Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
Isaac Alobu, Sarah N. Oshi, Daniel C. Oshi, Kingsley N. Ukwaja
1National Tuberculosis and Leprosy Control Programme, Ministry of Health, Abakaliki, Ebonyi State, Nigeria
2Centre for Development and Reproductive Health, Enugu, Enugu State, Nigeria
3Department of Internal Medicine, Federal Teaching Hospital, Abakaliki, Ebonyi State, Nigeria
Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
Isaac Alobu1, Sarah N. Oshi2, Daniel C. Oshi2, Kingsley N. Ukwaja3*
1National Tuberculosis and Leprosy Control Programme, Ministry of Health, Abakaliki, Ebonyi State, Nigeria
2Centre for Development and Reproductive Health, Enugu, Enugu State, Nigeria
3Department of Internal Medicine, Federal Teaching Hospital, Abakaliki, Ebonyi State, Nigeria
Objective:To evaluate the rates, timing and determinants of default and death among adult tuberculosis patients in Nigeria.Methods:Routine surveillance data were used. A retrospective cohort study of adult tuberculosis patients treated during 2011 and 2012 in two large health facilities in Ebonyi State, Nigeria was conducted. Multivariable logistic regression analyses were used to identify independent predictors for treatment default and death.Results:Of 1 668 treated patients, the default rate was 157 (9.4%), whilst 165 (9.9%) died. Also, 35.7% (56) of the treatment defaults and 151 (91.5%) of deaths occurred during the intensive phase of treatment. Risk of default increased with increasing age (adjusted odds ratio (aOR) 1.2; 95% confidence interval (CI) 1.1-1.9), smear-negative TB case (aOR 2.3; CI 1.5-3.6), extrapulmonary TB case (aOR 2.7; CI 1.3-5.2), and patients who received the longer treatment regimen (aOR 1.6; 1.1-2.2). Risk of death was highest in extrapulmonary TB (aOR 3.0; CI 1.4-6.1) and smear-negative TB cases (aOR 2.4; CI 1.7-3.5), rural residents (aOR 1.7; CI 1.2-2.6), HIV co-infected (aOR 2.5; CI 1.7-3.6), not receiving antiretroviral therapy (aOR 1.6; CI 1.1-2.9), and not receiving cotrimoxazole prophylaxis (aOR 1.7; CI 1.2-2.6).Conclusions:Targeted interventions to improve treatment adherence for patients with the highest risk of default or death are urgently needed. This needs to be urgently addressed by the National Tuberculosis Programme.
ARTICLE INFO
Article history:
Received 10 September 2014
Received in revised form 15 October 2014
Accepted 15 November 2014
Available online 20 December 2014
Tuberculosis
Epidemiology
Treatment default
Mortality
Treatment outcome
Health services
Nigeria
1. Introduction
According to the World Health Organisation (WHO), tuberculosis (TB) treatment default defined as interrupting treatment for at least two consecutive months[1], remains a major threat to TB control. Individuals who default from treatment can undermine efforts to control TB because such persons have an increased risk of clinical deterioration and complications, TB recurrence, relapse, treatment failures, and mortality[2,3]. Moreover, they may remain infectious; develop drug-resistant TB, and continue to be a source of transmission of TB in the community[4]. Of particular importance in Nigeria, default/sub-optimal adherence, and treatment failure may increase the risk of acquired drugresistant TB[4]. Of all previously treated TB cases registered in 2010, in Nigeria, 14.3% of them had multi drug-resistant TB[5].
Nigeria ranks tenth among the 22 high-burden TB countries, with an estimated prevalence rate of 161 per 100 000 population in 2012[6]. Although treatment success rates among new TB cases reached 85% in 2011[6], default rates may be as high as 30% in some settings[7]. Little is known about the predictors of treatment default in Nigeria[7,8]. Knowledge of the determinants of TB treatment default and mortality is critical for informing health policy solutions needed to improve the outcomes of TB care and contain the spread of the disease. Studies in other settings have identified several determinants for treatmentdefault, including at the individual (male sex, older age, rural residence, human immunodeficiency virus (HIV) coinfection, low socio-economic status, homelessness), and health system levels (poor health care accessibility, attitudes of health care workers)[9-18]. However, only few studies have evaluated the determinants of TB-related mortality in sub-Saharan Africa[18-20], and none in Nigeria. Since these factors can vary between settings and health systems, there is a need for site-specific research in order to develop targeted interventions.
A previous study suggested that a shorter TB treatment regimen can substantially decrease default rates[21]. In 2010 the WHO recommended a change in its treatment guidelines for TB from an 8-month anti-TB regimen 2RHZE/6EH (consisting of 2 months of rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E), followed by 6 months of ethambutol and isoniazid) to a 6-month regimen containing 6 months of rifampicin (2RHZE/4RH)[22]. The Nigerian National TB Control Programme (NTP) adopted the current WHO guideline in January 2012[23]. Since these changes were adopted, it is unclear if the shorter treatment regimen lowers the risk of default or death during treatment. Therefore, there is a need to re-assess the determinants of treatment default and mortality among TB patients. Also, in the last five years, the use of antiretroviral therapy (ART) and cotrimoxazole preventive therapy (CPT) have been scaled-up among HIV-infected TB patients through TB/HIV collaborative activities in high-burden countries[6]. The contribution of these factors to default or death during TB treatment is not known.
The aim of this study was to describe the epidemiological characteristics of TB patients who defaulted or died during treatment. Specific objectives were to describe among adults (age ≥15 years) TB patients in Nigeria in 2011 and 2012: (1) the number and proportion of TB patients who defaulted, and died during TB treatment; (2) the rate of treatment default and death stratified by age, gender, residence, TB regimen received, residence, treatment category, HIV status and HIV treatment; and (3) the independent predictors of default, and death during TB treatment.
2. Materials and methods
2.1. Study area and population
The study was conducted in Ebonyi State, one of the 36 states of Nigeria, located in the southeastern part of the country. The state has a human population of more than 2.5 million people and 80% of them reside in the rural area[24]. The NTP follows the directly observed treatment, short course (DOTS) strategy and uses recognised international standards for the diagnosis and treatment of patients with TB. DOTS coverage in the state has reached 100%; however, its case detection rate remained around 40%[25]. The study sites included one rural secondary care (not-for-profit/ mission) private hospital and the only tertiary (public) hospital in Ebonyi State. The study sites were selected due to high TB notification rates and to allow for public-private mix comparison. Together they accounted for 50% of total TB notification in the state in 2009 and they serve an estimated 1.5 million people in the state[26].
2.2. Study design
This was a retrospective cohort study using a routinely collected data from the national surveillance system to analyse treatment default and death among registered adult patients who received TB treatment in one tertiary (public) and one secondary (private) health facility in Ebonyi State, Nigeria, between 1 January 2011 and 31 December 2012. The outcomes followed standard WHO definitions[1]. Treatment default was defined as the interruption of treatment for 2 consecutive months, whilst death was defined as death for any reason during treatment[1].
2.3. Ethical approval
This study was approved by the Ethics and Research Advisory Committee of the National Tuberculosis Control Programme, Ministry of Health, Ebonyi State, Nigeria.
2.4. Treatment regimen
All new TB patients diagnosed before 2012 received an 8-month anti-tuberculosis regimen consisting of 2RHZE/6EH[22]. In line with the recent WHO guideline, from 1st January 2012, the six-month regimen containing 6 months of rifampicin: 2RHZE/4RH was started[22-23]. Previously treated TB patients had a 3-month intensive phase treatment with the addition of streptomycin to RHZE in the first two months[22,23]. The treatment given is observed using the community-DOTS approach where a family member (DOTS supporter) and/or a volunteer community health worker living in the patient’s community observe the patient intake of their drugs. All HIV-infected TB patients are given trimethoprim/sulfamethoxazole (CPT) to prevent other opportunistic infections. HIV treatment follows national and WHO guidelines with antiretroviral therapyadministered between two weeks and two months of starting TB treatment[22,23].
2.5. Data analyses
Data from the 2011 and 2012 TB registers from the study sites were double-entered, checked, and analyzed using Epi Info 3.4.1 (CDC, Atlanta, GA USA). Data were analysed by frequencies and percentages for demographic, clinical characteristics and treatment outcomes. The main outcome variables, classified as dichotomous, were treatment default (Yes versus No), and death (Yes versus No). Explanatory variables were gender, age, residence, patient classification (new versus retreatment), treatment regimen (2RHZE/6EH versus 2RHZE/4RH), facility (public versus private), HIV status, ART use and CPT use. Univariate analyses (Chisquare andFisher’sexact tests) were used to determine significant associations between each of the outcome variables and the explanatory variables. We performed a stratified analysis to determine the occurrence of interaction and confounding between each outcome variable (default, and death) and the explanatory variables. Multivariable logistic regression models were then constructed, including all variables of clinical importance and all with univariate P<0.25. A backward elimination approach was used to find the best model. P<0.05 were considered statistically significant
3. Results
3.1. Study population
During the study period, 1668 TB patients registered for treatment at the study sites. About 64% (1065) were 40 years old or less and 963 (57.7%) were male. Also, 1 180 (70.7%) resided in a rural area, and 1 390 (83.3%) of them received care at the rural facility (Table 1). Overall, the treatment default rate was 157 (9.4%), whilst 165 (9.9%) of the patients died. Patients who defaulted had a mean (SD) age of 41.4 (16.1) years while non-defaulters had a mean age of 38.2 (14.6 years); P=0.02. Also, patients who died during treatment had a mean (SD) age of 41.3 (14.8) compared to a mean of 38.2 (14.7) years among those who survived; P=0.003. Of the 157 patients who defaulted; 134 (85.4%) had pulmonary tuberculosis, 100 (63.7%) were rural resident, 91 (58%) were male, 97 (61.8%) received the longer treatment (2RHZE/6EH) regimen, 123 (78.3%) were HIV-infected, and 115 (73.2%) received care in the private facility. Also, of the 165 patients who died during treatment, 100 (60.6%) were male, 129 (78.2%) were rural residents, 94 (57%) received the longer anti-TB (2RHZE/6EH) regimen, 150 (90.9%) registered as new patients, 100 (60.6%) were HIV-negative, and 122 (73.9%) received care at the private facility.
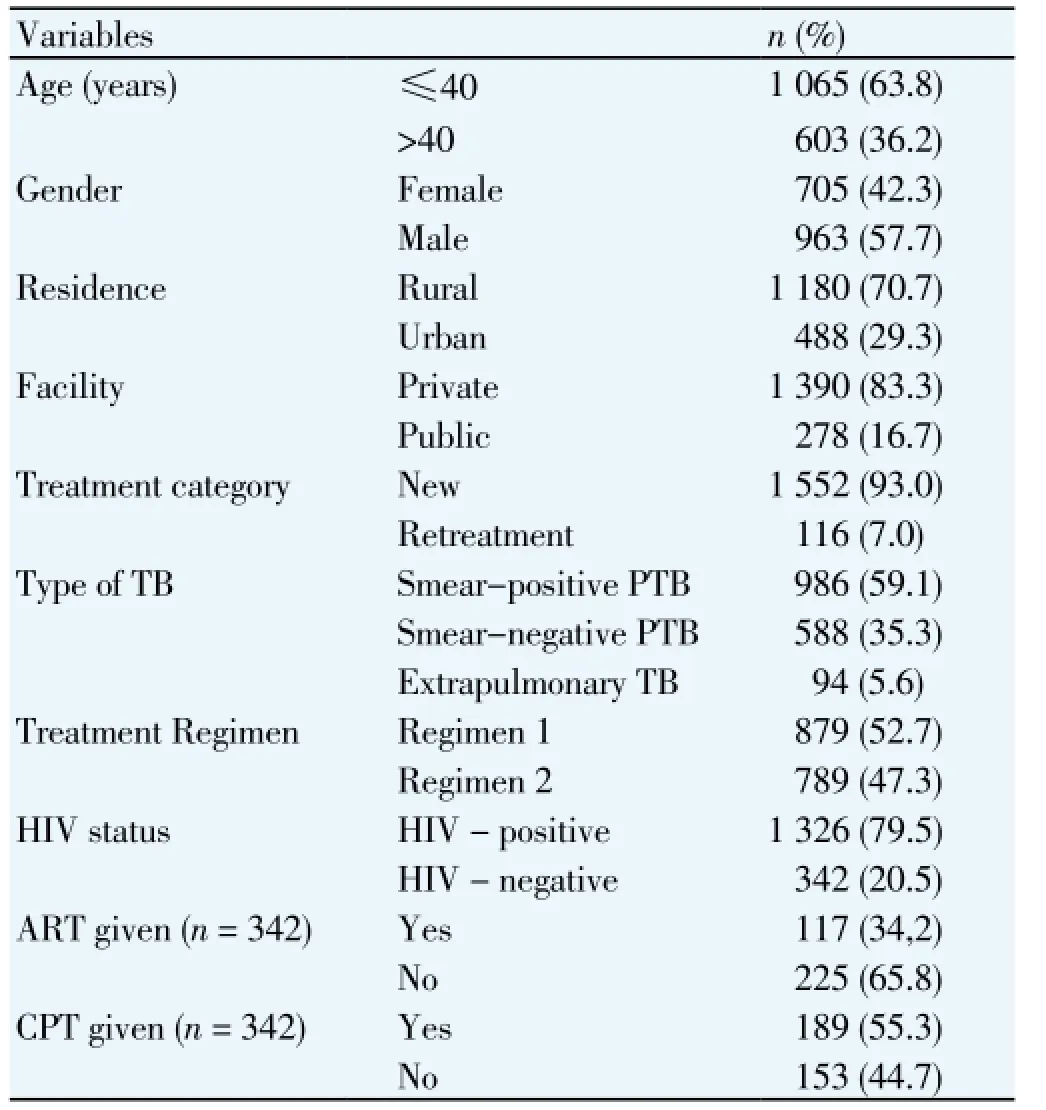
Table 1 Demographic and clinical profile of 1668 TB patients, Nigeria, 2011 - 2012.
3.2. Duration of treatment before default and death
The rate of treatment default for patients receiving both 6 and 8-month regimes was high during the early months of treatment and generally decreased each consecutive month (Figure 1). Of 157 patients who defaulted during treatment, 16 (10.2%) and 40 (25.5%) abandoned treatment during the first and second months respectively, thus 35.7% (56) defaulted during the intensive phase. Of the 60 patients on the 6-month regimen, 27 (45%) defaulted within the first two months of treatment higher than the 29.9% (29/97) of patients on the 8-month regimen; P=0.05 (Figure 1). Also, of the 165 patients who died during treatment, 109 (66.1%) and 42 (25.4%) died during the first and second months respectively, thus 151 (91.5%) died during the intensive phase. Of 71 cases on the 6-month regimen, 93.0% (66) died during the first two months of treatment comparable to 90.4% (85/94) of patients on the 8-month regimen; P=0.6 (Figure 2).

Figure 1. Proportion of defaulters by time of default (months) for both six (n = 60) and eight-month (n = 97) regimens in Nigeria, 2011-2012.
3.3. Univariate analyses of factors associated with treatment default and death among TB patients
Univariate analyses showed that residence, facility where care was given, type of TB and treatment regimen were significantly associated with treatment default (Table 2). Treatment default rates were higher among urban residents compared with rural residents (11.7%vs.8.5%; P=0.04); patients treated at the tertiary (public) hospital had higher rates of treatment default compared with patients managed at the private (mission) hospital (15.1%vs.8.3%; P< 0.001). Also, default rates were higher among patients with extrapulmonary TB compared with pulmonary TB (25.3%vs.8.3%; P<0.001); and patients treated using the longer anti-TB regimen had higher rates of treatment default compared with patients treated with the shorter regimen (11%vs. 7.6%; P=0.02). Death rates were significantly associated with rural residence (P=0.03), care at the public facility (P<0.001), smear-negative TB/extrapulmonary TB (P<0.001), and HIV-positive status (P<0.001) in univariate analysis (Table 3).
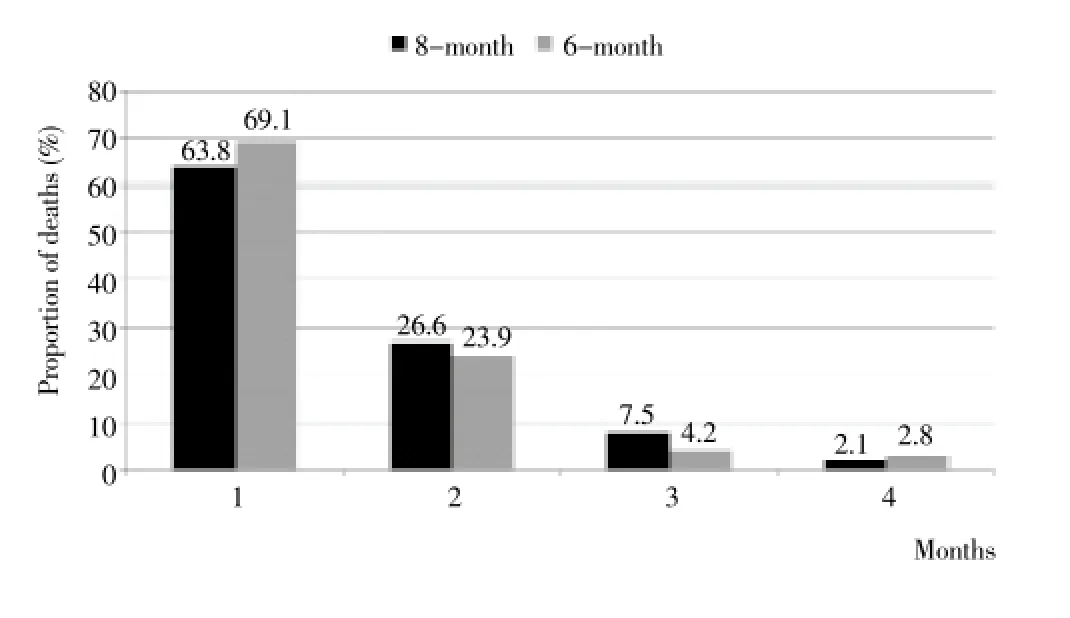
Figure 2. Proportion of death by time of death (months) for both six (n = 71) and eight-month (n = 94) regimens in Nigeria, 2011-2012.
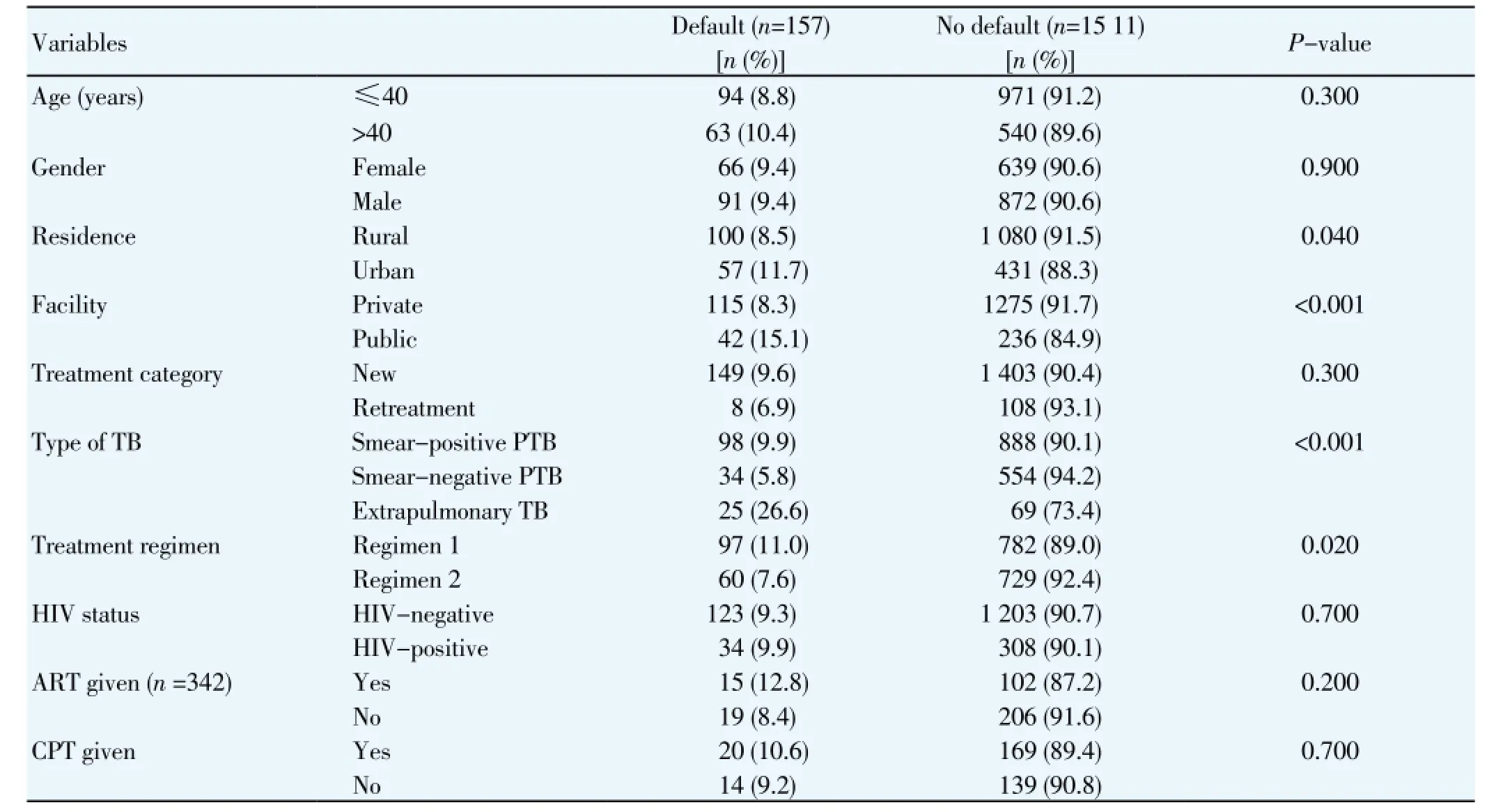
Table 2 Univariate analysis of factors associated with TB treatment default in Nigeria, 2011-2012.
3.4. Risk factors of treatment default and death among TB patients
The crude and adjusted ORs of treatment default determined by multivariable logistic regression analysis are shown in Table 4. The risk of default was higher in older patients [adjusted odds ratio (aOR) 1.2; 95% confidence interval (CI) 1.1 - 1.9)], smear-negative TB cases (aOR 2.3;CI1.5 - 3.6), extrapulmonary TB cases (aOR 2.7;CI1.3 -5.2), and patients who received the longer treatment regimen (aOR 1.6;CI1.1 - 2.2). Female gender, urban residence, patient classification, hospital where care was received, and HIV status and care did not predict treatment default. Also, in a multivariable logistic regression model (Table 4), having extrapulmonary TB was a strong predictor of death during treatment (aOR 3.0;CI1.4 - 6.1), as was rural residence (aOR 1.7;CI1.2 - 2.6), smear-negative TB (aOR 2.4;CI1.7 - 3.5), HIV co-infection (aOR 2.5;CI1.7 - 3.6), not receiving ART among HIV-infected TB patients (aOR 1.6;CI1.1 - 2.9), and not receiving CPT among HIV-infected TB patients (aOR 1.7;CI1.2 - 2.6).
4. Discussion
Reducing TB-related loss to follow-up and mortality during treatment in TB control programmes in low-resource, high-incidence settings that have fully implemented the DOTS strategy may require targeted interventions tailored to local needs[12]. In this study, we aimed to determine subpopulations of TB patients at risk for mortality or default in two large health facilities in Nigeria. We found that 9.4% of the patients treated for TB at the study sites defaulted from treatment and 9.9% died during treatment. The study showed treatment default to be associated with older age, smear-negative TB, extrapulmonary TB and receiving the 8-month antituberculosis regimen; and death to be associated with extrapulmonary TB, smear-negative TB, rural residence, HIV co-infection, not receiving ART, or CPT among TB/HIV patients.
More than one third of the patients who defaulted abandoned treatment too early after treatment was started. Thus, a substantial number of patients who had sputum smear-positive pulmonary TB may still have had positive smears at the time they discontinued treatment[12]. Early abandonment of treatment may lead to poor treatment outcomesie. treatment failure, death and the emergence of drug resistant TB. In contrast to previous studies in Kenya, Uzbekistan and Nigeria[6,12,16], most patients in this study defaulted during the continuation phase of treatment. Our finding agrees with recent studies in Morocco, Moldova, Indonesia and a systematic review which suggested thatmost patients tend to default during the continuation phase of treatment in low-and middle-income countries[9-11,21]. Higher default rates during the continuation phase of treatment have been suggested to be due to ongoing direct and indirect costs of care and symptom resolution[10,11,21]. More intensive patient education especially during the second to the fourth month of treatment and possibly economic support are needed to reduce treatment default

Table 3 Univariate analysis of factors associated with death during TB treatment, Nigeria, 2011-2012.
The risk of default and death increased in patients with smear-negative TB and extrapulmonary TB. These clinical factors have been associated with death in several studies[13-19]. This may be due to increased co-morbidities associated with smear-negative TB and extrapulmonary TB. It may also be that some of these cases had other diseases and were misdiagnosed and treated for TB resulting in poor outcomes. However, these have been reduced through the use of standardised algorithms in making a diagnosis of smearnegative TB; and histology for extrapulmonary TB[22,23]. Use of molecular diagnostics techniques (for example Gene Xpert MTB/RIF) may enhance early and appropriate diagnosis of smear-negative and extrapulmonary TB in low-income settings and should be scaled-up in these settings. The reason why patients with smear-negative and extrapulmonary TB patients had higher default rates is not clear. Thus, special care for such patients is therefore necessary for a successful control programme.
We found that older age was associated with an increased risk of default. This observation has been suggested to be due to increasing co-morbidities and general physical disability associated with old age[27]. Older age has been shown to increase default rates in other settings. In China, older patients were more likely to default than younger ones; and in northeastern Thailand, default rates were higher in older individuals[27,28]. Also, our finding on the protective effect of the shorter anti-tuberculosis regimen against default is reassuring and have confirmed previously held belief that shorter treatment regimen could reduce default rates[21]. Interventions to lower default rates in low-income settings are likely to include special care for older patients and sustained use of shorter treatment regimen.
Patients residing in a rural area had a higher risk of death. The health system in high burden countries into which TB control is fully integrated suffers from lack of human resources and limited outreach services for therural population - this is particularly seen in rural areas of Ethiopia, Indonesia, Pakistan and Nigeria[6]. Also, the poorer socioeconomic status of rural residents may constitute a hindrance in their ability to access health services[8,29,30]. The reasons why rural residents TB patients had higher risk of death needs to be evaluated in further studies.
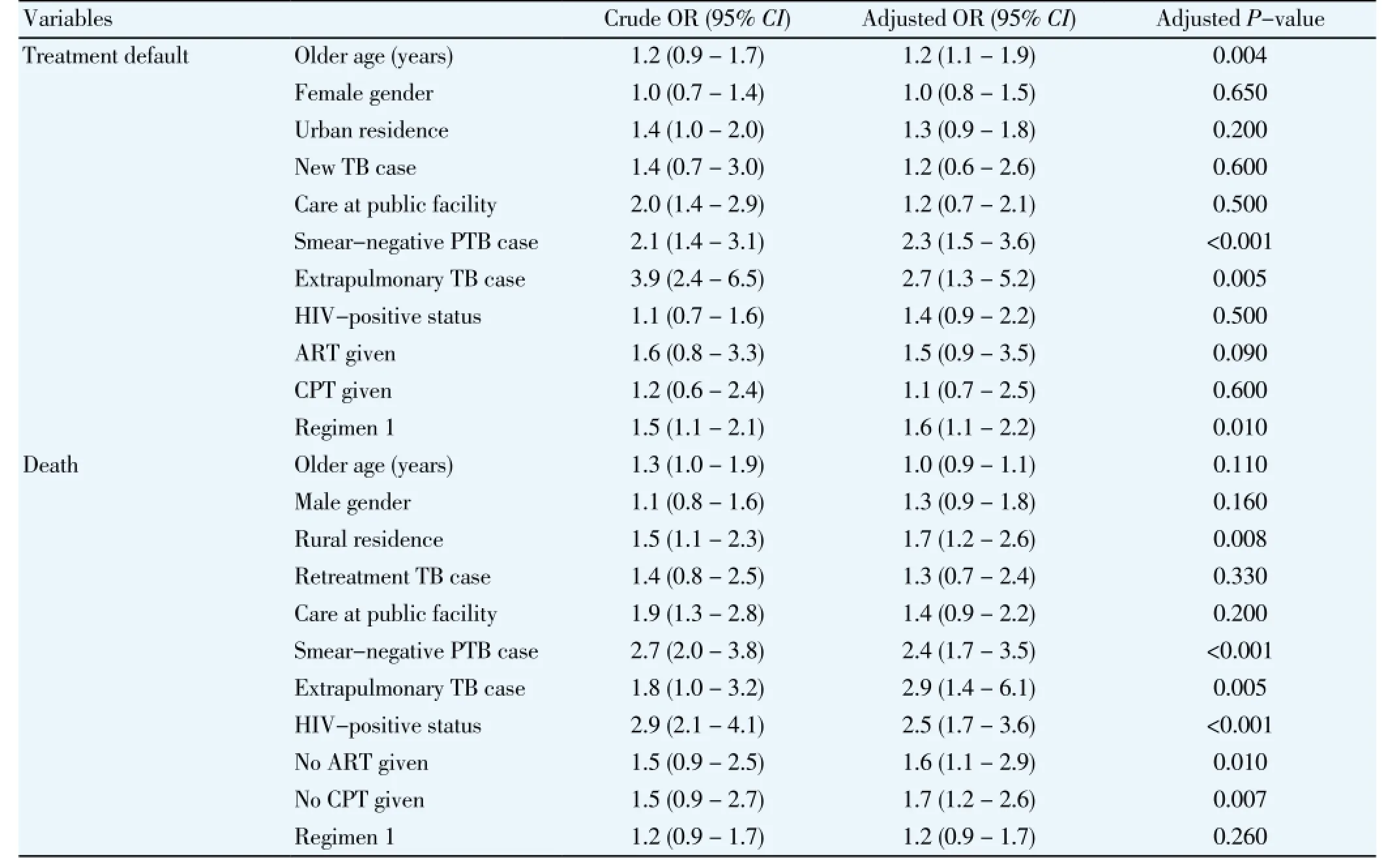
Table 4 Multivariable logistic regression analysis of factors associated with treatment default and death among TB patients, Nigeria, 2011-2012.
Consistent with previous studies[17-20], HIV co-infection increased the risk of death. However, these deaths may be due to other opportunistic infections as have been shown by postmortem studies[20,31]. In individuals with early stages of HIV, the effects of TB co-infection are most detrimental as they have accelerated decline in immune function resulting in high all-cause mortality even after the completion of anti-tuberculosis treatment[20]. Cotrimoxazole prophylaxis and antiretroviral therapy use are two very important interventions to reduce mortality in HIV-infected TB patients[32,33]. Although the medium to long term benefits of cotrimoxazole preventive therapy and antiretroviral therapy have been demonstrated in TB/HIV patients, data so far show that none of these interventions significantly decrease death in the early months of anti-tuberculosis treatment[20,34]. In our study, more than 90% of TB/HIV deaths in patients who received either the shorter or longer anti-TB regimen occurred within the first two months of treatment. Therefore, identifying the clinical and biochemical determinants of death in the first two months of treatment among HIV-infected TB patients in high-burden countries is crucial for a successful control programme and needs to be further explored.
Although this study provides important information for the improvement of TB treatment outcome in Nigeria, our analysis is limited by the use of retrospective data available from TB registers. The registers used did not contain information on patient income, employment status, alcohol consumption, tobacco use, malnutrition, co-morbidities, patients’ knowledge of TB, distance to health facility, and patients’ perception of TB services. For HIV-infected patients, there were no information on combination of drugs received and CD4 count. These factors have been found to predict default and/or death in other settings[7-20,28]. Future quantitative and qualitative studies on how and why default occurs and what interventions may be most effective may improve upon these limitations.
We conclude that treatment default and death remains a barrier to successful TB control in Nigeria; and it is driven by many remediable factors. Interventions that provide special care for older patients and patients with smearnegative and extrapulmonary TB patients may help to reduce default rates in this and similar settings. Furthermore, patients with smear-negative TB and extrapulmonary TB, rural residents, HIV co-infection require special attention to reduce mortality in this and similar settings; and collaborative TB/HIV activities including the use of cotrimoxazole prophylaxis needs to be scaled-up among TB/ HIV co-infected individuals to improve treatment outcomes.
Conflict of interest statement
All authors declare that there are no competing interests to declare
Acknowledgments
We acknowledge all the Staff of the National Tuberculosis Control Programme, Ebonyi State, the Centre for Development and Reproductive Health (CDRH) and all health workers who participated in the meticulous data collection and reporting for their contributions
[1] World Health Oragnization. Definitions of tuberculosis cases and treatment outcomes. Geneva, Switzerland: World Health Organization, 2013.
[2] Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005; 171(12): 1430-1435.
[3] O’Brien JK, Sandman LA, Kreiswirth BN, Rom WN, Schluger NW. DNA fingerprints from Mycobacterium tuberculosis isolates of patients confined for therapy noncompliance show frequent clustering. Chest 1997; 112(2): 387-392.
[4] Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med 1994; 330(17): 1179-1184.
[5] Federal Ministry of Health, Nigeria. National TB and leprosy control program, National drug-resistant tuberculosis prevalence survey. Abuja, Nigeria: Federal Ministry of Health, 2012.
[6] World Health Organization. Global tuberculosis control: WHO report 2013. Geneva, Switzerland: World Health Organisation 2013
[7] Ifebunadu NA, Ukwaja KN. Tuberculosis treatment default in a large tertiary care hospital in urban Nigeria: Prevalence, trend, timing and predictors. J Infect Public Health 2012; 5(5): 340-345.
[8] Ibrahim LM, Hadejia IS, Nguku P, Dankoli R, Waziri NE,Akhimien MO, et al. Factors associated with interruption of treatment among pulmonary tuberculosis patients in Plateau State, Nigeria. 2011. Pan Afr Med J 2014; 17: 78.
[9] Tachfouti N, Slama K, Berraho M, Elfakir S, Benjelloun MC, El Rhazi K, et al. Determinants of tuberculosis treatment default in Morocco: results from a national cohort study. Pan Afr Med J 2013; 14: 121.
[10] Rutherford ME, Hill PC, Maharani W, Sampurno H, Ruslami R. Risk factors for treatment default among adult tuberculosis patients in Indonesia. Int J Tuberc Lung Dis 2013; 17(10): 1304-1309.
[11] Jenkins HE, Ciobanu A, Plesca V, Crudu V, Galusca I, Soltan V, et al. Risk factors and timing of default from treatment for nonmultidrug-resistant tuberculosis in Moldova. Int J Tuberc Lung Dis 2013; 17(3): 373-380.
[12] Muture BN, Keraka MN, Kimuu PK, Kabiru EW, Ombeka V, Oguya F. Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: a case control study. BMC Public Health 2011; 11: 696.
[13] Garrido Mda S, Penna ML, Perez-Porcuna TM, de Souza AB, Marreiro Lda S, Albuquerque BC, et al. Factors associated with tuberculosis treatment default in an endemic area of the Brazilian Amazon: a case control-study. PLoS One 2012; 7: e39134.
[14] Sendagire I, Schim Van der Loeff M, Kambugu A, Konde-Lule J, Cobelens F. Urban movement and alcohol intake strongly predict defaulting from tuberculosis treatment: an operational study. PLoS One 2012; 7: e35908.
[15] Vijay S, Kumar P, Chauhan LS, Vollepore B H, Kizhakkethil UP, Rao SG. Risk factors associated with default among new smear positive TB patients treated under DOTS in India. PLoS One 2010; 5: e10043.
[16] Hasker E, Khodjikhanov M, Usarova S, Asamidinov U, Yuldashova U, Van der Werf MJ, et al. Default from tuberculosis treatment in Tashkent. Uzbekistan; who are these defaulters and why do they default? BMC Infect Dis 2008; 8: 97.
[17] Nahid P, Jarlsberg LG, Rudoy I, de Jong BC, Unger A, Kawamura LM, et al. Factors associated with mortality in patients with drugsusceptible pulmonary tuberculosis. BMC Infect Dis 2011; 11: 1.
[18] Burton NT, Forson A, Lurie MN, Kudzawu S, Kwarteng E, Kwara A. Factors associated with mortality and default among patients with tuberculosis attending a teaching hospital clinic in Accra, Ghana. Trans R Soc Trop Med Hyg 2011; 105(12): 675-682.
[19] Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis 2011; 15(7): 871-885.
[20] Horne DJ, Hubbard R, Narita M, Exarchos A, Park DR, Goss CH. Factors associated with mortality in patients with tuberculosis. BMC Infect Dis 2010; 10: 258.
[21] Kruk ME, Schwalbe NR, Aguiar CA. Timing of default from tuberculosis treatment: a systematic review. Trop Med Int Health 2008; 13(5): 703-712.
[22] World Health Organisation. Treatment of tuberculosis guidelines. Fourth Edition. Geneva, Switzerland: World Health Organization; 2010.
[23] Federal Ministry of Health, Nigeria. National Tuberculosis and Leprosy Control Programme: workers manual. Revised 5th ed. Abuja, Nigeria: Federal Ministry of Health, 2010.
[24] National Population Commission. The 2006 population and housing census of the Federal Republic of Nigeria. Priority Tables (Volume I). Abuja, Nigeria: The National Population Commission; 2009.
[25] Ukwaja KN, Alobu I, Ifebunandu NA, Osakwe PC. Trend in case detection rate for all tuberculosis cases notified in Ebonyi, Southeastern Nigeria during 1999 - 2009. Pan Afr Med J 2013; 16: 11.
[26] Ukwaja K, Alobu I, Ifebunandu N, Osakwe C, Igwenyi C. From DOTS to the stop TB strategy: DOTS coverage and trend of tuberculosis notification in Ebonyi, southeastern Nigeria, 1998-2009. Pan Afr Med J 2011; 2: 12.
[27] Abuaku B, Tan H, Li X, Chen M, Huang X. Treatment default and death among tuberculosis patients in Hunan, China. Scand J Infect Dis 2010; 42(4): 281-287.
[28] Anunnatsiri S, Chetchotisakd P, Wanke C. Factors associated with treatment outcomes in pulmonary tuberculosis in northeastern Thailand. Southeast Asian J Trop Med Public Health 2005; 36(2): 323-330.
[29] Ukwaja KN, Modebe O, Igwenyi C, Alobu I. The economic burden of tuberculosis care for patients and households in Africa: a systematic review. Int J Tuberc Lung Dis 2012; 16(6): 733-739.
[30] Ukwaja KN, Alobu I, Nweke CO, Onyenwe EC. Healthcareseeking behavior, treatment delays and its determinants among pulmonary tuberculosis patients in rural Nigeria: A crosssectional study. BMC Health Serv Res 2013; 13: 25.
[31] Martinson N A, Karstaedt A, Venter WD, Omar T, King P, Mbengo T, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS 2007; 21(15): 2043-2050.
[32] Suthar AB, Granich R, Mermin J, Van Rie A. Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Organ 2012; 90(2): 128C-138C.
[33] Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr 2006; 43(1): 42-46.
[34] Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362(8): 697-706.
ment heading
10.1016/S1995-7645(14)60172-3
*Corresponding author: Dr. Kingsley N. Ukwaja, Department of Internal Medicine, Federal Teaching Hospital, Abakaliki, Ebonyi State, Nigeria.
Tel: +234 08036243196
E-mail: ukwajakingsley@yahoo.co.uk
This research was supported through an operational research writing grant, which is a component of the Wave 3 TBREACH grant from the WHO/ Stop TB Partnership with funds from the Canadian International Development Agency (CIDA).
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Assessment of traumatic brain injury degree in animal model
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
