Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
Bao-Qin Liu, Xin Gong, Zhe Jin*
1Beijing University of Chinese Medicine, Beijing 100029, China
2Department of Gynecology, Oriental Hospital of Beijing University of Chinese Medicine, Beijing 100029, China
Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
Bao-Qin Liu1, Xin Gong2, Zhe Jin1*
1Beijing University of Chinese Medicine, Beijing 100029, China
2Department of Gynecology, Oriental Hospital of Beijing University of Chinese Medicine, Beijing 100029, China
Objective:To investigate the effects of traditional Chinese medicine, Danzhi decoction, on the expression angiogenesis factors in human endometrial cells during the sequelae of pelvic inflammatory disease (SPID) and explore the role of Danzhi decction in improving the blood stasis microenvironment of SPID.Methods:A three-dimensional (3D) co-culture system including human vascular endothelial cells (VECs), endometrial stromal cells and glandular epithelial cells was established in vitro and treated with Danzhi decoction, sterilized water and aspirin respectively. A Milliplex multifunctional liquid chip technique was used to measure the expression levels of vascular endothelial growth factor (VEGF)-A/C/D, fibroblast growth factor -1/2, angiopoietin-2, epidermal growth factor (EGF), HB-EGF, bone morphogenetic protein-9, endoglin, endothelin-1, granulocyte colony stimulating factor, hepatocyte growth factor, interleukin-8, follistatin, placenta growth factor and leptin. The location of angiogenesis factors was monitored by immunofluorescence labeling and confocal laser scanning microscope 3D reconstruction.Results:Endometrial stromal cells and glandular epithelial cells were isolated and primary cultured for establishing a 3D co-culture system. The levels of VEGF-A/ C/D in Danzhi decoction group and aspirin group were significantly lower than those in mock group (P<0.05), while there was no significant difference between Danzhi decoction group and aspirin group (P>0.05). Furthermore, the alterative location of VEGF-A/C/D was observed in the cytoplasm of endometrial glandular epithelial cells.Conclusions:Danzhi decoction may inhibit the expression of VEGF in the blood stasis microenvironment of SPID by targeting the cytoplasm of endometrial glandular epithelial cell.
ARTICLE INFO
Article history:
Received 24 September 2014
Received in revised form 10 October2014
Accepted 15 November 2014
Available online 20 December 2014
Danzhi decoction
Sequelae of pelvic inflammatory disease
Endometrial cells
Angiogenesis factor
Microenvironment
1. Introduction
Sequelea of pelvic inflammatory disease (SPID), once called chronic pelvic inflammatory disease (CPID), is the legacy lesions of CPID. SPID is a common disease and frequentlyoccurring disease in the department of gynecology. Its clinical features include chronic pelvic pain and recurrent inflammation. SPID leads to infertility, tubal pregnancy and pelvic congestion syndrome, which seriously influences the health of women and the quality of life and increases the economic burden of family and society[1,2]. There is no definition of SPID in Chinese traditional medicine but some symptoms, such as abdominal pain, abdominal mass, irregular menstruation, infertility and morbid leucorrhea[3]. Chinese medical science considers that causes including cold and dampness damage uterus and uterine collateral and lead to Qi obstruction, stagnation of blood stasis, recurrent and refractory lingering. The stagnation of blood stasis is the main pathogenesis of SPID. Thus, promoting dredging the channel, circulation and removing stasis are principles for SPID treatment[4].
Chinese medicinal formulae, Danzhi decoction has a role in stasis-reducing and venation-flowing[5]. It is an effective medicine for improving the blood stasis microenvironment of SPID. In traditional Chinese medicine, promoting dredging the channel, circulation and removing stasis are principles for SPID treatment[5]. Danzhi decoction, a kindof Chinese medicinal formulae for disperses blood stasis and dredges collateral, is consisted with danshen root, mulberry twig, szechwan lovage rhizome, himalayan teasel root, weeping forsythia fruit, lychee seed, corydalis tuber and nutgrass galingale rhizome. It is an effective treatment for SPID and pelvic congestion syndrome. In these Chinese medicinal formulae, danshen root activate blood and resolve stasis. Mulberry twig dispel wind to free the collateral vessels. Szechwan lovage rhizome and himalayan teasel root promote Qi to activate blood. Weeping forsythia fruit and lychee seed are good at antipyretic detoxicate and reducing swelling. Compendium of Materia Medica has indicated that corydalis tuber have analgesic effects. Nutgrass galingale rhizome plays a role in regulation of painful menstruation. All these drugs have a role in activating blood circulation to dissipate blood stasis, antipyretic detoxicate, reducing swelling and analgesia. Modern pharmacological studies have found that dan-shen root expand the coronary artery and improve microcirculation[6]. Otherwise, it has a role in anti-bacteria and anti-tumorigenesis[6]. Mulberry twig exert anti-inflammatory effect by regulating mitogenactivated protein kinases signaling pathway[7]. Szechwan lovage rhizome increase blood flow in coronary artery and brain and improve blood circulation in heart and brain[8]. Himalayan teasel root can stop bleeding and alleviate pain[9]. Weeping forsythia fruit have a role in anti-bacteria and anti-viruses[10]. Lychee seed show functions in reducing blood glucose, blood fat regulation, anti-oxidant and antiviruses[11]. Corydalis tubers not only have analgesic effects but reducing the fever[12]. Nutgrass galingale rhizomes have estrogen-like activity in regulation of painful menstruation[13].
In this study, the human endometrial cells were 3D culturedin vitro. Then we established 3D cultured model of endometrial stromal cells and glandular epithelial cells in the blood stasis microenvironment of SPID. The effects of Danzhi decoction on angiogenesis factors were observed in this model. Moreover, we investigated the role of Danzhi decoction in improving the blood stasis microenvironment of SPID. All these experiments are aimed to provide theory basis for treatment of SPID.
2. Materials and methods
2.1. Clinical samples
Endometrial samples were collected from patients, who underwent complete hysterectomy for simple uterine fibroids or received hysteroscopy for benign diseases in the Department of Gynecology, Oriental Hospital of Beijing University of Chinese Medicine. All patients with menstrual regularity were under 45 years old and did not receive preoperative sex hormone in 3 months. All tissues were confirmed to be proliferative phase of endometrium but not endometrial lesions by postoperative pathological examination. The uterine endometrium was got from the bottom of uterus using a curet under aseptic conditions. All samples were used after obtaining informed consent. The Oriental Hospital of Beijing University of Chinese Medicine Ethics Committee approved all protocols according to the Helsinki Declaration (as revised in Edinburgh 2000).
2.2. Danzhi decoction preparation
Danzhi decoction included 10 g danshen root, 10 g mulberry twig, 6 g szechwan lovage rhizome, 15 g himalayan teasel root, 10 g weeping forsythia fruit, 10 g lychee seed, 10 g corydalis tuber and 6 g nutgrass galingale rhizome. All the traditional Chinese medicine was obtained from the Dispensary of Traditional Chinese Medicine, Oriental Hospital of Beijing University of Chinese Medicine. The doses of these traditional Chinese medicines comply with the Chinese pharmacopoeia. Eight drugs were concentrated to 100 mL (77 g/100 mL) after 1 hour soaking and 1hours decocting. The administration concentration was confirmed as 5 mg/mL by CCK8 (Pep- tide Institute, Inc., Osaka, Japan) toxicity test.
2.3. Primary culture of endometrial stromal cells and glandular epithelial cells
The endometrium tissues were washed and divided into pieces (0.5-1.0 mm3). Then tissues were mixed with 0.1% collagenase Ⅰ (Sigma, St-Louis, MO, USA) and 40 μg/mL Deoxyribonuclease I (Sigma). The cell suspension was subjected to Dynabeads® CD45 (DynalBiotech LLC, Brown Deer, WI) for removing white blood cell. Tissues were purified using CELLection Kit™ (Dynal, Oslo, Norway) Epithelial Enrich. Glandular epithelial cells were obtained by positive selection, while endometrial stromal cells were collected by negative selection. The cell suspension of endometrial stromal cells and glandular epithelial cells were incubated with vimentin and keratin, respectively. Flow cytometer was used to detect cell purity and the purity of them exceeded 90% indicated successful isolation of primary cell. After that, cells were cultured in 6-well NUNC UpCell plates (Nunc, Rochester, NY, USA) in a humidified containing of 5% CO2incubator at 37 ℃. When cell confluence was observed under inverted microscope, membranes were placed on the cell layer and incubated for 5-6 min at room temperature (20-25 ℃). Then cells were transferred from NUNC UpCell to membranes.
2.4. Culture of human vascular endothelial cells (VECs)
Human VEC cell line (HUVEC), was purchased from the Cell Center of Peking Union Medical University and cultured in ECM complete medium (Gibco, Grand Island, NY, USA). Cells were divided into three groups: Danzhi decoction, aspirin and mock. 5 mg/mL Danzhi decoction and 4 mmol/mL aspirin were filtered by 0.22 μm filter membrane for degerming and treated cells for 24 hours. But cells in mock group were treated with sterile water. Treated cells were cultured in 6-well NUNC UpCell plates in a humidified containing of 5% CO2incubator at 37 ℃. When cell confluence was observed under inverted microscope, membranes were placed on the cell layer and incubated for 5-6 min at room temperature (20-25 ℃). Then cells were transferred from NUNC UpCell to membranes.
2.5. Establishment of a 3D co-culture system
Membrane-carried HUVEC cells were placed on the surface of NUNC UpCell culture plates for 30min at 37 ℃. Then HUVEC cells were seeded in NUNC UpCell culture plates. Endometrial stromal cells were seeded on the surface of HUVEC cells and glandular epithelial cells were placed on the top floor to establish a 3D co-culture system.
2.6. Detection of angiogenesis factor
Cells in 3D co-culture system were treated with cytokine, IL-17A/IL-17F (Sigma). The supernatant was collected and subjected to Milliplex multifunctional liquid chip technique (Millipore, Billerica, MA, USA) for angiogenesis factors, such as vascular endothelial growth factor (VEGF)-A/C/D, fibroblast growth factor (FGF)-1/2, angiopoietin-2, epidermal growth factor (EGF), HB-EGF, bone morphogenetic protein(BMP)-9, endoglin, endothelin (ET)-1, granulocyte colony stimulating factor (G-CSF), hepatocyte growth factor (HGF), interleukin (IL)-8, follistatin, placenta growth factor (PLGF) and leptin.
2.7. Immunofluorescence (IF)
Cells were fixed with 3% paraformaldehyde and permeabilized using 0.2% Triton X-100. Then the fixed cells were incubated with the VEGF (Cell Signaling, Danvers, MA, USA) (1:500) and keratin (Cell Signaling) (1:500) primary antibody. The secondary antibody is an Alexa Fluor-conjugated IgG (Invitrogen, Carlsbad, CA, USA). Fluorescence confocal images were captured using a LSM 5 Pascal Laser Scanning Microscope (Zeiss Germany, Oberkochen, Germany) using a×10 lens and Laser Scanning Microscope LSM PASCAL software (version 4.2 SP1)[14].
2.8. Statistical analysis
Results are expressed as Mean±SD. Significance was established, with the SPSS statistical package for Windows Version 16 (SPSS, Chicago, IL, USA), using one-way ANOVA and LSD-tor Tambane’s T2 test when appropriate. Difference were considered significant when P<0.05.
3. Results
3.1. Isolation of endometrial stromal cells and glandular epithelial cells
The endometrial stromal cell shape was round under light microscope. After 30 min attachment, cells showed spindle-shaped like fibroblasts. The glandular epithelial cells showed tubular or grape-like growth. After 24 hours attachment and 3 days growth, cells were arranged in swirl (Figure 1A and 1B). Immunostaining revealed that endometrial stromal cells showed negative staining of keratin and positive staining of fibronectin. However, glandular epithelial cells showed positive staining of keratin and negative staining of fibronectin (Figure 1C and 1D). The flow cytometer indicated that the purity of endometrial stromal cell and glandular epithelial cell was 94% and 92%, respectively (Figure 2).

Figure 1. Immunostaining of endometrial stromal cells and glandular epithelial cells (original magnification×400).A) The microscopic view of endometrial stromal cells. B) The microscopic view of glandular epithelial cells. C) Immunostaining of fibronectin in endometrial stromal cells. D) Immunostaining of keratin in glandular epithelial cells.
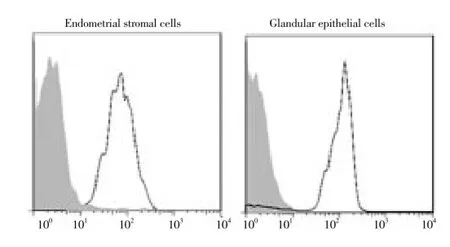
Figure 2. Purity of endometrial stromal cells and glandular epithelial cells.The purity of endometrial stromal cells was analyzed by flow cytometry using a vimentin antibody. The purity of glandular epithelial cells was detected by flow cytometry using a keratin antibody. The dashed area showed control antibodies.
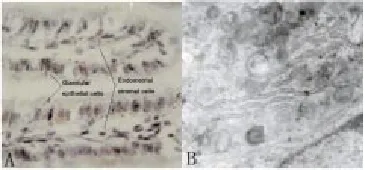
Figure 3. 3D model of endometrium in vitro.A) The light microscopic view of the 3D model of endometrium (original magnification×400). B) The electron microscopic view of the 3D model of endometrium (original magnification×10 000).
3.2. A 3D model of endometrium in vitro
Cells showed a 3D growth under the light microscope. Glandular epithelial cells showed a single columnar growth and endometrial stromal cells located at collagen matrix under the glandular epithelial cells. Under the electron microscope, we found that the surface of co-culture system was covered by glandular epithelial cells. These cells had polarity and microvillus on the plasma membrane. There was intercellular tight junction, intermediate junction and desmosome junction. Cytoplasm was rich in mitochondrion, ribosome, lipid droplet, big nuclear with obvious nucleolus (Figure 3).
3.3. Comparison of angiogenesis factor expression among three groups
As shown in Table 1, the levels of VEGF-A/C/D in Danzhi decoction group were significantly lower than those in mock group (P<0.05). Similarly, these three angiogenesis factors in aspirin group were obviously lower than those in mock group (P<0.05). However, there was no significant difference between Danzhi decoction group and aspirin group (P>0.05).
3.4. Glandular epithelial cell is sensitive to Danzhi decoctionAbove results indicated that Danzhi decoction inhibited VEGF expression. Next we sought to investigate which cell was the therapeutic target of Danzhi decoction. Double IF for VEGF (green) and keratin or vimentin (red) revealed that the glandular epithelial cell was sensitive to Danzhi decoction (Figure 4). An obvious reduction of VEGF level in glandular epithelial cells with keratin staining was observed afterDanzhi decoction treatment (Figure 4).
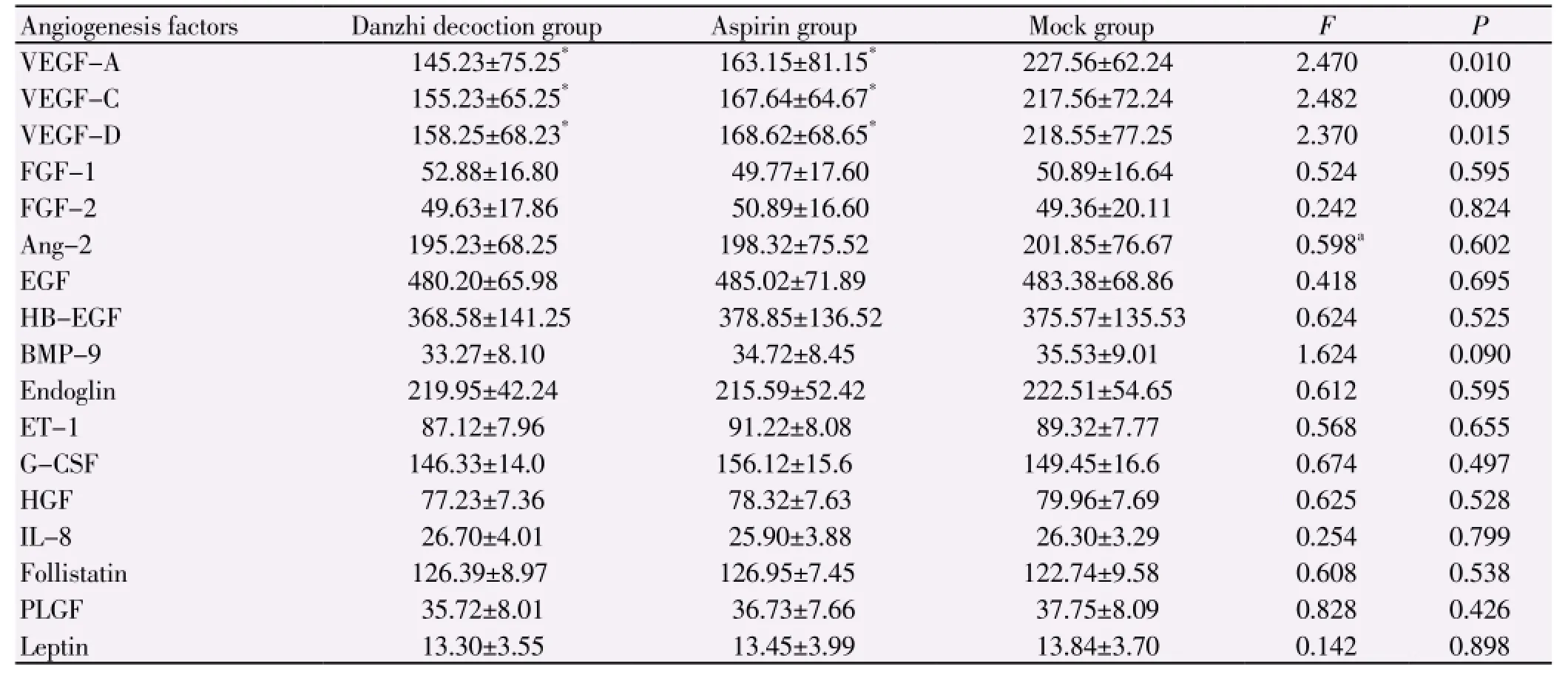
Table 1 Comparison of angiogenesis factor expression (pg/mL).
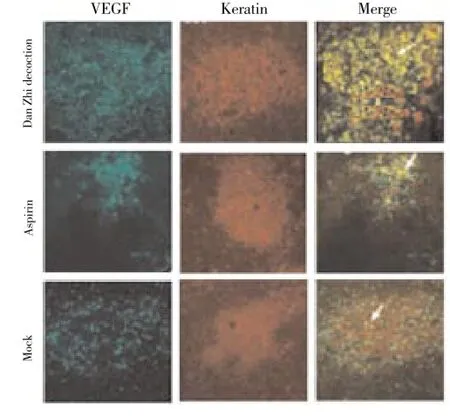
Figure 4. Double IF for VEGF and keratin in glandular epithelial cells (original magnification×100).Cells from different group (Dan Zhi decoction, aspirin or mock group) were subjected to double IF for VEGF and keratin. These data showed that Danzhi decoction led to a prominent reduction of VEGF expression in glandular epithelial cells.
4. Discussion
In order to investigate the mechanisms involved in Danzhi decoction improved blood stasis microenvironment of SPID, human endometrial cells in proliferative phase were 3D culturedin vitro. A blood stasis microenvironment of SPID model containing endometrial stromal cells and glandular epithelial cells was establishedin vitro. Milliplex multifunctional liquid chip technique was performed to determine the expression of angiogenesis factors, such as VEGF-A/C/D, FGF-1/2, angiopoietin-2, EGF, HB-EGF, BMP-9, endoglin, ET-1, G-CSF, HGF, IL-8, follistatin, PLGF and leptin. Compared with mock group, Danzhi decoction (5 mg/mL) inhibited VEGF-A/C/D expressions in human endometrial cells within blood stasis microenvironment of SPID. Previous studies have considered that the development of blood stasis microenvironment in SPID was correlated with vascular endothelium function[15]. Dysfunction of vascular endothelium is the key factor in the initiation and progression of blood stasis. However, VEGF is the only specific biomarker for vascular endothelium injury. VEGF stimulates VEC division and proliferation and subsequently changes microcirculation and leads to diseases. Previous studies reported that the Chinese medicinal formulae including lobed kudzuvine root, Chinese angelica, malaytea scurfpea fruit, prepared rhubarb and leech could prevent VECs from anoxia induced damage[16]. Lobed kudzuvine root increased physiological activity of VECs. Chinese angelica, malaytea scurfpea fruit, prepared rhubarb and leech had obvious protective effect on VECs. Another Chinese medicinal formulae including peach seed, safflower, pangolin scales, cowherb seed, blue turmeric rhizome, Chinese angelica, Chinese thorowax root, immature tangerine fruit, nutgrass galingale rhizome, common selfheal spike, kelp, chekiang fritillary bulb and loofah was applied to treat cyclomastopathy by reducing VEGF expression, suggesting that Chinese medicinal formulae with disperse blood stasis and dredge collateral function inhibits neovascularization by reducing VEGF expression[17]. Our studies indicated that Danzhi decoction decreased the level of VEGF expression in endometrial cells in the blood stasis microenvironment of SPID. Accordingly, it may improve endothelium function through regulating the endocrine function of VECs. Subsequently Dan Zhe decoction may exert positive effects on improving the blood stasis microenvironment of SPID. But the precise mechanisms need to be further investigated.
Modern medicine studies have considered that inflammation plays a critical role in the initiation and development of SPID[18, 19]. Several studies showed that most of SPID patients did not get bacterial infections, but illness had continued development[20]. Thus, bacteria-infective inflammation cannot explain the causes of SPID. Now, most of researchers consider that inflammatory injury is the key pathological change of SPID. Persistent activation of inflammatory factors and effusion of inflammatory cells lead to inflammatory autoimmune injury and subsequently indirectly or directly influences the initiation, progression and prognosis of SPID. Persistent inflammation results in blood stasis. In turn, blood stasis promotes inflammation. Prostaglandin epoxy enzyme inhibitor, aspirin, induces thrombosis in peripheral vessels by inhibiting platelet aggregation and accordingly increases the volume of blood flow. Recent researches have reported that aspirin is used to improve thrombosis in obstetrical and gynecological diseases. In this study, we chose aspirin (4mmol/mL) as a positive control for medicine treated SPID cell model. Our data indicated that the comparison of VEGF expression between Danzhi decoction and aspirin group was not statistically significant, suggesting that both Danzhi decoction and aspirin could evidently reduce VEGF expression in endometrial cell in the blood stasis of SPID. Danzhi decoction may repair injured VECs and improve local microcirculation to achieve the same clinical outcome of aspirin. Furthermore, double IF found that the reductionof VEGF-A/C/D was observed only in the cytoplasm of glandular epithelial cell. These results provide a potential target for Danzhi decoction mediated VEGF reduction.
In conclusion, we establish a 3D endometrial cell model with the blood stasis of SPID via endometrial stromal cells and glandular epithelial cellsin vitroculture. We find that Danzhi decoction improves the blood stasis microenvironment of SPID by reducing VEGF expression in glandular epithelial cells. All these data provide theoretic basis for the clinical treatment of SPID.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Gradison M. Pelvic inflammatory disease. Am Fam Physician 2012; 85(8): 791-796.
[2] Liu Z. Guideline of pelvic inflammatory disease. Zhonghua Fu Chan Ke Za Zhi 2014; 49(6): 401-403.
[3] Yao XQ, Yang HE. Clinical observation on integrated traditional Chinese and Western medicine therapy in treating patients with pelvic inflammatory disease: a report of 28 cases. Zhong Xi Yi Jie He Xue Bao 2006; 4(2): 199-201.
[4] Yu ZJ. Combined traditional Chinese medicine and western medicine in the treatment of inflammatory diseases in gynecology. Zhong Xi Yi Jie He Za Zhi 1984; 4(4): 231-233.
[5] Lucas DN, Robinson PN, Nel MR. Sepsis in obstetrics and the role of the anaesthetist. Int J Obstet Anesth 2012; 21(1): 56-67.
[6] Wen XD, Wang CZ, Yu C, Zhang Z, Calway T, Wang Y, et al. Salvia miltiorrhiza (dan shen) significantly ameliorates colon inflammation in dextran sulfate sodium induced colitis. Am J Chin Med 2013; 41(5): 1097-1108.
[7] Guo C, Liang T, He Q, Wei P, Zheng N, Xu L. Renoprotective effect of ramulus mori polysaccharides on renal injury in STZ-diabetic mice. Int J Biol Macromol 2013; 62: 720-725.
[8] Zengyong Q, Jiangwei M, Huajin L. Effect of Ligusticum wallichii aqueous extract on oxidative injury and immunity activity in myocardial ischemic reperfusion rats. Int J Mol Sci 2011; 12(3): 1991-2006.
[9] Niu YB, Li YH, Kong XH, Zhang R, Sun Y, Li Q, et al. The beneficial effect of Radix Dipsaci total saponins on bone metabolism in vitro and in vivo and the possible mechanisms of action. Osteoporos Int 2012; 23(11): 2649-2660.
[10] Zhang HY, Piao XS, Zhang Q, Li P, Yi JQ, Liu JD, et al. The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult Sci 2013; 92(8): 1981-1988.
[11] Ichinose T, Musyoka TM, Watanabe K, Kobayashi N. Evaluation of antiviral activity of Oligonol, an extract of Litchi chinensis, against betanodavirus. Drug Discov Ther 2013; 7(6): 254-260.
[12] Ma H, Zhao Q, Wang Y, Guo T, An Y, Shi G. Design and evaluation of self-emulsifying drug delivery systems of Rhizoma corydalis decumbentis extracts. Drug Dev Ind Pharm 2012; 38(10): 1200-1206.
[13] Kim HG, Hong J, Huh Y, Park C, Hwang DS, Choi JH, et al. Cyperi Rhizoma inhibits the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced reduction in nigrostriatal dopaminergenic neurons in estrogen-deprived mice. J Ethnopharmacol 2013; 148(1): 322-328.
[14] Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, et al. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer 2014; 13: 110.
[15] Dhasmana D, Hathorn E, McGrath R, Tariq A, Ross JD. The effectiveness of nonsteroidal anti-inflammatory agents in the treatment of pelvic inflammatory disease: a systematic review. Syst Rev 2014; 3: 79.
[16] Li P, Ren J, Duan C, Lin C, Liu J. Effects of four components of Rhizoma Corydalis on anoxia and peroxidation injuries in neonatal cardiomyocytes. Zhongguo Zhong Yao Za Zhi 2010; 35(1): 84-88.
[17] Wen JM, Xu YP, Dong JW, Pan GC, Sun YS, Sang ZC, et al. Effects on VEGF and VEGF mRNA expression in issues of callus in treating fracture of rabbit with three-period treatment theory of bone fracture in traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2007; 32(24): 2640-2645.
[18] Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am 2013; 27(4): 793-809.
[19] Walker CK, Wiesenfeld HC. Antibiotic therapy for acute pelvic inflammatory disease: the 2006 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2007; 44( Suppl 3): S111-S122.
[20] Shih TY, Gaydos CA, Rothman RE, Hsieh YH. Poor provider adherence to the Centers for Disease Control and Prevention treatment guidelines in US emergency department visits with a diagnosis of pelvic inflammatory disease. Sex Transm Dis 2011; 38(4): 299-305.
ment heading
10.1016/S1995-7645(14)60173-5
*Corresponding author: Zhe Jin, M.D, Beijing University of Chinese Medicine, Beijing 100029, China.
E-mail: jinzhe3223@163.com
Foundation project: This study is supported by a grant from the National Natural Science Foundation of China (81373677).
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Assessment of traumatic brain injury degree in animal model
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
- Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
