Serum inflammatory factor and cytokines in AECOPD
Tie-Gang Jia, Jian-Qing Zhao, Jian-Hua Liu
Department of Respiratory Medicine, First Affiliated Hospital of Hebei North University, Zhangjiakou, Hebei 075061, China
Serum inflammatory factor and cytokines in AECOPD
Tie-Gang Jia1, Jian-Qing Zhao2*, Jian-Hua Liu2
Department of Respiratory Medicine, First Affiliated Hospital of Hebei North University, Zhangjiakou, Hebei 075061, China
Objective:To explore the serum levels of IL-32, MMP-9,PCT and CRP in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).Methods:A total of 50 patients with AECOPD and 45 cases with acute asthma attack admitted from October 2013 to August 2014 were selected, and the serum levels of IL-32, MMP-9, PCT and CRP were determined and compaed by using Double antibody sandwich Enzyme linked immunosorbent assay, immunofluorescence double antibody sandwich assay and immunoturbidimetry assay.Results:Serum levels of IL-32, MMP-9, PCT and CRP were significantly higher in AECOPD group than acute asthma attack group (P<0.05). IL-32 and MMP-9 were negatively correlated with lung function. MMP-9 in AECOPD patients was increased more significantly.Conclusions:Serum levels of IL-32 and MMP-9 were negatively correlated with lung function, and the worse the lung function is, the more significant the increase is.
ARTICLE INFO
Article history:
Received 24 September 2014
Received in revised form 10 October 2014
Accepted 15 November 2014
Available online 20 December 2014
MMP-9
IL -32
PCT
CRP
Serum
AHTMA
AECOPD
PF
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease with an increasing incidence rate year by year. This disease is mainly manifested as persistent airway obstruction showing progressive and irreversible development. With the gradual decline in lung function, the frequency of acute exacerbation increases in some patients with severe lung tissue damage, resulting in decreased activity tolerance, which not only increases the financial burden of the patients, but also seriously affects the quality of life of the patients. Currently it is concluded that the pathogenesis of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is mainly related to pulmonary inflammatory reaction and many other factors, including cytokines and inflammatory factors such as IL-32, MMP-9, PCT, CRP,etc[1-3]. Therefore, decreased levels of these factors in the treatment may reduce the frequency of AECOPD. In addition, the difference in serum IL-32, MMP-9, PCT and CRP levels of patients with AECOPD and those with acute asthma attack, and the relationship between these levels and the severity of pulmonary function impairment are unknown, which need further study.
2. Materials and methods
2.1. General data
Fifty patients (Aged between 59-80 years old) with AECOPD admitted from October 2013 to August 2014 were selected, including 27 males and 23 females. The diagnosis and severity assessment were based on the Guideline for Diagnosis and Treatment in COPD[4]. In acute asthma attack group, there were 45 patients (20 males and 25 females). The diagnostic criteria for patients in acute asthma attack group were based on Guidelines for diagnosis and treatment of bronchial asthma (2013)[5]. The patients in the two groups above had not received anti-leukotriene receptorantagonists, corticosteroids and antibiotics within 4 weeks before admission. The patients with the following diseases were excluded: chronic lung diseases such as tuberculosis, bronchiectasis,etc., rheumatic autoimmune disease, inflammatory response syndrome and other complications, and fungal infections. All patients had no pulmonary infection confirmed by chest X-ray or CT. At the same time, 45 healthy volunteers (Aged between 53-74 years old) were selected and enrolled in the control group, including 19 males and 26 females, who had no history of COPD, asthma or allergies, and the pulmonary function was normal. There was no history of respiratory infection and no any medicines within past 4 weeks were taken. There were no differences in age, sex and occupation structure among three groups.
2.2. Methods
Before treatment, 5 mL of blood was collected from the cubital vein of patients with AECOPD and acute asthma attack admitted and healthy volunteers into a dry tube without anticoagulant. The blood was placed at room temperature for natural coagulation for 20 minutes, and then centrifuged for 20 minutes (3 000 rpm). The blood sample was centrifuged again if precipitate appeared during the preservation process. Then the serum was extracted and cryopreserved at -70 ℃ for later tests. The instruments used were (1) ELIASA: Perlong Medical (Beijing), model: DNM-9602; (2) Plate washer: Finland (Thermo Labsystems), model: AC8; (3) Centrifuge: high-speed micro-centrifuges (domestic), model: TG16W; (4) Incubator: water-jacket incubator (domestic), model: GNP-9080. Serum IL-32 and MMP-9 was determined by double antibody sandwich ELISA assay, PCT was quantified by immunofluorescent double antibody sandwich assay, and CRP was determined by immunoturbidimetry. The detection range of was 5 ng/L-100 ng/L for IL-32 and 0.4 μg/L-20 μg/L for MMP. The normal range was < 0.5 ng/L for PCT and < 10 mg/L for CRP. The kits for IL-32 and MMP-9 were provided by Beijing Baiaolaibo Science and Technology Co., Ltd. PCT kit was provided by Guangzhou Wondfo Biotech Co., Ltd, and CRP kit was provided by Beijing Strong Biotechnologies, Inc. All tests were conducted strictly in accordance with instructions. Pulmonary function test was conducted using German Jaeger MS-PFT breathing machine after inhaling bronchodilator (ventolin 5 mg). The severity of pulmonary function impairment was indicated by FEV1/FVC5 and FEV1%.
2.3. Statistical methods
The continuous variables were normally distributed which were expressed as mean±standard deviation. ANOVA was used for comparison among groups and LSD test was used for comparison between two groups. Enumeration data was analyzed usingChi-square test. The correlation between the grading of pulmonary function, smoking index and serum levels of MMP-9 and IL-32, CRP and PCT in AECOPD group was analyzed using Spearman rank test. The best choice point for determining the sensitivity of AECOPD was area under ROC curve. AUC of 0.5-0.7 indicated lower accuracy, 0.7-0.9 indicated moderate accuracy and > 0.9 indicated higher accuracy. All data were processed using statistical software SPSS 21.0 and analyzed by two-sided test. Significant level was defined as α=0.05 and P<0.05 was considered statistically significant.
3. Results
The levels of IL-32, MMP-9, PCT and CRP in AECOPD group were significantly higher than those of asthma group and control group (P<0.05). however, no difference in the levels of PCT and CRP was observed between asthma group and control group (P>0.05) (Table 1). The levels of MMP-9 and IL-32 were negatively correlated with pulmonary function and positively correlated with smoking index with correlation coefficients of 0.493, 0.663, 0.181 and 0.221, respectively, suggesting that the worse pulmonary function was, the higher smoking index was, the higher the levels of MMP-9 and IL-32 were. The levels of PCT and CRP had no correlation with pulmonary function but had positive correlation with smoking index. Further analysis revealed that the levels of IL-32 and MMP-9 increase with the grade of pulmonary function increasing, which were the highest in patients with grade 4 pulmonary function, significantly higher than that in patients with grade 1-3 pulmonary function (P<0.05). The level of IL-32 in patients grade 3 pulmonary function was higher than that in grade 1 and 2 (P<0.05); however, the level of MMP-9 in patients with grade 3 pulmonary function was higher than that in grade 1 (P<0.05), but had no differences with that in grade 2 (P>0.05) (Table 2). PCT, CRP, MMP-9 and IL-32 have diagnostic value for AECOPD. MMP-9 was more sensitive in AECOPD patients, followed by CRP, then IL-32, and finally PCT (Table 3).

Table 1 Comparason in IL-32 , MMP-9, PCT and CRP among AECOPD group, athma group and control group.
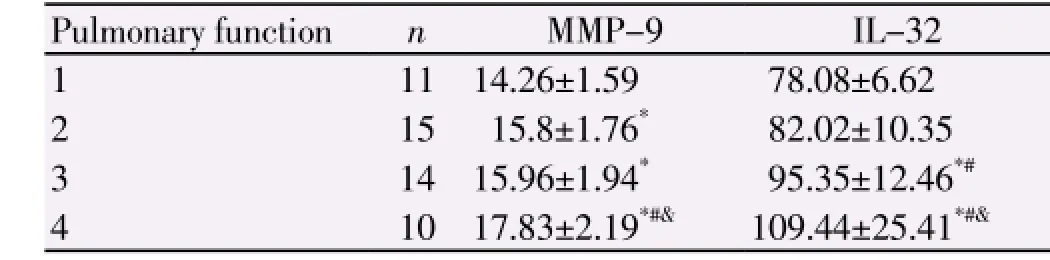
Table 2 Relationship between PF and MMP-9, IL-32.
4. Discussion
Currently it is believed that AECOPD is a kind of chronic inflammatory reactions in airways, pulmonary parenchyma and vessels are caused and mediated by a variety of cytokines and inflammatory factors are produced by various cells, such as macrophages, neutrophils and T lymphocytes,etc. Cytokines and inflammatory factors are involved in the progressive development of airflow limitation and structural damage of airway wall and lung parenchyma[6]. IL-32 is an important cytokine, which is expressed in a number of immune tissues, such as spleen, thymus, leukocytes, small intestine,etc. IL-32, which could be produced by endogenous secretion by T cells, monocytes and NK cells and gene recombination, plays an important role in the regulation of inflammation, and it can induce the immune cells to produce a variety of other cytokines. In addition, the expression of IL-32 in the airway wall is related to the infiltration of neutrophils and CD8+cells and the obstruction of airway, suggesting IL-32 may be involved in specific immune response of COPD[7]. MMP-9, a calcium and zinc-dependent endopeptidase which is mainly synthesized and secreted by neutrophils and macrophages with main function in maintaining normal metabolism of extracellular matrix, it is involved in airway inflammation reaction and airway remodeling process. Moreover, the excessive secretion of MMP-9 may lead to the destruction of pulmonary tissue structure and the expansion of air chamber and result in airflow obstruction[8]. PCT, a glycoprotein, is the precursor molecule of human calcitonin. Under normal condition, PCT is produced by thyroid C cells with low serum level (<0.05 ng/mL). In case of bacterial infections and septicopyemia, most of PCT is produced by the tissues beside the thyroid. The level of PCT is generally not elevated in autoimmunity, fungal and viral infections and allergies, but is significantly elevated in bacterial infections. PCT is not influenced by hormones and immunosuppressive agents, and therefore, it has high accuracy and specificity[9]. It can be be used to evaluate the severity of disease effectively and to judge prognosis of patients with AECOPD. It is consider as bacterial infection if PCT>0.25 μg/L; when the level is 0.26-0.5 μg/L, relevant anti-infective therapy is recommended. Strongly recomand anti-infective therapy is strongly recommended with antibiotics if PCT>0.5 μg/ L. CRP is a non-specific acute phase protein synthesized by the liver, which is mainly mediated by IL-6. Under physiological state, CRP in 99% of the population is less than 10 mL which is not easy to detect. However, in case of bacterial infections and other inflammatory stimulation may cause elevated synthesis of CRP in the liver leading to persistent elevated level of CRP. However, the level of CRP rapidly declines after the infection is controlled, and usually returns to normal in 1 week. Therefore, serum level of CRP can be used as a sensitive indicator for bacterial infections in AECOPD, which is significant to the diagnosis and treatment of AECOPD[10,11].
This study showed that serum levels of IL-32, MMP-9 in the patients with AECOPD and the patients with acute asthma attack were higher than those of healthy people, suggesting that both these two types of patients had different degrees of airway structural damage. In addition, serum levels of IL-32 and MMP-9 in AECOPD patients were higher than those in patients with acute asthma attack, suggesting the degree of airway damage of the former’s structure may be more serious than the latter. The reasons may lie in one important cause in the pathogenesis of COPD: the imbalance of protease/anti-protease. Moreover, MMP-9 can degrade extracellular matrix, resulting in airway wall damage, expansion of alveolar cavity and finally leading to airway remodeling. Another important cause in the pathogenesis of COPD is activation of the immune inflammatory reactions, and IL-32- involved specific immune response may cause the apoptosis of epithelial cells of airways and lung tissue, causing airway structure damage and lung tissue remodeling[12]. In addition, serum levels of IL-32 and MMP-9 in patients with AECOPD was negatively correlated with FEV1%, further suggesting that the higher concentrations of IL-32 and MMP-9 cause greater damage to the airways and lung tissue and more severe airflow limitation[13].However, the level of MMP-9 was not significantly changed in patients with grade 1 and 2 pulmonary function, possibly due to lesser damage of airways and lung tissue in these patients. MMP-9 is more sensitive than the other three Index as to AECOPD patients,further represent that the relationship between MMP-9 and airway wall damage,airway remodeling is closer, can be used as an important index to diagnose AECOPD. As markers of inflammation, PCT and CRP were elevated more significantly in patients with AECOPD, because the infection was the primary cause of AECOPD, while acute attack of asthma may be related to inhaled allergens. Therefore, PCT and CRP as indicators for infection can not only be used to determine the degree of infection in patients with AECOPD, but also guide the use of antibiotics[14]. The patients with higher smoking index had higher concentrations of IL-32, MMP-9, PCT and CRP, because compared with healthy smokers, the patients with COPD have large amounts of MMP-9 with increased activity released by alveolar macrophages, and the expression of IL-32 is related to neutrophil infiltration in the airway wall. Therefore, the patients with higher smoking index had higher concentrations of IL-32 and MMP-9 in airways and lung tissue, leading to more severe structural damage to the airways and lung tissue and more significant airflow limitation[15]. Moreover, smoking worsens the damage to airways and lung tissue, which aggravate the infection in the patients, leading to elevated levels of PCT and CRP.
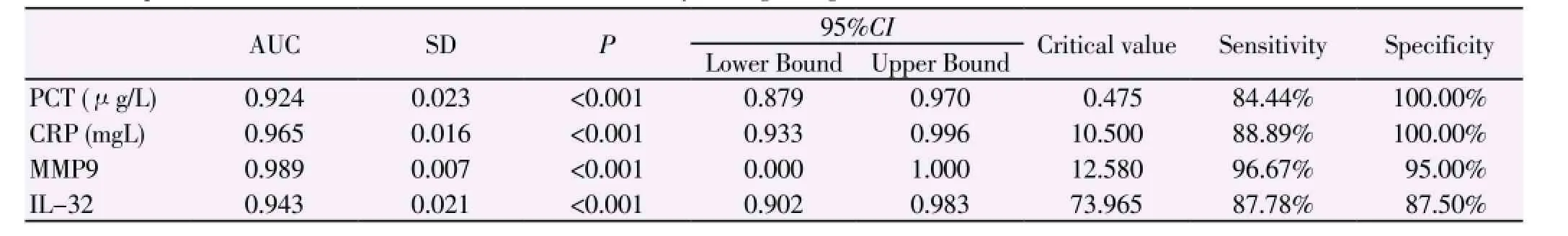
Table 3 Relationship between IL-32, MMP-9, PCT, CRP and sensitivity in diagnosing AECOPD.
In summary, the levels of serum IL-32, MMP-9, PCT and CRP in patients with AECOPD are higher than those in patients with acute asthma, suggesting that the increase of the levels of IL-32 and MMP-9 has certain relationship with AECOPD and can be used as a marker for acute exacerbation of COPD to a certain extent. The infection is the leading cause of AECOPD; therefore, PCT and CRP can be used as an indicator to determine the severity of AECOPD. Meanwhile, the increase of levels of IL-32 and MMP-9 were negatively correlated with pulmonary function. This study is aimed to provide a theoretical basis for the use of matrix metalloproteinase regulators and IL32 inhibitors to treat and delay the decline in pulmonary function in patients with AECOPD. In addition, the levels of IL-32 and MMP-9 levels were positively correlated with smoking index, further suggesting that long-term heavy smoking may cause more serious structural damage to lung tissue; therefore, early active quitting is important to improve the quality of life and reduce the financial burden of patients with COPD. However, further study is needed to investigate the role of the above 4 indicators in the long-term control of patients with COPD.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, AnzuetoA, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347-365.
[2] Mocchegiani E, Giacconi R, Costarelli L. Metalloproteases/antimetalloproteases imbalance in chronic obstructive pulmonary disease: genetic factors and treatment implications. Curr Opin Pulm Med 2011; 17(Suppl 1): S11-S19.
[3] Gao Z, Halmurat U, Jing J, Liao CY, Xu D, Li FS. Inflammatory responses of the rat lungs in cold-dryness syndrome in the northwest of China. J Tradit Chin Med 2012; 32(2): 234-237.
[4] Chinese Medical Association Guidelines for the diagnosis and treatment of chronic obstructive pulmonary disease (revision, 2013). Chin J Tuberculosis Respiratory 2013; 36(4): 1-10.
[5] Chinese Medical Association. Chinese experts’ common understanding of bronchial athma control Chin. J Internal Med 2013; 52(5): 440-443.
[6] Moermans C, Heinen V, Nguyen M, Henket M, Sele J, Manise M, et al. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine 2011; 56(2): 298-304. doi: 10.1016/j.cyto.2011.07.010.
[7] Kudo M, Ogawa E, Mishima M. Oxidative stress induced Interleukin-32 mRNA expression in human bronchial epithelial cells. Respir Res 2012; 13(1): 19.
[8] Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, et al. Differential expression of tis-sue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181(12): 1329-1335.
[9] Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kühn F, et al. Effectiveness and safety of procalcitonin -guided antibiotic therapy in lower respiratory tract in-fections in “real life”: an international,multicenter poststudy survey(ProREAL). Arch Intern Med 2012; 172(17): 715-722.
[10] Arinzon Z, Peisakh A, Schrire S, Berner Y. C-reactive protein (CRP): an important diagnostic and prognostic tool in nursinghome-associated pneumonia. Arch Gerontol Geriatr 2011; 53(3): 364-369.
[11] Xiao CY. Clinical significance of C-reactive protein and prealbumin detection during the acute exacerbation of COPD. J Clin Pulmonary Med 2012; 17(2): 252-253.
[12] Deng XQ, Shao JY, Gu W, Liu M, Cheng DS, Ling MR, et al. IL-32 level change and meaning in serum and Induced sputum from AECOPD paients. Chin J Prat Diagnosis Treatment 2012; 26(1): 18-20.
[13] Yıldırım E, Kormi I, Başoğlu ÖK, Gürgün A, Kaval B, Sorsa T, et al. Periodontal health and serum,saliva matrix metalloproteinases in patients with mild chronic obstructive pulmonary diseas. J Periodontal Res 2013; 48(3): 269-275.
[14] Jiang YM, Guo C, Sun L, Yang K, Dong L, Gao SH, et al. Application of Crp and calciton in assay in aecopd antibacterial treatment. J Hebei Med 2014; 36(9): 1365-1366.
[15] Cheng X. Approach of relationship between IL-6, IL-8, hs-CRP level in COPD stable phase patients’serum and smoking. Chin Clin Study 2010; 23(4): 273-274.
ment heading
10.1016/S1995-7645(14)60177-2
*Corresponding author: Jian-Qing Zhao, Professor, Postgraduate Supervisor, Respiratory Medicine Department of First Affiliated Hospital of Hebei North University, Zhangjiakou, Hebei 075061, China.
E-meal: jq.zhao66@163.com
Tel: 15530396510
Foundation project: It is supported by Sub-topic of ZhangJiaKou Technological and Seismological Bureau Instruct Project (Number12110043D-3).
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Assessment of traumatic brain injury degree in animal model
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
- Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
