基质金属蛋白酶的基因多态性与腹主动脉瘤关联的研究进展
左尚维,郭 伟
解放军总医院 血管外科,北京 100853
基质金属蛋白酶的基因多态性与腹主动脉瘤关联的研究进展
左尚维,郭 伟
解放军总医院 血管外科,北京 100853
腹主动脉瘤是一种受遗传与环境因素共同影响的复杂疾病,基质金属蛋白酶家族(matrix metalloproteinases, MMPs)是腹主动脉瘤壁细胞外基质破坏的关键因素之一。本文回顾了MMPs在腹主动脉瘤发生过程中的作用机制和既往MMPs基因多态性位点与腹主动脉瘤的关系。分析表明,MMPs家族多种蛋白在腹主动脉瘤的病理过程中具有重要作用;MMP2、MMP3、MMP9等多个基因的多态性位点与腹主动脉瘤的发生有关。
腹主动脉瘤;单核苷酸多态性;基质金属蛋白酶
腹主动脉瘤(abdominal aortic aneurysm,AAA)是腹主动脉发生永久性、节段性的扩张,是受遗传与环境因素共同影响的复杂性疾病。美国大型筛检研究估计,18 ~ 85岁人群AAA患病率约为1.4%[1]。腹主动脉瘤患者最大的风险是瘤体破裂,破裂后的死亡率可达80% ~ 90%[2-3]。动脉瘤壁组织能够观察到的典型病理改变包括慢性透壁性炎症、细胞外基质破坏性重构、血管平滑肌细胞凋亡等,其中动脉瘤壁的细胞外基质的破坏是其关键机制之一,与此机制联系最紧密的是基质金属蛋白酶家族(matrix metalloproteinases,MMPs)[4]。MMPs是一系列对细胞外基质各种成分有降解作用的酶类,其可以降解腹主动脉壁组织中的胶原及弹力蛋白成分,造成动脉壁重塑,进而使动脉壁逐渐扩张形成动脉瘤。本文主要介绍MMPs在AAA发生过程中的作用机制,并综述既往MMPs基因多态性位点与AAA的关联研究。
1 MMPs在腹主动脉瘤病程中的作用
MMPs是一类包含锌离子的内源性蛋白水解酶,目前已发现20多种,根据其生物学功能将其分为6类:明胶酶(MMP2、MMP9)、胶原酶(MMP1、MMP8、MMP13、MMP18)、基质溶解素(MMP3,MMP10)、基质分解素(MMP7,MMP11,MMP26)、膜型MMP(MMP14 ~ MMP17、MMP24、MMP25)以及其他种类。目前MMPs家族中已知与AAA关系较密切的有MMP2、MMP3、MMP9等。
主动脉壁中的MMPs主要来源于巨噬细胞、单核细胞及中层平滑肌细胞,参与多种生理过程,包括伤口愈合、组织重构等[5-6]。生理情况下MMPs可在动脉血流量增加的情况下浓度增高,从而降解动脉壁基质,最终增加动脉直径和扩张性[7]。MMPs的生物学功能可被组织型基质金属蛋白酶抑制物(tissue inhibitors of metalloproteinases,TIMPs)抑制,MMPs与TIMPs通常处于平衡状态,确保细胞外基质降解与增殖的平衡。但在病理情况下,一旦MMPs与TIMPs间的平衡被打破,MMPs水平增高并持续作用于动脉壁,细胞外基质降解速度将大于修复速度,动脉壁发生重构,在血流的冲击下逐渐扩张直至形成动脉瘤[7]。另有实验证实,MMPs和TIMPs在动脉瘤壁组织中处于失衡状态[8]。早在1991年,Vine等[9-13]发现明胶酶、胶原酶及基质溶解素等在主动脉瘤瘤壁组织中水平升高。另外MMPs在不同直径的动脉壁组织的表达水平存在差异,提示MMPs的过量表达可能对腹主动脉直径的增加有促进作用[14-15]。除动脉瘤壁组织外,研究者同样发现外周血MMP水平也与AAA的发生及发展存在关联[16-18]。由此可见,各种不同来源细胞分泌的MMPs在腹主动脉瘤的发生、发展乃至最后的破裂中起重要作用。
2 MMP基因的单核苷酸多态性与AAA
单核苷酸多态性(single nucleotide polymorphism,SNP)主要是指在基因水平上由单个核苷酸的变异所引起的DNA序列多态性,是人类可遗传的变异中最常见的一种。由于在蛋白质和mRNA水平上MMPs与AAA具有明确的相关性,近年来随着候选基因策略的完善和全基因组关联研究(genome wide association studies,GWAS)技术的兴起,MMPs与腹主动脉瘤的关联已上升到了基因水平,多个MMPs的多态性位点与腹主动脉瘤的关联性研究已开展。MMPs家族的多个基因均有涉及,大部分研究针对MMP2、MMP3、MMP9等基因。
2.1 MMP2 MMP2基因位于染色体16q13区域,近年来MMP2基因多态性位点与AAA的关联受到较多关注(表1)。既往多针对MMP2 -1306C/T(rs243865)位点进行研究,其位于MMP2编码基因的启动子区域。基础研究发现,rs243865-T等位基因会使启动子活性大大降低,从而影响MMP2表达水平[19]。流行病学研究方面,Saracini等[20]发现此位点与腹主动脉瘤的发生存在相关性,rs243865-T等位基因能够减小AAA发生风险(adjusted OR=0.55,95% CI:0.34 ~ 0.85)。然而Smallwood等[21]在澳大利亚人群中的研究并未发现rs243865多态性与AAA的发生存在相关性。英国针对腹主动脉瘤扩张进行了遗传流行病学研究,未发现此位点与腹主动脉瘤的扩张率具有相关性[22]。有关MMP2基因的其他多态性位点与腹主动脉瘤的关联性研究,均未获得突破性发现。2005年Hinterseher等[23]研究了MMP2启动子及全外显子区域18个多态性位点与AAA的关联,但并未获得阳性结果。因此目前有关MMP2的基因多态性位点与腹主动脉瘤的关联尚无一致结论,需要继续进行大样本的深入研究。
2.2 MMP3 MMP3基因位于染色体11q22.3。其启动子区域5A/6A序列,是近年来被重点关注的位点(表2)。很早就有学者发现MMP3启动子区5A/6A序列与动脉硬化的进展速度相关,且其和MMP3基因的转录速度相关[25]。此外MMP3 5A/6A序列还和冠心病等多种血管疾病的发生、转归具有相关性[26]。自1999年起,多位学者开始进行该位点和AAA的关联性研究。Deguara等[27]在英国人群中发现此位点与AAA有显著的相关性(OR=1.32,95% CI:1.09 ~ 1.61),且5A序列携带者的动脉壁MMP3含量较对照高。Saracini等[20]和Saratzis等[28]分别在意大利和希腊人群中也获得了类似的结果。然而Yoon等[29]在芬兰人群中虽然发现AAA患者中5A序列携带率高于对照组,但结果无统计学意义(P=0.06)。既往Meta分析发现MMP3 5A/6A是AAA的易感基因位点[30]。
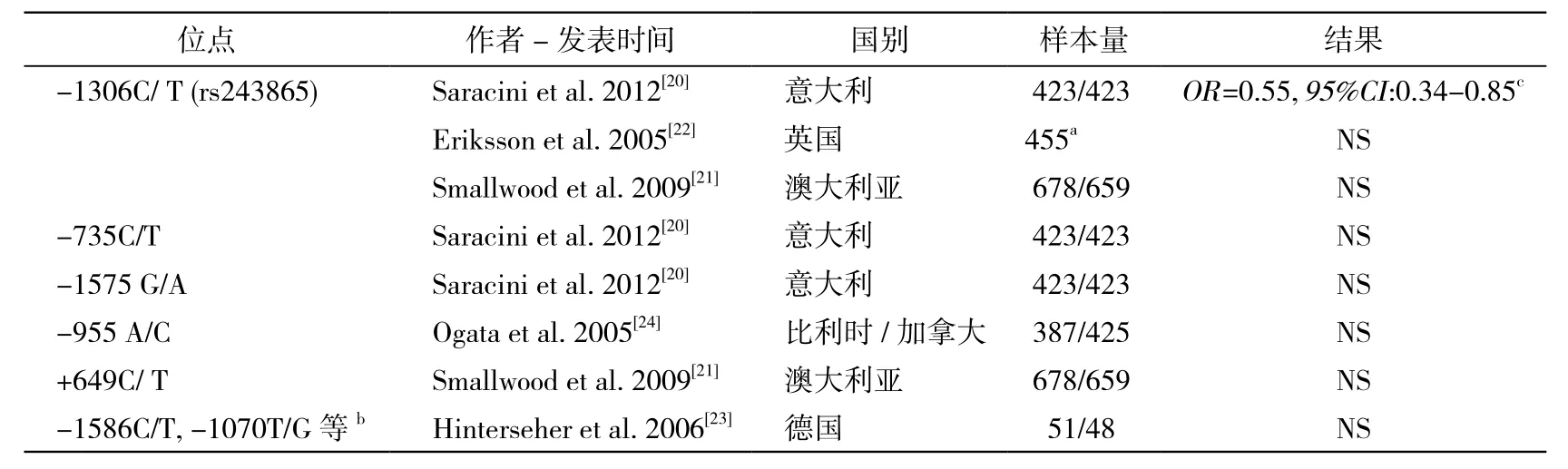
表1 MMP2基因多态性位点与AAA的关联性研究
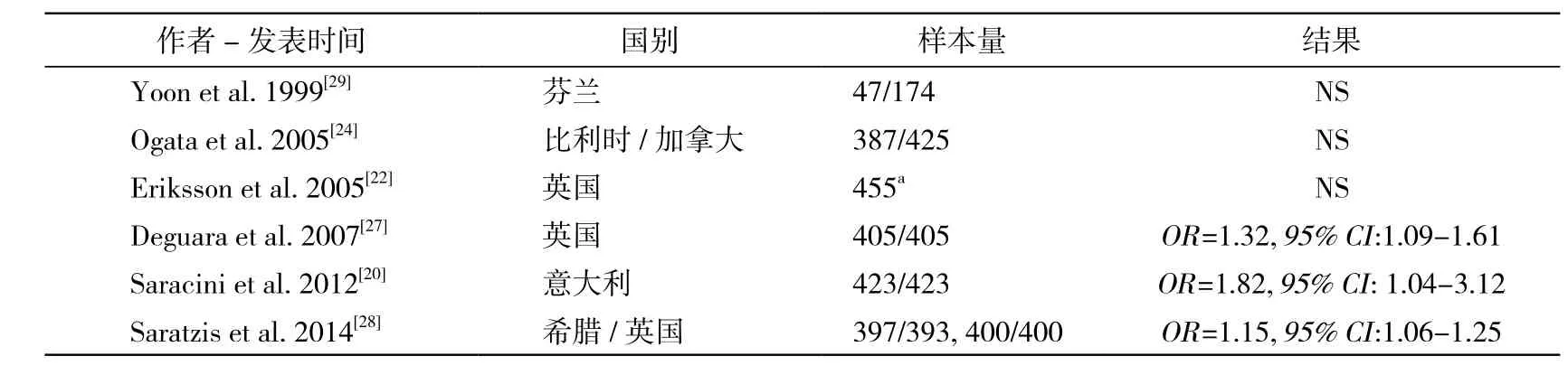
表2 MMP3基因多态性5A/6A位点与AAA的关联性研究

表3 MMP9基因多态性位点与AAA的关联性研究
2.3 MMP9 MMP9基因位于人类染色体20q11.2-13.1区域,多个研究针对该基因的(CA)n和-1562 C/T (rs3918242)位点展开了较为深入的研究(表3)。(CA)n是位于其第一个外显子前131到90个碱基对位置出现的重复序列,重复数介于14 ~ 27,但既往研究并未发现(CA)n与AAA有相关性[29,31]。MMP9 -1562 C/T (rs3918242)位点位于MMP9启动子区域,基础性研究发现该多态性位点可能与MMP9基因mRNA转录水平有关[32]。Jones等[33]在新西兰人群中发现-1562 C/T与AAA的发生具有相关性(OR=2.41,95% CI:1.44 ~4.02),但其他针对该位点的研究均无阳性结果[20,22,24,31]。目前Meta分析结果尚不能证实MMP9-1562T等位基因为腹主动脉瘤的易感基因,有待于在大样本中进一步探讨[29]。
3 国内研究现状
我国大陆地区腹主动脉瘤与MMP相关性研究已有一定基础,研究主要集中在动物实验、体外实验方面,以人群为基础的遗传流行病学研究较少。我国绝大多数研究均着眼于蛋白质水平,但亦有部分研究已逐渐深入。陈峰等[34]研究了实验性动脉瘤瘤壁MMP2和MMP9的蛋白质水平和mRNA水平,并发现其与AAA相关。王曰伟等[35]研究了人腹主动脉瘤组织中的MMP2、MMP9的表达数量和位置,且发现MMP-2与AAA的形成及早期扩张密切相关,MMP-9与AAA的持续扩张及破裂密切相关。然而仍没有基于中国大陆人群的MMPs基因多态性研究,此方面仍属空白。
4 结语
MMPs家族多种蛋白在腹主动脉瘤的病理过程中具有重要作用;MMP2、MMP3、MMP9等多个基因的多态性位点与腹主动脉瘤的发生有关。然而现有大多数遗传流行病学研究人群为高加索人种,对中国人群尚没有相应研究,此方面空白亟待填补。
1 Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals[J]. J Vasc Surg, 2010, 52(3): 539-548.
2 Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm[J]. Lancet, 2005, 365(9470):1577-1589.
3 Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening[J]. Br J Surg, 2012, 99(12):1649-1655.
4 Steinmetz EF, Buckley C, Thompson RW. Prospects for the medical management of abdominal aortic aneurysms[J]. Vasc Endovascular Surg, 2003, 37(3):151-163.
5 Irizarry E, Newman KM, Gandhi RH, et al. Demonstration of interstitial collagenase in abdominal aortic aneurysm disease[J]. J Surg Res, 1993, 54(6):571-574.
6 Keen RR, Nolan KD, Cipollone M, et al. Interleukin-1 beta induces differential gene expression in aortic smooth muscle cells[J]. J Vasc Surg, 1994, 20(5): 774-784.
7 Cronenwett J, Johansen K. Rutherford’s vascular surgery[M]. 7th ed. Philadephia: Saunders/Elsevier, 2010:124-127.
8 Kadoglou NP, Liapis CD. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms[J]. Curr Med Res Opin, 2004, 20(4): 419-432.
9 Vine N, Powell JT. Metalloproteinases in degenerative aortic disease[J]. Clin Sci (Lond), 1991, 81(2): 233-239.
10 Thompson RW, Holmes DR, Mertens RA, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages[J]. J Clin Invest, 1995, 96(1):318-326.
11 Patel MI, Melrose J, Ghosh P, et al. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms[J]. J Vasc Surg,1996, 24(1): 82-92.
12 Mao D, Lee JK, Vanvickle SJ, et al. Expression of collagenase-3(MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture[J]. Biochem Biophys Res Commun,1999, 261(3): 904-910.
13 Carrell TW, Burnand KG, Wells GM, et al. Stromelysin-1 (matrix metalloproteinase-3) and tissue inhibitor of metalloproteinase-3 are overexpressed in the wall of abdominal aortic aneurysms[J]. Circulation, 2002, 105(4): 477-482.
14 Freestone T, Turner RJ, Coady A, et al. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm[J]. Arterioscler Thromb Vasc Biol, 1995, 15(8):1145-1151.
15 Mcmillan WD, Tamarina NA, Cipollone M, et al. Size matters - The relationship between MMP-9 expression and aortic diameter[J]. Circulation, 1997, 96(7): 2228-2232.
16 Lindholt JS, Vammen S, Fasting H, et al. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study[J]. Eur J Vasc Endovasc Surg, 2000, 20(3):281-285.
17 Petersen E, Gineitis A, Wågberg F, et al. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture[J]. Eur J Vasc Endovasc Surg, 2000, 20(5):457-461.
18 Petersen E, Wågberg F, Angquist KA. Proteolysis of the abdominal aortic aneurysm wall and the association with rupture[J]. Eur J Vasc Endovasc Surg, 2002, 23(2):153-157.
19 Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation[J]. J Biol Chem,2001, 276(10):7549-7558.
20 Saracini C, Bolli P, Sticchi E, et al. Polymorphisms of genes involved in extracellular matrix remodeling and abdominal aortic aneurysm[J]. J Vasc Surg, 2012, 55(1): 171-179.
21 Smallwood L, Warrington N, Allcock R, et al. Matrix metalloproteinase-2 gene variants and abdominal aortic aneurysm[J]. Eur J Vasc Endovasc Surg, 2009, 38(2):169-171.
22 Eriksson P, Jormsjö-Pettersson S, Brady AR, et al. Genotypephenotype relationships in an investigation of the role of proteases in abdominal aortic aneurysm expansion[J]. Br J Surg, 2005, 92(11):1372-1376.
23 Hinterseher I, Bergert H, Kuhlisch E, et al. Matrix metalloproteinase 2 polymorphisms in a caucasian population with abdominal aortic aneurysm[J]. J Surg Res, 2006, 133(2):121-128.
24 Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms[J]. J Vasc Surg, 2005, 41(6):1036-1042.
25 Ye S, Eriksson P, Hamsten A, et al. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression[J]. J Biol Chem, 1996, 271(22):13055-13060.
26 Li M, Shi JP, Fu LY, et al. Genetic polymorphism of MMP family and coronary disease susceptibility: A meta-analysis[J]. Gene,2012, 495(1): 36-41.
27 Deguara J, Burnand KG, Berg J, et al. An increased frequency of the 5A allele in the promoter region of the MMP3 gene is associated with abdominal aortic aneurysms[J]. Hum Mol Genet, 2007, 16(24):3002-3007.
28 Saratzis A, Bown MJ, Wild B, et al. Association between seven single nucleotide polymorphisms involved in inflammation and proteolysis and abdominal aortic aneurysm[J/OL]. http://www.sciencedirect.com/science/article/pii/S0741521414001700.
29 Yoon S, Tromp G, Vongpunsawad S, et al. Genetic analysis of MMP3, MMP9, and PAI-1 in finnish patients with abdominal aortic or intracranial aneurysms[J]. Biochem Biophys Res Commun,1999, 265(2): 563-568.
30 隗瑛琦,左尚维,秦雪英,等.腹主动脉瘤易感基因多态性位点的系统综述与Meta分析[J].中华疾病控制杂志,2013,17(9):737-742.
31 Armani C, Curcio M, Barsotti MC, et al. Polymorphic analysis of the matrix metalloproteinase-9 gene and susceptibility to sporadic abdominal aortic aneurysm[J]. Biomed Pharmacother, 2007, 61(5):268-271.
32 Zhang BP, Ye S, Herrmann SM, et al. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis[J]. Circulation, 1999, 99(14): 1788-1794.
33 Jones GT, Phillips VL, Harris EL, et al. Functional matrix metalloproteinase-9 polymorphism (C-1562T) associated with abdominal aortic aneurysm[J]. J Vasc Surg, 2003, 38(6):1363-1367.
34 陈峰, 熊江, 郭伟, 等. 氢气饱和生理盐水抑制大鼠腹主动脉瘤形成的研究[J]. 中华外科杂志, 2013, 51(5): 437-441.
35 王曰伟,李君,赵宗刚,等.人腹主动脉瘤组织MMP-2和MMP-9表达及意义[J].青岛大学医学院学报,2013(4):303-306.
Relationship between matrix metalloproteinases gene polymorphisms and abdominal aortic aneurysm
ZUO Shang-wei, GUO Wei
Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, Beijing 100853, China
GUO Wei. Email: pla301dml@vip.sina.com
Abdominal aortic aneurysm is a complex disease affected by genetic and environmental factors. Matrix metalloproteinases (MMPs) is one of the key factors of the aorta wall extracellular matrix degeneration. This article reviews the mechanism of MMPs in abdominal aortic aneurysm progression and the relationship between MMPs gene polymorphisms and abdominal aortic aneurysm. Analysis shows that the MMPs family proteins play an important role in the pathology process of abdominal aortic aneurysm. MMP2, 3, 9 gene polymorphisms are associated with the occurrence of abdominal aortic aneurysm.
abdominal aortic aneurysm; single nucleotide polymorphisms; matrix metalloproteinases
R 543
A
2095-5227(2014)12-1278-04
10.3969/j.issn.2095-5227.2014.12.029
时间:2014-09-12 11:06
http://www.cnki.net/kcms/detail/11.3275.R.20140929.1712.003.html
2014-08-06
全军医药卫生科研项目(09BJZ04)
Supported by the Project of Medicine and Health Science Research of Chinese PLA(09BJZ04)
左尚维,男,在读博士。研究方向:血管外科。Email: lksybzsw@hotmail.com
郭伟,男,硕士,主任医师,博士生导师。Email: pla301dml@vip.sina.com

