptericolajiangsuensisZMUT11.
Screened bacterial were selected for enantioselective hydroxylation of EB. A loop inoculated strain was transferred from M9 agar plate into 10 ml LB liquid medium. The culture was allowed to grow for 24 h at 30 ℃ and 300 rpm. 1 ml LB seed culture was then transferred into 50 ml M9 medium supplemented with trace element in 250 ml conical flask containing 1% (v/v) toluene in a plastic tube. The cells were grown at 30°C and 250 rpm for 48h and harvested by centrifugation at 8000 rpm for 10 min. The cells of microorganism were suspended in 5 ml 50 mM KH2PO4-K2HPO4buffer (pH 7.0) to a density of 5 g dcw/L. 2 mM EB was added and the mixtures were shaken at 30 °C and 300 rpm for 24h. 1 ml samples were taken, cells were removed by centrifugation, and the product was extracted with 1 ml ethyl acetate and analyzed by chiral HPLC.
Compound (R)-PE and (S)-PE : DaicelTMOD-H chiral column (250×4.6 mm, 5 μm) at 25 °C with a flow rate of 1 ml/min and UV detection at 220 nm. Retention time: 6.115 min for (R)-PE and 6.775 min for (S)-PE (OD-H column, 10% isopropanol:90%n-hexane); GC-MSm/zvalue: 122 (Molecular ion peak), 107, 79, 77. The configuration was assigned with authentic (R)-1-PE andrac-1-PE and also established by comparison with authentic standard and the references[11].
2 Results and discussion
As shown in Figure 1, 17 strains were discovered for directly oxidation of EB on 2 mM substrate concentration. Its whole cell biocatalyst was able to catalyze the asymmetric hydroxylation of ethylbenznene to give the corresponding (R)-PE with 67%-86%eeat a cell density of 5 g dcw/L and pH 7.0 for 24 h. Surprisingly,IsoptericolajiangsuensisZMUT11 showed (S)-preference for enantioselective hydroxylation of EB with 98%eeunder 2 mM substrate concentration.
This probably demonstrates thatIsoptericolajiangsuensisZMUT11 had contained aS-selective monooxygenase for hydroxylation of EB. Therefore,IsoptericolajiangsuensisZMUT11 was investigated for further enantioselective oxidation of racmic 1-PE. No ketone was detected even extending reaction time to 48 hours. It indicates that this biotransformation was direct hydroxylation of benzylic C-H bond, and no alcohol dehydrogenase fromIsoptericolajiangsuensisZMUT11 was existed for further oxidation of in 1-PE.IsoptericolajiangsuensisZMU-T11 was found to be a promising hydroxylation biocatalyst with great synthetic potential due to its excellent chemical yield and enantiomeric excess (Fig 2).
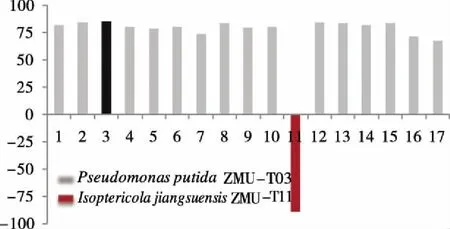
Fig 2 Screening of R and S-selective microbial strains for enantioselective hydroxylation
Following the interesting results above, the reaction parameters were optimized, such as substrate concentration, cell density, reaction temperature, and pH value (Table 1). As shown in Table 1, biooxidation of 2-8 mM EB at a cell density of 5 g dcw/L and pH 7.0 for 24 h (entries 1-4), The best results were obtained with 2 mM of EB, leading to the formation of (S)-PE in 98%ee. When the substrate concentration was increased from 2 mM to 4-8 mM, the conversion and enantioselective of (S)-PE was decreased. In addition, different cell density had investigated for the effect of this biotransformation, no obvious positive effect on yield (entries 5-7). Moreover, pH of 7.0 was shown to be better than pH 8.0 (entry1, 9).Moreover, reaction at 30 °C resulted in better conversion and enantioselectivity at 35 °C (entry 1, 8). Therefore, the optimized reaction conditions were 2 mM substrate concentration, 5 g dcw/L cell density, pH 7.0, and 30 °C reaction temperature. (R)-PE and (S)-PE was obtained with 90%-91% conversion in 86%-98%ee, respectively.
Table1OptimizationofreactionconditionswithPseudomonasputidaZMUT03andIsoptericolajiangsuensisZMUT11
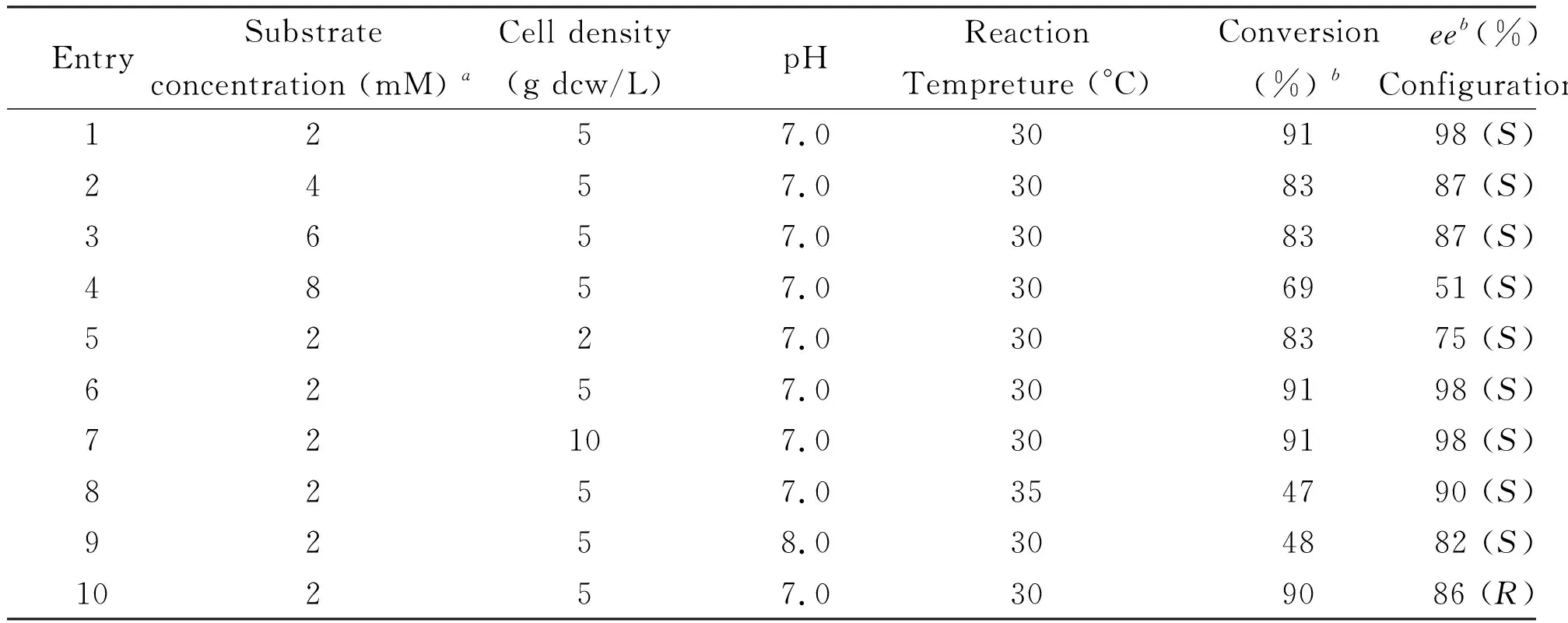
EntrySubstrate concentration (mM) aCell density(g dcw/L)pHReaction Tempreture (°C)Conversion(%) beeb(%) Configurations1257.0309198 (S)2457.0308387 (S)3657.0308387 (S)4857.0306951 (S)5227.0308375 (S)6257.0309198 (S)72107.0309198 (S)8257.0354790 (S)9258.0304882 (S)10257.0309086 (R)
aUnless otherwise noted, the reaction was performed with 2~8 mM substrate concentration in 5 ml cell suspension (2-10 g dcw/L) ofPseudomonasputidaZMUT03 (entry 10) andIsoptericolajiangsuensisZMUT11 (entries 1~9) in 50 mM KH2PO4~K2HPO4buffer (pH 7.0-8.0) at 30-35 °C and 300 rpm for 1h.
bDetermined by HPLC analysis with chiral OD-H column.
3 Conclusions
In summary, several toluene-degrading strains were employed as high efficient biocatalyst for the enantioselective oxidation of EB. Especially,PseudomonasputidaZMUT03 andIsoptericolajiangsuensisZMUT11 were used as an enantioselective complimentary biocatalyst with 90%-91% conversion and 86%-98%ee, respectively. Optimal reaction conditions with the resting cells as biocatalysts have been established. We are currently undertaking further enzymology investigations to fully understand the origin of catalytic behavior and whyIsoptericolajiangsuensisZMUT11 showsS-selectivity.
[References]
[1] Gotor V. Biocatalysis applied to the preparation of pharmaceuticals[J]. Org Proc Res Dev, 2002, 6 (4): 420-426.
[2] Jurĉek O, Wimmerová M, Wimmer Z. Selected chiral alcohols: Enzymic resolution and reduction of convenient substrates[J]. Coord Chem Rev, 2008, 252(5): 767-781.
[3] Groves J, Viski P.Asymmetric hydroxylation by a chiral iron porphyrin[J]. J Am Chem Soc,1989, 111(22): 8537-8538.
[4] Li Z, Chang D. Recent advances in regio- and stereoselective biohydroxylation of non-activated carbon atoms[J]. Curr Org Chem, 2004, 8(17): 1647-1658.
[5] Holland H, Bergen E, Henchaiash P, et al.Side chain hydroxylation of aromatic compounds by fungi. 1. Products and stereochemistry[J]. Can J Chem,1987, 65(3): 502-507.
[6] Uzura A, Katsuragi T, Tani Y.Conversion of various aromatic compounds by resting cells ofFusariummoniliformestrain MS31[J]. J Biosci Bioeng, 2001, 92(4): 381-384.
[7] Adam W,Lukacs Z,Harmsen D,et al.Biocatalytic asymmetric hydroxylation of hydrocarbons with the topsoil-microorganismBacillusmegaterium[J]. J Org Chem, 2000, 65(3): 878-882.
[8] Kluge M, Ullrich R, Scheibner K, et al. Stereoselective benzylic hydroxylation of alkylbenzenes and epoxidation of styrene derivatives catalyzed by the peroxygenase ofAgrocybeaegerita[J]. Green Chem, 2012, 14(2): 440-446.
[9] Yadav S, Yadav R, Yadava S, et al. Stereoselective hydroxylation of ethylbenzene to (R)-1-phenylethanol using mycelia of Aspergillus niger as catalyst[J]. Catal Commun, 2011,12(9): 781-784.
[10] Lie F, Chen Y Z, Wang Z, et al. Enantioselective benzylic hydroxylation of indan and tetralin with Pseudomonas monteilii TA-5[J]. Tetrahedron Asymmetry, 2009, 20(10): 1206-1211.
[11] Chen Y, Lie F, Li Z. Enantioselective benzylic hydroxylation withPseudomonasmonteiliiTA-5: A simple method for the syntheses of (R)-benzylic alcohols containing reactive functional groups [J]. Advanced Synthesis Catalysis, 2009, 351(13): 2107-2112.
[12] Chen Y Z, Tang W, Mou J, et al. High-throughput method for determining the enantioselectivity of enzyme-catalyzed hydroxylations based on mass spectrometry[J]. Angew Chem Int Ed, 2010, 122(13) : 5406-5411.
[13] Chen Y Z, Yang M, Zhuo J R, et al. Asymmetric synthesis of chiral 4-chlorobenzhydrol by C-H bonds oxidation with microbial enzymes [J]. J Zunyi Med Univ, 2014, 37 (1): 67-71.
[14] Lee K, Gibson D T. Toluene and ethylbenzene oxidation by purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4[J]. Appl Environ Microbiol, 1996, 62(9): 3101-3106.
[15] Szaleniec M, Hagel C, Menke M, et al. Kinetics and mechanism of oxygen-independent hydrocarbon hydroxylation by ethylbenzene dehydrogenase[J]. Biochemistry, 2007, 64(25):7637-7646.

