水螅细胞外基质及其在发生和再生中的作用
张晓明
(美国堪萨斯大学医学院 解剖及细胞生物学系,3901 Rainbow Blvd., Kansas City, Kansas 66160, USA)
In a multicellular organism, the extracellular matrix (ECM) is generally composed of cells, fibers, and ground substances. ECM functions as a structural supporting framework to the cells and tissues surrounding it. It bears the tension from the cells and tissues, stores materials and molecules, provides pathways for cells and molecules to migrate, and stands as a “ground” for cells during growth and regeneration.
Embryogenesis starts with one single cell - a fertilized egg. Along with cell division, ECM is produced and laid around cells. The more complicated the embryo develops, the more ECM appears. What is the function of the ECM during this developmental process?
Tissue injuries cause cell loss and create gaps in tissues. Trauma may cause loss of body parts such as limbs. When repairing wounds or healing trauma, cells proliferate and ECM is also produced. Can missing body parts be regenerated? What is the function of ECM during wound healing and regeneration process?
Extensive research work has been carried out to answer these questions. One model system provides a great advantage in this effort. This model system is a fresh water animal,hydra.
Hydra, as a member of Cnidarian, arose early during metazoan evolution in approximately 580 million years ago before the divergence of protosomes and deuterostomes.Hydrapolyps’ body plan is organized as a hollowed tube with a gastric (body) region in the middle, a mouth (also known as the hypostome) and a tentacle ring (which is usually composed of 6 to 8 tentacles) at one end known as the head pole, and a peduncle and basal disk at the other known as the foot pole (Fig 1). The tubular body plan ofHydraallows the animal has a radial symmetry at any horizontal body plane. Therefore, the study of development and regeneration onHydrais mainly focused on the longitudinal axis.Hydrapolyps use their basal discs to adhere to a surface while their tentacles can catch preys and deliver them to the mouth opening. Nutrients are digested inside the gastric tube and wastes are expelled from either the mouth or the basal disc openings.
The wall that surrounds the entire polyp from the tip of the tentacles to the bottom of the basal disc is structured as an epithelial bilayer with an intervening ECM (Fig 1). The ectodermal epithelial cells contain muscle fibers arranged longitudinally whereas the endodermal epithelial cells have muscle fibers arranged circularly. At normal stage, these two groups of muscle fibers contract alternatively making hydra polyps exercise periodic elongation and retraction. In the gastric region, in between the epithelial cells, are the interstitial cells (I-cells) that include such phenotypes as nerve cells, nematocytes, secretory cells, gametes, and differentiating I-cell precursors[1].
Hydrapolyps reproduce most of the time through budding at the “Budding zone”, which is located at the junction between body and peduncle (Fig 1) although sexual reproduction via gametes is possible under special environmental conditions. The development of a bud starts with a tissue evagination followed by tentacle formation, body elongation, and base region constriction[2]. A detached bud is proportionally the same as an adult polyp. Therefore, the budding process of aHydrapolyp has drawn great interests from developmental biologists because it is similar to a developmental process involving cell-cell interaction, molecular signaling, and pattern formation.
When the head or the foot is cut off, aHydrapolyp can regenerate the missing structure in 30-36 hours[3]. A complete normalHydrapolyp can be regenerated from a gastric region tissue as small as 5% of the entire polyp[4]. In 1972, Gierer et al[5].achieved regeneratingHydrapolyps from aggregated cell pellets that were formed by non-enzymatically dissociatedHydracells. This robust regenerating capacity grantedHydraa powerful model to study regeneration. However, studies have indicated that the high regenerative capacity ofHydrais solely due to the epithelial cells because polyps lacking any I-cells are fully capable of complete body regeneration[6]although they are unable to feed themselves because of the lack of nerve cells.

A Hydra polyp’s body plan is shown in the left diagram. The diagram to the right utilizes a transmission electron micrograph of Hydra ECM interposed between a drawing of two cell layers (ectoderm and endoderm). Hydra laminin and Type IV Collagen are localized to the two subepithelial zones while Hydra fibrillar collagens (e.g. Hcol-I) are localized to the central fibrous interstitial matrix. Fig 1 Hydra body plan formed of an epithelial bilayer with an intervening extracellular matrix (ECM)
Hydrahas been used as a model to study the ECM functions during development and regeneration. Over the last three decades, biochemical and molecular studies have established that the ECM ofHydrais composed of a broad spectrum of matrix components reflective of that seen in more complicated vertebrate systems. Combining the early structural analyses with recent molecular and biochemical studies, the current understanding ofHydraECM is a highly flexible matrix with elastic properties.
This review includes discussions ofHydraECM in regard to 1) its general structure, 2) its molecular composition, 3) the biogenesis ofHydraECM during regeneration, 4)HydraECM in development, 5) cell-ECM interaction inHydra, and 6)HydraECM remodeling during development.
1 The general structure of Hydra ECM
1.1 Early studies on the morphological structure and biochemical composition ofHydraECM In 1950s and 1960s, ultrastructural studies ofHydraECM using electron microscopy technique identified a broad spectrum of structural components.HydraECM, also called mesoglea, was described as an amorphous matrix of low density containing fine fibrils ranging from 5-50 nm in diameter[7-9]. From both sides, ectodermal and endodermal cells send cellular processes from their basal surfaces into the ECM and form cell-cell contacts across the mesoglea[7-8,10].
In 1968, Davis and Haynes[11]provided a detailed analysis ofHydraECM with polyps being either relaxed or contracted. In agreement with previous studies, they reported thatHydraECM was approximately 0.5 to 2.0 μm in thickness and was thickest in the body region and thinnest in the tentacles. Using TEM analysis, they indicated that theHydraECM had three structural components, namely, an amorphous ground substance, particulate materials, and fibrils. The fibrils consisted of three types. The smallest fibrils were 5-9 nm in diameter and their density in the ECM varied depending on whether the polyps were in a relaxed or contracted state. When a polyp is contracted these fibrils were randomly arranged and more densely packed while in a relaxed state the fibrils were in a more orderly arrangement and less densely packed. A second type of fibril that was less abundant was a thicker banded fibril that ranged from 36-45 nm in diameter and had a periodicity of approximately 30 nm. The third class of fibrils consisted of short thin fibrils that formed bundles oriented perpendicular to the longitudinal axis of the polyp. These fibrils extend from the basal surface of the ectodermal cells into the ECM suggesting some type of linker function.
Studies by Shostak et al[12]. showed that isolatedHydraECM was very elastic and could be stretched to twice its original length. It would retract to its original length when released from tension. This elastic feature agrees with the ultrastructural studies of Davis and Haynes[11]that in a contracted state, the fibrils inhydraECM become irregular and fold upon themselves.
In 1970s, biochemical studies targeting at the macromolecular composition ofHydraECM by Barzansky and Lenhoff[13]and Barzansky et al[14]. provided additional insight to its structure. Utilizing a combination of techniques including gel chromatography, amino acid analysis and thin layer chromatography for sugar moiety analysis in conjunction with radiotracer precursor-labeling, they reported thatHydraECM had biochemical characteristics similar to vertebrate basal lamina. They also concluded that the amino acid profiles suggested the presence of collagens inHydraECM. Experiments using lathyritic agents known to reduce cross-linking of collagens indicated a role for these macromolecules inHydramorphogenesis[13].
By the end of 1970s and early 1980s, the combination of morphological and biochemical studies provided such a view thatHydraECM exists as a highly flexible and elastic structure as compared to normal ECM of vertebrates. Also in contrast to vertebrate ECM, the collagens ofHydraECM are more easily extracted due to a reduced amount of cross-linking as will be discussed later in theHydracollagen sections[15]. The unique properties ofHydraECM provide the necessary rigidity for maintenance of body shape while at the same time permitting sufficient plasticity to allow extensive but reversible shape changes along the longitudinal and radial axis.
Since early 1990s, with a purpose to further clarify the macromolecular structure ofHydraECM and to elucidate the role of cell-ECM interactions during cell differentiation and morphogenesis, Sarras and his colleagues published a series of papers[16-18]that utilized a combination of biochemical, molecular, and cell biological approaches. Below is an overview of what is currently known about the overall structure ofHydraECM.
1.2HydraECM is a tri-laminar structure composed of two subepithelial basal lamina matrices and a central interstitial matrix In order to investigate the structural details ofHydraECM, it is essential to have a more in-depth understanding of the molecular composition of it. Once ECM molecules are in hand, antibodies and probes can be developed as tools for structural studies.Therefore, a series of studies toward this goal was started in late 1980s. The work involved: 1) isolation ofHydraECM and use of biochemical and immunological approaches to analyze the purified matrix preparation, 2) use of the purifiedHydraECM preparation to generate a battery ofHydra-specific polyclonal and monoclonal antibodies, and 3) use of theseHydraspecific antibodies as reagents to screen expression cDNA libraries and as probes to characterize the distribution of matrix components inHydraECM using morphological techniques. In addition,Hydra-specific antibodies and isolated matrix component domains were also used as blocking reagents to study the role of cell-ECM interactions inHydrausing a number of regeneration bioassays. These functional studies were later complimented with antisense RNA studies to selectively knockdownHydraECM components during regenerative processes.
Initial biochemical and immunological studies using antibodies generated to vertebrate ECM components indicated thatHydraECM had a spectrum of matrix components similar to those observed in more complicated invertebrates and in vertebrates. Specifically, evidence for the presence of collagen type IV, laminin, heparan sulfate proteoglycan and fibronectin-like molecules was present[16]. Pulse-chase autoradiographic studies in conjunction with translational and post-translational processing inhibitor studies supported the presence of collagen and proteoglycan components[17]. Use ofHydra-specific monoclonal antibodies[18]in combination with special ultrastructural staining techniques[19]revealed thatHydraECM is composed of distinct structural regions. A central zone that appeared fibrous and similar to interstitial matrix occupies the majority of HydraECM. Flanking the central interstitial matrix, adjacent to the basal plasma membrane of each epithelial cell layer, there are two much thinner basal lamina-like regions named the subepithelial zones (Fig 1). Subsequent studies showed that laminin chains were confined to the subepithelial zones (i.e. basal lamina)[19]and type I-like collagen was confined to the central fibrous zone (i.e. interstitial matrix)[15]. A recent study using vertebrate monoclonal antibody to the NC1 domain of collagen type IV has localized this macromolecule in the basal lamina layers as well, co-localizing with laminin[20].
Using confocal microscopy, scanning electron microscopy, and immunohistochemistry techniques, a study usingHydra-specific antibodies to laminin,collagen type IV, and collagen type I has demonstrated thatHydraECM is a porous structure with multiple trans-ECM pores ranging from 0.5 to 1 μm in diameter and about six pores per 100 mm2in density. Cellular processes from the ectoderm and endoderm utilize these pores to form cell-cell connections within the ECM. These cellular processes are still in contact with the basal lamina which forms a cylinder around the cellular processes as they pass through the ECM(Fig 2)[20]. The most up-to-date understanding of the overall structure ofHydraECM is shown in Figure 1 and Figure 2. A list of the major components ofHydraECM with their general properties is shown in Table 1.

Cellular processes extend through the ECM from both the ectoderm and endoderm. These processes are sheathed by the basal lamina and thereby separated from direct contact with interstitial matrix. Fig 2 Model of Hydra ECM structure
Table1MajorcomponentsofHydraECM*

ECMComponentBasalLaminaECMcomponentsGeneralPropertiesLamininContainsα,β,andγsubunitsinatrimericcruciate(HLM)structureasviewedbyrotaryEM.Cloningstudiesindicatethealphasubunitisavertebrateα5-likeorDrosophila-likechainwhilethebetasubunitisavertebrateβ1-likechaincontainingatleastonedefinedcellbindingdomain(FTGTQ).CollagentypeIV(Hcol-IV)AhomotrimericglycoproteinformedfromthreecollagenIVα1-likechains.EachsubunitcontainsacollagendomainatitsN-terminusandasmallernon-collagenousdomain(NC1)atitsC-terminus.ThecollagendomaincontainsRGDcellbindingmotifs.TEMro⁃taryshadowstudiesindicatethatpolymerizationofHcol-IVmoleculeswithintheECMin⁃volvesinteractionoftheNC1domains.Atypical7SdomainattheN-terminusappearsnottobepresentinthematureECM.InterstitialECMcomponents(Fibrillarcollagens)FibrillarCollagenstypeI(Hcol1)AhomotrimericglycoproteinformedfromthreecollagentypeIα1-likechains.EachchaincontainsanN-terminalpropeptideandC-terminalpropeptide.DuringprocessingtheC-ter⁃minalpro-peptideisremoved,buttheN-terminalpropeptideisretainedinthematuremole⁃cule.Thisresultsintheformationofflexiblefibrilsbutnotthickenedbandedfibrilsastypi⁃callyseeninvertebratetypeIcollagens.Hcol-1'sflexibilityisenhancedbyareductioninitsprolinecontentandalossofcriticallysinesinvolvedinlysyl-crossbridgingHydraCollagentypes2,3,5,&6(Hcol2;Hcol3;Hcol5&Hcol6)HydrafibrillarcollagensHcol2,3,5,and6werecharacterizedonlyfromcDNAsequenceanalysisandsubsequentcomputermodeling,andsounlikeHcol1wheretheproteinwasal⁃sopurifiedandbiochemicallyanalyzed,thereissomedegreeofspeculationabouttheinvi⁃vonatureoftheseproteins.
*:See the proposed structures of the fibrillar collagens in Fig 3.
2 Molecular Components of Hydra ECM
2.1HydraLamininHydralaminin chains have been cloned and functionally analyzed[18-19,21]. As stated above,Hydralaminin is localized to the two subepithelial zones (basal lamina) ofHydraECM. A partial sequence for aHydraβ1-like chain was initially reported[19]. Studies have since been completed for description of the entire ORF of the β1 chain and partial description of a α-5-like chain[21]. No γ chain has thus far been identified, although the typical trimeric form for isolatedHydralaminin has been observed by rotary shadow TEM analysis[21]. WhileHydralaminin is localized to the two subepithelial zones (basal lamina) ofHydraECM, it is synthesized exclusively by the endoderm, which means that the molecules have to diffuse through the mesoglea to reach the ectodermal layer[18-19]. The location at the basal region of both cell layers suggests that laminin is required for cell-ECM interaction. DuringHydraECM regeneration, laminin secretion from the endoderm precedes the secretion ofHydracollagen-I that arises from the ectoderm[15]and inhibition of laminin secretion through a RNA antisense technique will block collagen secretion[22]. Earlier studies have already established that antibodies toHydralaminin will blockHydramorphogenesis[18]and other ECM-related processes such as cell migration[23].
While the mechanism(s) of signal transduction inHydrais not fully known, some published data[24]and unpublished studies (Sarras laboratory) suggest the involvement of integrins, the primary class of ECM-receptors in higher animals[25-26]. TheHydraECM receptor data mainly pertains to a region in the short arm ofHydralaminin β1-like chain. In this regard, sequence analysis of the β1-like chain indicates the substitution of a FTGTQ sequence for the YIGSR receptor-binding sequence observed in vertebrates. Although the role of the YIGSR sequence in signal transduction-mediated processes has been questioned, published studies do indicate 1) its potential use as an inhibitor of human pre-B leukemic cell growth and metastasis using SCID mice models[27]and 2) its role in the guidance of axon growth cones[28]. Such studies and others support its involvement in cell signaling processes. More recent studies also validate the existence of the YIGSR ECM-receptor sequence[29].
The substituted FTGTQ sequence inHydralaminin β1-like chain has also been shown to interact with the cell surface under both in vitro and in vivo conditions[19].Affinity purification studies indicated that the FTGTQ sequence can interact with aHydraintegrin-like protein[24]. To fully understand the relationship between pattern formation and ECM structural assembly inHydra, further analysis of 1) laminin-mediated cell signaling processes and 2) the role of laminin in the biogenesis and assembly ofHydraECM is required. As an update, it is important to note that based on publication of theHydragenome in 2010, it is now known that true integrin molecules are present in the genome of this organism[30].Based on this sequence data, more extensive structural and functional studies regarding Cell/ECM interaction can now be conducted.
2.2HydraCollagens While indirect evidence had suggested the existence of collagens in the ECM ofHydra[13,31], more recent structural and functional analysis has provided a clear understanding of the types of collagens that exist in this invertebrate[15,32]. These collagens include a basement membrane-type (Hydracollagen type IV, Hcol-IV) and an interstitial-type (Hydrafibrillar collagen, Hcol-1). OtherHydrafibrillar collagens have been more recently identified[33]. As will be discussed, Hcol-IV and Hcol-1 collagens have been characterized at both the cDNA and protein level.
2.3 Collagen Type IV (Hcol-IV) As reported by Fowler et al[32].HydraECM also contains a collagen type IV (Hcol-IV). Analysis of the cDNA clone revealed a protein of 1723 amino acids, including an interrupted 1455 residue collagenous domain and a 228 residue carboxyl-terminal non-collagenous domain. Hcol-IV is similar to all known α(IV) chains, but again, most closely resembles vertebrate and invertebrate α1(IV) chains. LikeHydrafibrillar collagen, Hcol-IV also forms homotrimeric molecules. Electron microscopy reveals an irregular network of rod-like structures interrupted by globular domains. This network can be depolymerized by reducing agents to dimeric collagen molecules, joined via their C-terminal non-collagenous domains. Under extensive denaturing conditions, depolymerization can only be taken to the dimeric but not monomeric stage. This suggests that the individual polypeptide chains are quantitatively held together by non-reducible cross-links in addition to disulfide bonds. This behavior is quite different from the vertebrate collagen type IV that needs pepsin digestion for solubilization. For vertebrate collagen type IV, a model has been proposed in which four molecules aggregate via their N-terminal domains to form a spider-like structure. The interactions are stabilized via disulfide bonds and lysine derived cross-links, resulting in a highly protease resistant 7S domain. In addition, the C-terminal globular domain, NC1 domain, binds to itself, mainly via disulfide bridges, to form a linear dimer.
Both interactions at the N-terminal and C-terminal ends lead to the proposal of an open network structure that can further polymerize via lateral aggregation of the triple helical domains[34-36]. In contrast, inHydra, while C-terminal interactions and lateral aggregation occurs, a stable 7S domain is not formed. A similar collagen type IV has also been reported in the worm,Ascarissuum[37]. This form of collagen type IV favors a more flexible ECM than that seen in typical vertebrate matrices.
2.4 Fibrillar Collagen Type I (Hcol-1) Fibrillar collagens make up the majority of ECM components within the interstitial matrix of the animal kingdom.Likewise,Hydrafibrillar collagens are the major components ofHydraECM[15]. The cDNA for theHydrafibrillar collagen, Hcol-1, encodes a protein of 1412 amino acids. Hcol-1 was the first fibrillar collagen to be identified inHydra. The polypeptide isolated fromHydraECM has an apparent molecular weight of 155 kDa. The subunit chains of Hcol-1 form homotrimeric molecules that constitute the majority of the fibrils within the central fibrous zone (interstitial matrix). Sequence comparisons clearly define Hcol-1 as a fibrillar collagen. The highest similarity is to the α chains of vertebrate collagens type I and II. A similar degree of similarity is found between Hcol-1 and invertebrate sea urchin collagen[38]and a sponge fragment[39]. Corresponding to the similarity at the sequence level, Hcol-1 also exhibits the characteristic domain structure of fibrillar collagens, consisting of a central triple helical domain flanked by an N-terminal propeptide-like domain and a C-terminal propeptide (Fig 3). It is note worthy that the triple helical domain with 340 uninterrupted GLY-X-Y repeats has exactly the same length as the fibrillar collagens of vertebrates, suggesting similar fibril forming possibilities.
Despite marked similarities in the primary structure, there are distinct differences in the supramolecular organization of vertebrate fibrillar collagen networks as compared to that seen inHydraECM. Hcol-1 forms a network of fine fibrils rather than thicker banded fibrils as seen by electron microscopy of vertebrate interstitial matrices. In contrast to vertebrate collagens that require pepsin digestion for solubilization, large polymeric structures of Hcol-1 can be isolated from the ECM ofHydraunder native conditions.
Several factors are responsible for the special structure ofHydraHcol-1. These factors include: 1) a low content of proline in the triple helical domain that is only about 40% that of vertebrate collagens: 2) a reduced degree of inter-chain cross-linking due to the lack of classical consensus sequences for lysine/lysine-aldehyde derived covalent bonds; and 3) most importantly, altered post-translational processing that results in retention of the N-terminal propeptide-like domain in the mature molecule. Combined, these factors result in a more flexible fibrillar collagen that can bend on itself as suggested by the early ultrastructural studies of Davis and Haynes[11].
2.5 OtherHydraFibrillar Collagens: Hcol2, Hcol3, Hcol5, Hcol6 Next to be identified through molecular cloning techniques were four fibrillar collagens named, Hcol2, Hcol3, Hcol5 and Hcol6[33]. Hcol6 was only a partial sequence of a collagen gene with a unique structural organization consisting of multiple von Willebrand factor-A domains interspersed with interrupted collagenous triple helices. Hcol2 and Hcol5 have major collagenous domains of classical length (1020 amino acid residues), whereas the equivalent domain in Hcol3 is shorter (969 residues). The N-propeptide of Hcol2 contains a whey-acid-protein four-cysteine repeat (WAP) domain, and the equivalent domain of Hcol3 contains two WAP and two von Willebrand factor-A domains (Fig 3).
Phylogenetic analyses revealed that theHydrafibrillar collagen genes form a distinct clade that appeared related to the protostome/deuterostome A-clade of fibrillar collagens. Data base searches revealed that Hcol2, Hcol5, and Hcol6 are highly conserved, which also provided preliminary evidence for the expression of a B-clade fibrillar collagen. In situ hybridization indicated an ectoderm expression pattern along the entire longitudinal axis ofHydrafor the Hcol2 and Hcol3 fibrillar collagens as was previously reported for Hcol1[15].HydraHcol2 and Hcol3 also have high expression levels in the tentacles and forming buds. Fibrillar collagen Hcol5 has high expression levels in the tentacles, foot process, and forming buds of adult polyps while Hcol6 expression is high at the base of the tentacles and in forming buds. During head regeneration it is expressed in the ectoderm as observed with Hcol1. Hcol6 is expressed to a much lower degree than the other fibrillar collagens and its expression pattern is restricted to the base of the tentacles and to forming buds[15].

Fig 3 Summary chart of the proposed structure of each of the Hydra fibrillar collagens
2.6 Non-ECM collagens ofHydraTo be complete, it should be pointed out thatHydrahas a variety of what are called “mini collagens”. These collagens are localized within the capsule of nematocysts and are not associated withHydra’s ECM. They have a unique and interesting structure due to their role in maintaining high hydrostatic pressures within the cavity of the nematocyst capsule. Because these collagens are not associated withHydra’s ECM, they will not be further discussed in this chapter. Readers are referred to articles pertinent to their unique structure and function[40-42].
2.7 Evidence for other types of matrix components inHydraECM During functional studies using a pharmacological approach, evidence was obtained by Sarras et al.[16-18]and Zhang and Sarras[23]indicating that proteoglycans also exist in theHydraECM. These experiments involved analysis of the effect of molecules that block proteoglycan biosynthesis during head regeneration[17],Hydracell aggregate formation, and during cell migration as studied inHydragrafting experiments[23]. The validity of these pharmacological studies was confirmed by using pulse-chase autoradiographic techniques to demonstrate that proteoglycan-associated molecules such as SO4 were in fact blocked from appearing in the ECM following treatment with such agents as β-xyloside as compared to its inactive isomer, α-xyloside. These studies indicated that any blockage in proteoglycan biosynthesis resulted in a blockage inHydramorphogenesis (head regeneration and cell aggregate morphogenesis) and a retardation in normal I-cell migration, suggesting that proteoglycans are components of theHydraECM. Genbank search on NCBI website forHydraproteoglycan EST sequences and proteoglycan proteins returned with several results of predictedHydrasequences similar to vertebral proteoglycans. As of this date however, proteoglycans have not been isolated fromHydraECM and therefore these evidences are indirect in nature.
3 Biogenesis of Hydra ECM is intimately tied to regeneration
HydraECM synthesis and normal assembly is required for general morphogenesis, pattern formation, and cell differentiation to occur in the adult polyp. This has been determined through head and foot regeneration studies and through experiments utilizingHydracell aggregates. Development ofHydracell aggregates involves complete morphogenesis of severalHydrapolyps within 48-72 hours from a cell pellet formed from non-enzymatically dissociated cells obtained from the adult polyps[5,18]. In all processes studied, including head regeneration, foot regeneration, and development ofHydracell aggregates, formation of a new ECM must occur before morphogenesis can proceed. If ECM biogenesis is blocked or perturbed in any way, morphogenesis is stopped. This has been shown through a broad array of approaches including:1) use of pharmacological reagents[15], 2)blocking antibodies toHydraECM components[18],3)fragments of ECM components that are used to compete in the normal polymerization of the matrix components[43], and 4)anti-sense RNA to ECM components introduced through electrophoresis techniques[22].
The sequence of events associated withHydraECM biogenesis indicates that cross-talk occurs between the ectoderm and endoderm and through signals emanating from the ECM to both epithelial layers. A general description ofHydraECM biogenesis is depicted in Fig 4. Removal of the head or foot or a simple incision along the gastric tube wall ofHydraresults in a retraction of the ECM from the cut edge. This is explained by the unique elastic properties ofHydraECM. Together with epithelial cell migration to cover the wound, a region with sealed epithelial bilayer that has no ECM between the two cell layers is created[22]. This ECM-deficient bilayer changes its morphology so that cells become flattened compared to the more cuboidal to columnar cellular phenotype normally seen alongHydra’s body wall (Fig 4). Soon after sealing (within 1 hr post-decapitation), this bilayer immediately begins to synthesize a new ECM. The process involves initial up-regulation of ECM component genes within 3 hrs of decapitation (in the case of head regeneration experiments as shown in Fig 4). In this process, basal lamina mRNA expression (e.g. laminin and Hcol-IV) is specific to endodermal cells while interstitial matrix components expression (e.g. Hcol1, 2, and 3) is specific to the ectoderm layer of cells. Within 7 hrs of decapitation, basal lamina (BL) proteins are secreted from the endoderm and a newly formed BL is seen associated with the basal extracellular border of both the ectoderm and endoderm. Through ECM receptor systems[24]BL components trigger synthesis of fibrillar collagens, such as Hcol1, that are secreted from the ectoderm and polymerize in the interstitial matrix zone between the two BL layers[22]. The linkage between BL formation and interstitial matrix component translation and secretion is supported by RNA antisense experiments in which laminin translation is blocked and subsequent Hcol1 translation and secretion from the ectoderm is prevented[22], thereby blocking ECM formation and perturbing morphogenesis of a head or foot structure. The regeneration ofHydracell aggregates formed from dissociated cells goes through a cystic stage which has an epithelial bilayer that lacks an ECM. If one uses pharmacological agents, blocking antibodies toHydraECM components, or fragments of ECM components to perturb ECM polymerization inHydracell aggregate, morphogenesis of the aggregates beyond the cystic stage will not occur[43].
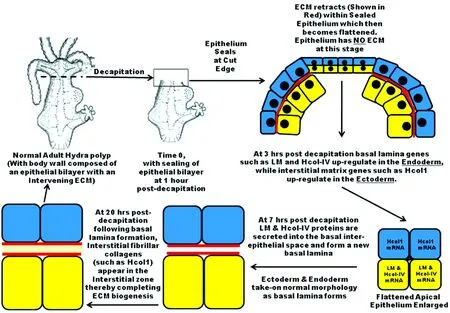
When decapitation is initiated (time 0), the head pole is open to the environment. The wound seals within 1 hour by fusion of the two apical epithelial bilayers. Within 3 hours, an up-regulation of ECM genes is observed in the ectoderm and endoderm. By 7 hours, laminin and collagen type IV (Hcol-IV) are translated and secreted into the ECM. At 20 hours, interstitial fibrillar collagens such as Hcol1 begin to be translated and appear between the two previously formed basal lamina layers. The ECM is now completely polymerized and the normal adult Hydra body wall structure is reformed.Fig 4 ECM biogenesis following decapitation of Hydra
Taken in total, these studies indicate that 1) ECM biogenesis is essential forHydramorphogenesis and 2) ECM biogenesis is a complicated process involving cross-talk between the endoderm and ectoderm as well as signaling from the ECM to the cells of the bilayer. This cross-talk involves sequential synthesis of basal lamina (endoderm) and interstitial matrix (ectoderm) components that will in turn signal appropriately timed translation and secretion of ECM proteins resulting in the polymerization of a well ordered matrix. As with higher invertebrates, this also involves ECM receptor complexes that trigger appropriate signaling pathways associated with ECM biogenesis processes.
4 Hydra ECM in development
Since embryogenesis inHydradoes not occur regularly, development inHydracan be studied through the processes of bud formation and tentacle growth. In addition,Hydracell aggregate regeneration involves the entire process of polyp development from de novo, and therefore, is also a good model to studyHydradevelopment.
When a bud forms, the evagination of the epithelial bilayer brings the ECM with it. As the epithelial bilayer protrudes quickly in budding[2], the ECM is stretched to a very thin layer[44]. This provides another evidence to support the elastic property ofHydraECM. At this time, the thickness of the ECM is only about 50%-75% of that of the body region. Nevertheless, the ECM is still associated with the bud and the trilaminar structure is likely maintained. While the ECM is stretched thin within the bud, the epithelial cells of the bud start to up-regulate the synthesis of ECM components; namely, laminin, collagens type I and IV. This is shown by in situ hybridization experiments using molecular probes to these ECM components[21,33]. During this process the sequence of events is proposed to involve the following steps:1) epithelial cell protrusion stretches the ECM thin; 2) the thinning of the ECM sends signal(s) to epithelial cells on both sides to make more ECM; and 3) as a result, the ECM increases in thickness within the bud which affects bud morphogenesis and tentacle formation. The nature and pathway(s) of this signaling process needs further study.
The ECM within tentacles is much thinner than that of the body region and remains thin during the life of the organism. In situ hybridization experiments[15,21,32-33]indicated that ECM synthesis is concentrated in the proximal region of the tentacles with the highest level of synthesis at the base. ECM synthesis of the tentacle gradually disappears at its distal region. Aufschnaiter et al[44].published a set of in vivo studies, which labeled the ECM of the tentacles and followed its movement. The data indicates that the ECM of tentacles moves from a proximal to distal position and finally disappears. Combining the two sets of data (in situ hybridization and in vivo ECM labeling), it is reasonable to propose that tentacle ECM is brought over from the body region when tentacles start to emerge, continue to be synthesized in the proximal region during tentacle extension, and is displaced toward the tentacle distal tips. There is no data indicating that tentacle emergence also involves ECM stretching as observed in budding; however, both biological events start with epithelial cell evagination and protrusion. It is possible that tentacle emergence and budding both employ the same signaling mechanism as a result of ECM stretching and thinning.
5 Cell-ECM Interactions in Hydra
Cell-ECM interactions are essential during normal tissue homeostasis, in a broad spectrum of disease states, as well as in developmental processes[26,45-48]. Here, we discuss cell-ECM interactions by including the following processes: 1) cell proliferation, 2) cell migration, 3) cell differentiation, and 4) morphogenesis. These cell-ECM interactions inHydraare listed in Table 2 which includes references of the studies. A role for the ECM in the modulation of cell proliferation inHydrawas reported by Zhang et al. in 1994[43]. These studies showed that introduction of exogenous matrix protein domains during ECM formation inHydraresulted in alterations in cell proliferation rates. Likewise, alteration of ECM structure or exogenous introduction of blocking antibodies to matrix components or matrix component domains would retard I-cell migration inHydragrafting experiments[23]. In vitro studies with isolated nematocytes have also indicated dependence of cell-ECM interactions for cell migratory processes inHydra[49-51]. As discussed above, a number of studies involving head regeneration[17]and morphogenesis ofHydracell aggregates[18]have clearly shown that any perturbation of ECM formation will result in blockage ofHydramorphogenesis. In addition, studies involvingHydracell aggregates[43]orHydrafoot regeneration[52]have shown that perturbation of ECM structure or ECM turnover can affect cell differentiation in the adultHydrapolyp. To understand the signaling process of the cell-ECM interactions inHydra, the signaling motifs on the cell surface or in the ECM are considered. These signaling motifs can be either exposed or cryptic.
Table2DevelopmentalprocessesinHydrathathavebeenreportedtoinvolvecell-ECMinteractions
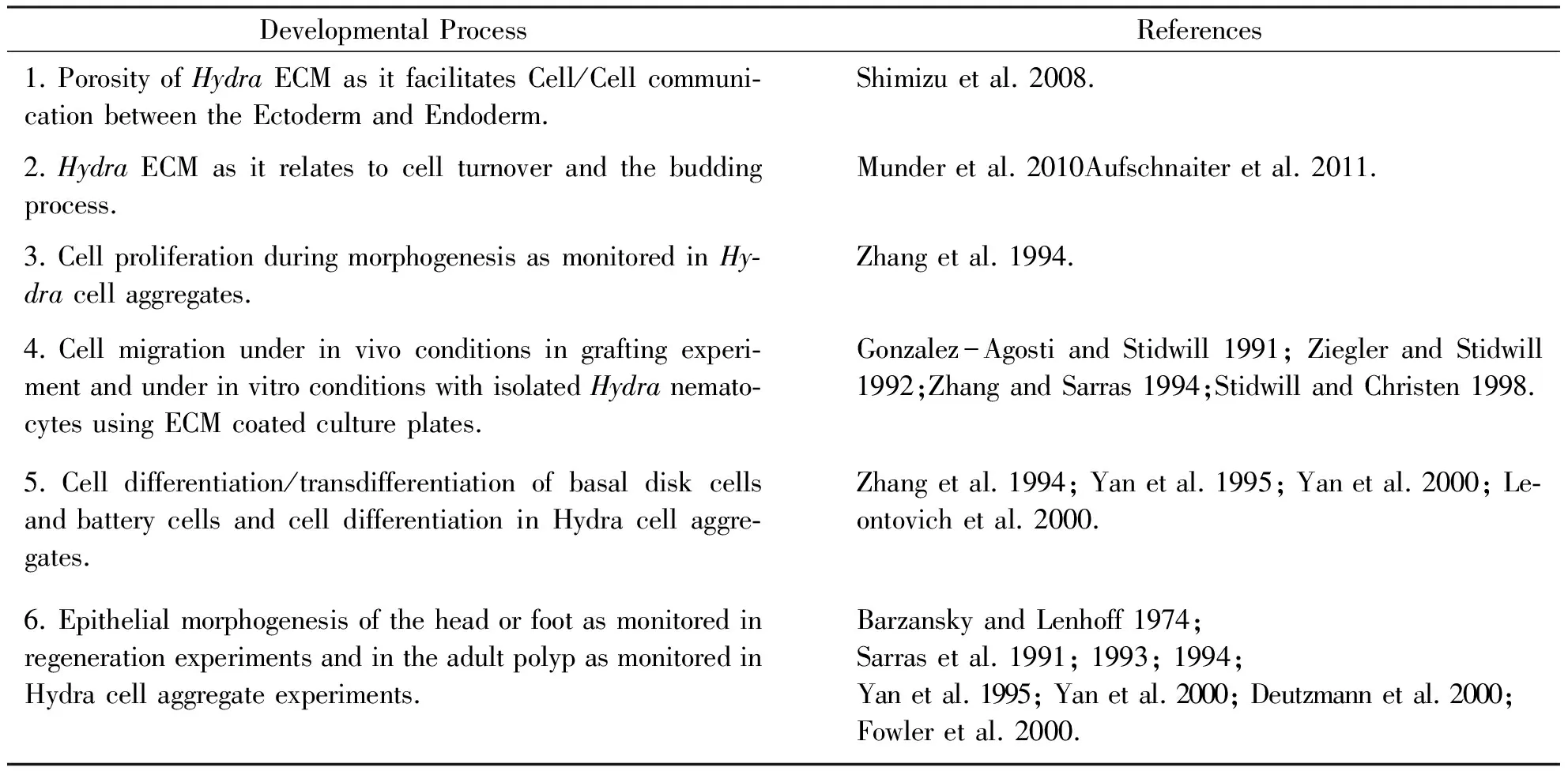
DevelopmentalProcessReferences1.PorosityofHydraECMasitfacilitatesCell/Cellcommuni⁃cationbetweentheEctodermandEndoderm.Shimizuetal.2008.2.HydraECMasitrelatestocellturnoverandthebuddingprocess.Munderetal.2010Aufschnaiteretal.2011.3.CellproliferationduringmorphogenesisasmonitoredinHy⁃dracellaggregates.Zhangetal.1994.4.Cellmigrationunderinvivoconditionsingraftingexperi⁃mentandunderinvitroconditionswithisolatedHydranemato⁃cytesusingECMcoatedcultureplates.Gonzalez-AgostiandStidwill1991;ZieglerandStidwill1992;ZhangandSarras1994;StidwillandChristen1998.5.Celldifferentiation/transdifferentiationofbasaldiskcellsandbatterycellsandcelldifferentiationinHydracellaggre⁃gates.Zhangetal.1994;Yanetal.1995;Yanetal.2000;Le⁃ontovichetal.2000.6.EpithelialmorphogenesisoftheheadorfootasmonitoredinregenerationexperimentsandintheadultpolypasmonitoredinHydracellaggregateexperiments.BarzanskyandLenhoff1974;Sarrasetal.1991;1993;1994;Yanetal.1995;Yanetal.2000;Deutzmannetal.2000;Fowleretal.2000.
The action ofHydramatrix metalloproteinases has been shown to be involved in exposing potentially cryptic signaling sites within ECM components[52].Hydramatrix metalloproteinase (HMMP) has been shown to specifically affect the maintenance of the phenotype of basal disk cells through mechanisms that remain unclear[52]. A similar observation has been reported forHydrametalloproteinase-1 that is localized to the tentacle ECM and has been shown to be involved in the maintenance of tentacle battery cell phenotypic markers[53-54]. As reviewed by Schmid et al[55].the importance of cell-ECM interactions during morphogenesis and cell differentiation extends beyondHydrato a number of classes within Cnidaria. Most recently, a new in vivo labeling technique forHydracollagen 1 and laminin was used to track the fate of ECM in all body regions of the animal and to track the relationship between epithelial cell movement and that of the underlying ECM[44]. It has been known that the epithelial cell layers inHydraare constantly displaced toward the tentacle tips, the basal disc, and into the buds[56-58]. Therefore, all cells in aHydrapolyp (except cells in the hypostome region) are constantly moving. What happens to the ECM during epithelial cell movement was not clearly understood before. These recent ECM studies revealed thatHydraepithelial cells moved together with the associated ECM although the pace of movement of the ECM varies at different regions of a polyp[44]. Sometimes, epithelial cells and ECM move together, whereas other times cells move faster than that of the ECM. During the evagination of buds and tentacles, extensive movement of epithelial cells relative to the matrix was observed together with local ECM remodeling[44]. From this study, we now confirm that theHydraECM is not a stationary skeleton that supports cells to move on it, but rather, it moves dynamically along with the epithelial cells.
6 ECM remodeling during development in Hydra
While the extracellular metalloproteinases work to expose the ligands for cell-ECM signaling, they are also involved in remodeling the ECM constantly to meet the needs for maintaining the polyp’s shape and structures. Several cDNA fragments ofHydramatrix metalloproteinases have been cloned and their expression patterns were observed at different regions of the polyp such as base of the tentacles, upper body column, lower body region, peduncles, and base of the bud (Zhang lab unpublished data). One matrix metalloproteinase, MMP-A3, was specifically expressed at the junction between the base of the bud and the body of the mother polyp. It appeared when the base of the bud started to constrict, which is a necessary step to make the bud detach, and lasted until shortly after the bud fell off from the mother polyp[59]. The constriction at the base of the bud is the beginning of a boundary setup between the bud tissue and that of the mother polyp. Several signaling pathways are involved in this boundary formation[59]. In addition, collagen biosynthesis and cross-linking is needed to carry out the constriction process[44]. When using 2,2-Dipyridyl, a chemical inhibitor of prolyl-hydroxylase, on a buddingHydrapolyp, the constriction stopped and the bud failed to detach[44]. Taking these two sets of data together, these studies suggest that ECM synthesis, assembly, and remodeling occur at the same time and at the same position for a common biological process to take place inHydra. This dynamic and intimate association between ECM synthesis and remodeling may happen throughout the body axis ofHydra.
7 Conclusion
Hydra ECM is a flexible and elastic matrix positioned between two epithelial layers. Hydra ECM has multiple pores that house epithelial cell processes that arise from both sides of the bilayer and that contact each other within the matrix. It is organized as a trilaminar structure with two thinner subepithelial zones and one thicker central interstitial matrix. Laminin and type IV collagen occupy the subepithelial zones whereas fibrillar type I collagen forms the majority of the interstitial matrix. Both epithelial cell layers contribute to ECM synthesis with laminin from the endoderm and Type I collagen from the ectoderm. The trilaminar structure is maintained throughout aHydrapolyp and moves with epithelial cells, but each at different rates.HydraECM is re-synthesized and assembled during regeneration and this process is absolutely needed before other developmental processes, such as morphogenesis and pattern formation, can happen. During development and regeneration,HydraECM interacts with epithelial cells via signaling processes between the two. Remodeling ofHydraECM happens constantly throughout the polyp by using a family of matrix metalloproteinases which are specifically located at different regions of the polyp.
The functional role ofHydraECM in a variety of its cellular and developmental processes is seen replicated in more complicated vertebrate systems[26,46-48,60]. Given the early divergence ofHydraduring metazoan evolution, this serves to reinforce the fundamental nature of ECM to the cell biology of all metazoans. This also reminds us that althoughHydrais considered a relatively “simple” organism, it does not mean that its cellular and molecular biology is anything but simple to understand. In this regard, future studies withHydrashould be directed at 1) understanding how cell signaling pathways coordinate the formation of ECM by the epithelial bilayers ofHydraand 2) the precise mechanisms underlying the role of ECM inHydramorphogenesis and cell differentiation.
Acknowledgements:The author wishes to thank Dr. Liu Jie for making the connection with Zunyi Medical University and Dr. Xie Peng for the invitation of this review. Many studies in this review were completed in Dr. Michael Sarras’ lab at the University of Kansas Medical Center. Dr. Dale Abrahamson and Dr. D. B. Borza provided monoclonal antibodies to hydra ECM. This work was partially funded by the National Institutes of Health, USA, including grant DK092721.
[References]
[1] Bode H R.The interstitial cell lineage of hydra: a stem cell system that arose early in evolution [J].J Cell Sci,1996,109 (Pt6):1155-1164.
[2] Otto J J, Campbell R D. Budding in Hydra attenuata: Bud stages and fate map [J].J Exp Zool,1977,200(3):417-428.
[3] Holstein T W, Hobmayer E, Technau U. Cnidarians: an evolutionarily conservative model system for regeneration? [J]. Dev. Dyn,2003,226(2):257-267.
[4] Bode H R. Head regeneration in Hydra [J]. Dev Dyn, 2003, 226(2):225-236.
[5] Gierer A, Berking S, Bode H, et al. Regeneration of hydra from reaggregated cells [J]. Nat New Biol, 1972, 239(91): 98-101.
[6] Bode H. Axis formation in Hydra [J]. Annu Rev Genet, 2011,45:105-117.
[7] Hess A, Cohen A I, Robson E A. Observations on the structure of Hydra as seen with the electron and light microscope [J]. Quart J Micr Sci, 1957,98(3): 315-326.
[8] Wood R L. The fine structure of intracellular and mesoglea attachments of epithelial cells in Hydra. In: Lenhoff HLW, editor[M]. The Biology of Hydra. Coral Gables, FL: Miami Press University, 1961.
[9] Gauthier G F. Cytological studies on the gastroderm of Hydra [J]. J Exp Zool, 1963,152(1):13-39.
[10] Haynes J F, Burnett A L, Davis L E. Histological and ultrastructural study of the muscular and nervous systems in Hydra. I. The muscular system and the mesoglea [J]. J Exp Zool, 1968,167(3): 283-293.
[11] Davis L E,Haynes J F.An ultrastructural examination of the mesoglea of Hydra [J]. Z Zellforsch Mikrosk Anat, 1968,92(2): 149-158.
[12] Shostak S, Patel N G, Burnett A L. The role of mesoglea in mass cell movement in Hydra [J]. Dev Biol,1965,12(3): 434-450.
[13] Barzansky B, Lenhoff H M. On the chemical composition and developmental role of the mesoglea of Hydra [J]. Amer Zool, 1974,14(2):575-581.
[14] Barzansky B, Lenhoff H M, Bode H. Hydra mesoglea: similarity of its amino acid and neutral sugar composition to that of vertebrate basal lamina [J]. Comp Biochem Physiol B, 1975,50(3):419-424.
[15] Deutzmann R, Fowler S, Zhang X, et al. Molecular, biochemical and functional analysis of a novel and developmentally important fibrillar collagen (Hcol-I) in hydra [J]. Development, 2000,127(21): 4669-4680.
[16] Sarras M P Jr, Madden M E, Zhang X M, et al. Extracellular matrix (mesoglea) of Hydra vulgaris. I. Isolation and characterization [J]. Dev Biol,1991,148(2): 481-494.
[17] Sarras M P Jr, Meador D, Zhang X M. Extracellular matrix (mesoglea) of Hydra vulgaris. II. Influence of collagen and proteoglycan components on head regeneration [J]. Dev Biol, 1991,148(2): 495-500.
[18] Sarras M P Jr, Zhang X, Huff J K, et al. Extracellular matrix (mesoglea) of Hydra vulgaris III. Formation and function during morphogenesis of hydra cell aggregates [J]. Dev Biol,1993,157(2): 383-398.
[19] Sarras MP Jr, Yan L, Grens A, et al. Cloning and biological function of laminin in Hydra vulgaris [J]. Dev Biol, 1994,164(1):312-324.
[20] Shimizu H, Aufschnaiter R, Li L, et al. The extracellular matrix of hydra is a porous sheet and contains type IV collagen [J]. Zoology(Jena), 2008,111(5): 410-418.
[21] Zhang X, Fei K, Agbas A, et al. Structure and function of an early divergent form of laminin in hydra: a structurally conserved ECM component that is essential for epithelial morphogenesis [J]. Dev Genes Evol, 2002,212(4):159-172.
[22] Shimizu H, Zhang X, Zhang J, et al. Epithelial morphogenesis in hydra requires de novo expression of extracellular matrix components and matrix metalloproteinases [J]. Development, 2002,129(6):1521-1532.
[23] Zhang X, Sarras M P Jr. Cell-extracellular matrix interactions under in vivo conditions during interstitial cell migration in Hydra vulgaris [J]. Development, 1994,120(2):425-432.
[25] Darribère T, Skalski M, Cousin H L, et al. Integrins: regulators of embryogenesis [J]. Biol Cell, 2000,92(1):5-25.
[26] Wickstrom S A, Radovanac K, Fassler R. Genetic analysis of integrins signaling [J]. Cold Spring Harb Perspect Biol, 2011,3(2):1-22.
[27] Yoshida N, Ishii E, Nomizu M, et al. The laminin-derived peptide YIGSR (Tyr-Ile-Gly-Ser-Arg) inhibits human pre-B leukaemic cell growth and dissemination to organs in SCID mice [J]. Br J Cancer,1999,80(12):1898-1904.
[28] Hopker V H, Shewan D, Tessier-Lavigne M, et al. Growth-cone attraction to netrin-1 is converted to repulsion by laminin- 1 [J]. Nature, 1999,401(6748): 69-73.
[29] Saleh A F, Aojula H S, Pluen A. Enhancement of gene transfer using YIGSR analog of Tat-derived peptide [J]. Biopolymers, 2008,89(1):62-71.
[30] Chapman J A, Kirkness E F, Simakov O, et al. The dynamic genome of Hydra [J]. Nature, 2010,464(7288):592-596.
[31] Hausman R E, Burnett A L. The mesoglea of Hydra: IV. A quantitative radiographic study of the protein component [J]. J Exp Zool, 1971,177: 435-446.
[32] Fowler S J, Jose S, Zhang X, et al. Characterization of hydra type IV collagen. Type IV collagen is essential for head regeneration and its expression is up-regulated upon exposure to glucose [J]. J Biol Chem, 2000,275(50):39589-39599.
[33] Zhang X, Boot-Handford R P, Huxley-Jones J, et al. The collagens of hydra provide insight into the evolution of metazoan extracellular matrices [J]. J Biol Chem, 2007,282(9):6792-6802.
[34] Yurchenco P D, O'Rear J J. Basal lamina assembly [J]. Curr Opin Cell Biol, 1994,6(5):674-681.
[35] Kühn K. Basement membrane (type IV) collagen [J]. Matrix Biol, 1995,14(6):439-445.
[36] Reinhart-King C A. How matrix properties control the self-assembly and maintenance of tissues [J]. Ann Biomed Eng, 2011,39(7):1849-56.
[37] Noelken M E, Wisdom B J Jr, Dean D C, et al. Intestinal basement membrane of Ascaris suum. Molecular organization and properties of the collagen molecules [J]. J Biol Chem,1986,261(10):4706-4714.
[38] Exposito J Y, Garrone R. Characterization of a fibrillar collagen gene in sponges reveals the early evolutionary appearance of two collagen gene families [J]. Proc Natl Acad Sci U S A, 1990, 87(17):6669-6673.
[39] Exposito J Y, D'Alessio M, Solursh M, et al. Sea urchin collagen evolutionarily homologous to vertebrate pro-alpha 2(I) collagen [J]. J Biol Chem, 1992,267(22):15559-15562.
[40] Kurz E M, Holstein T W, Petri B M, et al. Mini-collagens in hydra nematocytes [J]. J Cell Biol,1991,115(4):1159-1169.
[41] Holstein T W, Benoit M, Herder G V, et al. Fibrous mini-collagens in Hydra nematocysts [J]. Science, 1994,265(5170):402-404.
[42] Beckmann A,Özbek S. The Nematocyst: A molecular map of the cnidarian stinging organelle [J]. Int J Dev Biol, 2012,56(6-8):577-82.
[43] Zhang X, Hudson B G, Sarras M P Jr. Hydra cell aggregate development is blocked by selective fragments of fibronectin and type IV collagen [J]. Dev Biol,1994,164(1):10-23.
[44] Aufschnaiter R, Zamir E A, Little C D, et al. In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps [J]. J Cell Sci, 2011,124(Pt23),4027-4038.
[45] Matejas V, Hinkes B, Alkandari F, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum [J]. Hum Mutat, 2010,31(9):992-1002.
[46] Kim S H, Turnbull J, Guimond S. Extracellular matrix and cell signaling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor [J]. J Endocrinol,2011,209(2): 139-151.
[47] Watt F M, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin [J]. Cold Spring harb Perspect Biol, 2011,3(4), doi:pii: a005124.
[48] Yurchenco P D. Basement membranes: cell scaffoldings and signaling platforms [J]. Cold Spring Harb Perspect Biol, 2011,3(2), doi: 10.1101/cshperspect.a004911.
[49] Gonzalez Agosti C, Stidwill R. In vitro migration of Hydra nematocytes: the influence of the natural extracellular matrix (the mesoglea, of collagen type IV and type, laminin, and fibronectin) on cell attachment, migration parameters and patterns of cytoskeletal proteins [J]. Cell Motil Cytoskeleton, 1991,20(3):215-227.
[50] Ziegler U, Stidwill R P. The attachment of nematocytes from the primitive invertebrate Hydra to fibronectin is specific and RGD-dependent [J]. Exp Cell Res, 1992,202(2): 281-286.
[51] Stidwill R P, Christen M. Alteration of fibronectin affinity during differentiation modulates the in vitro migration velocities of Hydra nematocytes [J]. Cell Motil Cytoskeleton, 1998,41(1):68-73.
[52] Leontovich A A, Zhang J, Shimokawa K. A novel hydra matrix metalloproteinase (HMMP) functions in extracellular matrix degradation, morphogenesis and the maintenance of differentiated cells in the foot process [J]. Development, 2000,127(4):907-920.
[53] Yan L, Leontovich A, Fei K,et al.Hydra metalloproteinase 1: a secreted astacin metalloproteinase whose apical axis expression is differentially regulated during head regeneration [J]. Dev.Biol, 2000,219(1):115-128.
[54] Yan L, Pollock G, Nagase H, et al. Hydra metalloproteinase 1 (HMP1), a member of the astacin family, localizes to extracellular matrix in a head-specific manner and has a developmental function [J]. Development, 1995,121(6):1591-1602.
[55] Schmid V, Ono S I, Reber-Müller S. Cell-Substrate Interactions in Cnidaria [J]. Microsc Res Tech, 1999,44(4):254-268.
[56] Shostak S, Globus M. Migration of epithelio-muscular cells in Hydra [J]. Nature, 1966,210(5032):218-219.
[57] Campbell R D. Tissue dynamics of steady state growth in Hydra littoralis. II. Patterns of tissue movement [J]. J Morphol, 1967,121(1):19-28.
[58] Campbell R D. Vital marking of single cells in developing tissues: India ink injection to trace tissue movements in hydra [J]. J Cell Sci, 1973,13(3):651-661.
[59] Münder S, Käsbauer T, Prexl A. Notch signalling defines critical bourdary during budding in hydra [J]. Dev Biol,2010,344(1),331-345.
[60] Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices [J]. Cell Mol Life Sci,2010,67(17):2879-2895.

