Therapeutic effect of Captopril on rheumatoid arthritis in rats
Hong-Mei Liu, Kai-Jie Wang
Tangshan Clinic Medicine College of Hebei Medical University, Tangshan, Hebei, 063000, China
Therapeutic effect of Captopril on rheumatoid arthritis in rats
Hong-Mei Liu*, Kai-Jie Wang
Tangshan Clinic Medicine College of Hebei Medical University, Tangshan, Hebei, 063000, China
Objective:To investigate the therapeutic effect of the intervention treatment with different doses of Captopril on TNF-αcontents in serum of rheumatoid arthritis (RA) rats, and to provide the theoretical proofs for clinical application of Captopril in treatments of rheumatoid diseases.
Methods:Fifty Wistar rats were randomly divided into 5 groups, namely, Group A, Group B, Group C, Group D, Group E with 10 rats in each group. Injection of Freund’s complete adjuvant was employed to establish adjuvant-induced arthritis model in rats. Group A was model group; after model establishment, rats were treated with 20 mL normal saline as placebo (ip.). Rats in Group B were treated with 8 mg/kg cyclophosphamide (ip.). Rats in Group C, D and E were intraperitoneally injected with 30 mg/kg, 100 mg/kg and 300 mg/kg Captopril respectively. Rats in each group were subjected to continuous treatment for 3 weeks, and then sacrificed. Eyeballs of rats were excised and blood was collected. TNF-αcontent in serum were detected using ELISA; each group rats were compared for the hind legs arthrocele. Right ankle tissues of rats were collected to prepare section, and microscopic observation of pathological changes was performed.
Results:TNF-αcontent in serum of Group A rats was significantly higher than that of rats in other 4 groups (P<0.05). TNF-αcontent in serum of Group B rats was significantly lower compared with that of rats in Groups C, D and E. The highest TNF-αcontent in serum of rats treated with Captopril was found in Group C, followed by Groups D and E (P<0.05). Right ankle arthrocele of rats in Groups B, C, D and E in early stage showed no statistical difference compared with that of Group A rats (P>0.05). From Day 8, ankle arthrocele of rats in Groups B, C, D and E was obviously relieved compared with that of Group A rats; the anti-inflammatory effects were gradually enhanced with the extension of medication time. Treatments of Groups C, D and E showed significant activities against tardive arthrocele; the degree of ankle arthrocele in rats of these three groups was lower than that of Group A rats (P<0.01). Histological observation showed that large amount of inflammatory cells and plasmocyte infiltration was found in ankle synovial tissues of Group A rats. Relief of hyperaemia and edema of right ankle synovial tissues as well as significant decrease in synoviocyte layer hyperplasia, intra-articular inflammatory cells infiltration and cartilago articularis damage degree etc. were observed in Groups B, C, D and E.
Conclusions:Intervention treatment with Captopril can effectively reduce the TNF-αcontent in serum of rheumatoid arthritis rats and inhibit the generation of inflammatory factors, so as to achieve the therapeutic effect.
ARTICLE INFO
Article history:
Received 24 September 2014
Received in revised form 10 October 2014
Accepted 15 November 2014
Available online 20 December 2014
Captopril
1. Introduction
Rheumatoid arthritis (RA) is the common clinical disease with attack in arthrosis of arms and legs; it is a autoimmune disease with joint synovitis as the major clinical characteristic[1-3]. RA mainly invades joint synovium, and it can also influence visceral organs like heart, lung, kidney, artery and nerve etc. The clinical manifestations of RA usually include symmetrical, polyarticular and chronic inflammatory lesions; the main pathological changes include hyperplasia, expansion and hyperaemia of synoviocytes, inflammatory cell infiltration as well as degeneration and proliferation of fibrous tissuesetc[4,5]. Currently, the pathogenesis of RA has not been completely clinically confirmed yet. Some scholars believe that the attack of RA is related to infection, immunoregulation and heredity[6-9]. In addition, some other researchers thinkthat the attack of RA is related to immunodisorder of body fluid[10-12]. The immunodisorder of body fluid causes mass generation of immune complexes, such as rheumatoid factor, which damage the hyaline cartilage and fiber, leading to the destruction of cartilage and bone. Therefore, cytokines play an important role during the pathogenic process of RA. A study verified that TNF2A plays a particularly important role in different stages of RA synovitis as well as development of articular substrate degeneration[13]. Besides, TNF2A is considered as the key cytokine to regulate the express of other proinflammartory cytokines during the course of RA. Captopril is an angiotensin converting enzyme inhibitor with effects of reducing bradykinin degradation and inhibiting synthesis of angiotensin II. Captopril can significantly alleviate tissue edema and inflammatory exudation, possessing anti-inflammatory and anti-fibrosis activities. It is proven in a study that Captopril have, to some extent, therapeutic effects on rheumatoid disease. In order to investigate the therapeutic effects and mechanism of Captopril against rheumatoid arthritis, the present study used Wistar rats to establish RA model and give intervention treatment with Captopril. The results are reported as follows.
2. Materials and methods
2.1. Experimental animals
50 healthy adult Wistar rats of clean grade with either sex, weighting 150-200 g with average weight of (175±12) g, from experimental center of Hebei Medical University were selected. Rats had access to water ad arbitrium and treated with class II feeding. Handle of experimental animals during the experiments strictly followed the relevant provisions of Regulation for the Administration of Affairs Concerning Experimental Animals.
2.2. Apparatus and reagents
Olympus optical microscope and biomicroscope (Olympus, Japan) were used. Electronic analytical balances produced by Shanghai Balance Instrument, LD5-10 centrifuge produced by Beijing Centrifuge Factory and microplate reader (TECAN, Austria) were used. Tumor putrescence factor--α kit was purchased from Boster Biological Technology., LTD, and Captopril was purchased from Guangzhou Bai Yun Shan Pharmaceutical General Factory. 25% Urethane was provided by Beijing Zhongshan Golden Bridge Biotechnology Co., LTD; sodium carboxymethylcellulose and cyclophosphamide were provided by Shanghai Chemical Reagent Factory and Freund’s adjuvant was provided by Beijing Guoding Biotechnology Co., LTD.
2.3. Model establishment
Injection of Freund’s adjuvant was employed to establish RA model. Rats were injected with 0.05 mL Freund’s adjuvant by microsyringe in the posterior part of right hind limb. After injection, rats cannot walk by right hind feet, behaving restlessly. After 18 h, evident ankle swelling of right hind limb was observed; rats shown slow walk and decrease in taking food. Three days after injection, ankle swelling of right hind limb was alleviated, but became serious on Day 8, manifesting ankle swelling of left hind limb which was not injected with Freund’s adjuvant. Around 10 days later, multi-arthritis was observed in the rats, indicating the successful establishment of RA model.
2.4. Animal grouping
Fifty Wistar rats were randomly divided into 5 groups, namely, Group A, Group B, Group C, Group D, Group E with 10 rats in each group. Group A was model group; after model establishment, rats were treated with 20 mL normal saline as placebo (ip.). Rats in Group B were treated with 8 mg/ kg cyclophosphamide (ip.). Rats in Group C, D and E were intraperitoneally injected with 30 mg/kg, 100 mg/kg and 300 mg/kg Captopril respectively. Rats in each group were subjected to continuous treatment for 3 weeks.
2.5. Observation of the experimental animals
Displacement of left and right ankle of rats in each group was measured one hour before model establishment, 18 h after modeling and every other day after model establishment; the difference of displacement before and after inflammation indicates the degree of articular swelling. After experiments, rats were sacrificed by injecting with 2% pentobarbital sodium (ip.). Eyeballs of rats were excised and blood was collected. TNF-αcontent in serum were detected using ELISA, and detection procedures were performed strictly according to the operation instruction of reagents. Right ankle tissues of rats were collected to prepare section, stained by HE and microscopically observed for pathologic changes such as ankle synovial tissues proliferation, inflammatory cellular infiltration, destruction of cartilage and bone, and tissue edema around arthrosis.
2.6. Statistical analysis
The data obtained were analyzed by statistical software SPSS 17.0. Data were expressed as mean±SD. One-way analysis of variance was employed for comparison among groups.t-test was used and P<0.05 was considered to be statistically significant.
3. Results
3.1. Comparison of arthrocele in rats of each group at different time points
18 h after injection of adjuvant, evident ankle swelling was observed in Group A rats. Three days after injection, swelling was alleviated, but became serious on Day 8, and degree of arthrocele increased with the expansion of time. No arthrocele was observed in rats of Groups B, C, D and E 3 days prior to treatment, showing no statistical differencecompared with rats in Group A (P>0.05). However, 10 days after treatment, arthrocele in rats of Groups B, C, D and E was significantly relieved compared with rats in Group A (P<0.05) . There was no statistical difference among Groups B, C, D and E regarding arthrocele after treatment (P>0.05) (Table 1).
3.2. Comparison of TNF-αcontent in serum of each group rats in the early stage of treatment
TNF-αcontent in serum of Groups A, B, C, D and E rats at 21 d after modeling was (370.57±45.40), (305.71±26.37), (327.67±39.72), (318.71±24.02), and (314.10 ±29.18) ng/mL, respectively. TNF-αcontent in serum of Group A rats was significantly higher than that of other 4 group rats (P<0.05). TNF-αcontent in serum of Group B rats was significantly lower than that of Groups C, D and E (P<0.05). Decrease of TNF-αcontent in serum of Groups C, D and E rats showed a dose-dependent manner, namely, the higher dose, the lower TNF-αcontent in serum.
3.3. Histological changes
By microscopic observation, large amount of plasmocyte and inflammatory cell infiltrations were found in the right ankle synovial tissues of Group A rats. Relief of hyperaemia and edema of right ankle synovial tissues as well as significant decrease in synoviocyte layer hyperplasia, intra-articular inflammatory cells infiltration and cartilago articularis damage degree etc. were observed in Groups B, C, D and E. In regard to Groups C, D and E rats treated with Captopril, Group E rats showed the minimum ankle damage, followed by Group D, and Group C showed comparatively the most serious damage among the Captopril treated groups (Figure 1).
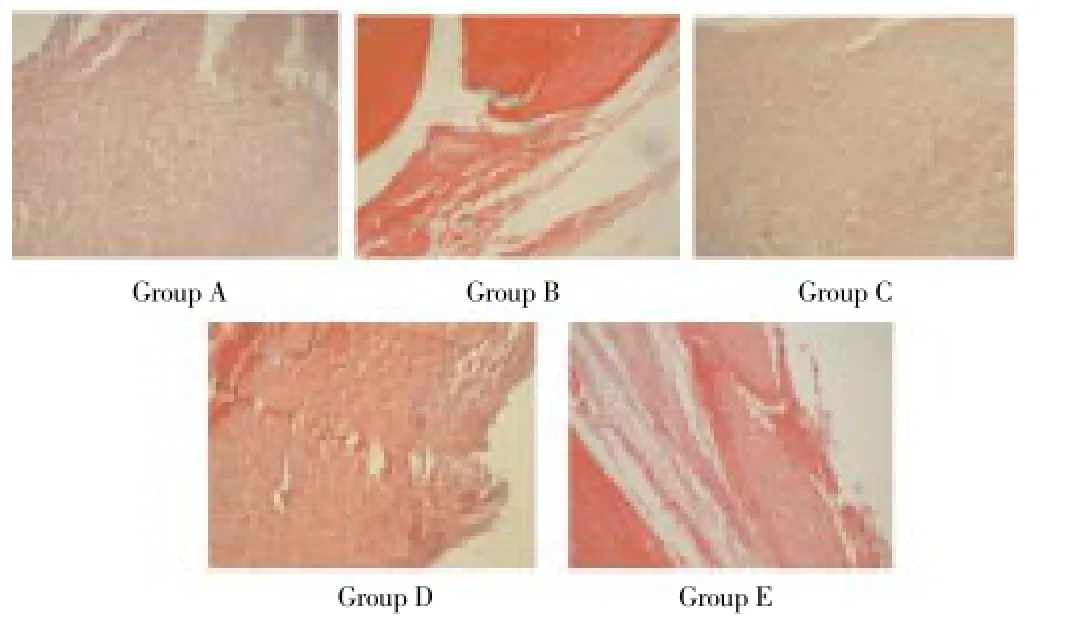
Figure 1. Observation for histological changes (HE, ×400).

Table 1 Comparison of arthrocele in rats of each group at different time points.
4. Discussion
RA, a common clinical disease with morbidity of about 1% worldwide, is the autoimmune disease characterized by disabling multi-joint synovitis[15]. The main pathologic change of RA is synovitis, manifesting mass trichoid protuberance on the surface of synovial membrane and generation of new vessels. Hence pannus is formed, damaging the bone and cartilage tissue[16]. The main clinical symptoms of RA include articular dyskinesia, swell and pain, and morning stiffness etc. During the late stage, RA can involve lung, heart, gastrointestinal tract, kidney, nervous system and blood system, causing multiple organs damage [17]. RA showed significant difference of course of disease, severity of disease and prognosis among different patients. If RA cannot be treated timely, it will aggravate gradually[18]. Therefore, early diagnosis and treatment of RA are significant for prognosis of patients. The present study adopted injection of Freund’s adjuvant to prepare RA model. Observations such as evident swelling of right hind limb ankle at 18 h after injection, aggravated swelling 8 days later and multiple arthritis observed around 10 days later indicate that this method for model establishment is fast and effective.
Currently, the pathogenesis of RA has not been completely clinically confirmed yet, but with the development of molecular biological technology, many researchers gradually realized the importance of cytokines and T lymphocyte subpopulation during the morbidity of RA[19]. A study stated that TND2A played a very significant role in the cytokine network and development of RA[20]. TNF-αis the key cytokine to regulate the express of other proinflammartory cytokines; it resists against TNF-αantibody and at the same time inhibit IL-1, IL-6 and GM-CSF produced by synoviocytes, thus reducing the diseased joint inflammatory reaction[21-22]. Captopril is an angiotensin converting enzyme inhibitor with effects of reducing bradykinin degradation and inhibiting synthesis of angiotensin II; it can lower pulmonary artery hyperpiesia and prevent pathological reproduction of angiocarpy. A study shown that Captopril possesses remarkable activities of scavenging oxygen free redicals and inhibiting lipid peroxidation etc. It can also reduce vascular permeability, tissue edema and inflammatory exudation, having anti-inflammatory and anti-fibrosis effects tosome extent[23]. Another research confirmed that Captopril can effectively decrease the TNF-αlevel in RA patients, inhibiting their inflammatory reaction[24]. In the present study, Groups C, D and E rats were treated with different doses of Captopril, and the TNF-αlevel was significantly lower compared with that of Group A rats (P<0.05). This verified that Captopril can indeed effectively decrease the TNF-αlevel in RA patients, and the effect was dosedependent, namely, the higher dose, the lower TNF-αlevel in serum. Results of the present study showed that the ankle swelling of rats treated with Captopril was better than that of Group A rats (P<0.05). Histological observation revealed relief of hyperaemia and edema of right ankle synovial tissues as well as significant decrease in synoviocyte layer hyperplasia, cartilago articularis damage degree and intraarticular inflammatory cells infiltration in rats treated with Captopril. The ankle injury of rats with treatment of Captopril was significantly alleviated compared with Group A rats, indicating the remarkable inhibitory activity of Captopril against secondary foot edema in RA rats. Captopril can effectively inhibit the arthritic damage, revealing certain anti-inflammatory and antirheumatic effects of Captopril. The activity of reducing the TNF-αlevel in serum may be responsible for anti-inflammatory and antirheumatic effects exhibited by Captopril.
Results of the present study showed that Captopril possess evident anti-inflammatory and antirheumatic effects. Treatment with Captopril can significantly reduce the TNF-αlevel in serum of RA rats, therefore inhibiting the production of inflammatory factor and decreasing its damage to joint.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012; 481(7381): 278-286.
[2] Gray DH, Kupresanin F, Berzins SP, Herold MJ, O’Reilly LA, Bouillet P, et al. The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity 2012; 37(3): 451-462.
[3] Ronnblom L, Aim GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol 2011; 23(2): 113-121.
[4] Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol 2012; 13(4): 333-342.
[5] Yu TY, Kong QX, Chen LZ, Liu BW. A brief analysis of rheumatoid arthritis pathomechanism. Heilongjiang Med J 2014; 27(1): 98-111.
[6] Inoue M, Williams KL, Oliver T, Vandenabeele P, Raj an JV, Miao EA, et al.Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal 2012; 5(225): ra38.
[7] Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS One 2011; 6(10): e27040.
[8] Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 2011; l7(6): 1359-13572.
[9] Guarda G, Braun M, Staehli F, Tardive 1 A, Mattmann C, Forster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011; 34(2): 213-223.
[10] Elkon KB, Wiedeman A. Type II FN system in the development and manifestations of SLE. Curr Opin Rheumatol 2012; 24(5): 499-505.
[11] Wang J, Wang Y, Guo XX. The diagnostic value of anti-cyclic citrullinated peptide antibody (CCP) for rheumatoid arthritis. China Foreign Med Treatment 2010; 29(8): 31.
[12] Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010; 32(3): 379-391.
[13] Ye S, Fang HJ, Tan J. Diagnosis and differential diagnosis of rheumatoid arthritis. J Navy Med 2013; 34(1): 51-53.
[14] Bauer C, Duewell P, Mayer C,Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010; 59(9): 1192-1199.
[15] Tong DH, Wang YL, Zhu P, Wu XL. The diagnostic value of anticyclic citrullinated peptide antibody and rheumatoid factor joint detection for rheumatoid arthritis. China Modern Doctor 2012; 50(34): 59-60.
[16] Guarda G, Zenger M, Yazdi A S,Schroder K, Ferrero I, Menu P, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol 2011; 186(4): 2529-2534.
[17] Zhu CM, Zhang HQ. Research process of laboratory diagnosis of rheumatoid arthritis. Chinese J Laboratory Diagnosis 2011; 15(4): 749-750.
[18] Allen IC, Tekippe EM, Woodford RT, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 2010; 207(5): 1045-1056.
[19] Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, et al. Necrotic cells trig-ger a sterile inflammatory response through the Nlrp3 in-flammasome. Proc Nat Acad Sci USA 2009; 106: 20388-20393.
[20] Shaw PJ, Mcdermott MF, Kanneganti T. Inflammasomes and autoimmunity. Trends Molecular Med 2011; 17(2): 57-64.
[21] Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 2011; 17(6): 1359-1372.
[22] Cai XH, Lv X, Qing ZJ. The diagnostic significance of IgMRF, IgG-RF, IgA-RF and anti-CCP on rheumatoid arthritis. Laboratory Med 2012; 27(12): 1066-1069.
[23] Ding JF. Diagnostic significance of anti-cyclic citrullinated peptide antibody (anti-CCP) and rheumatoid factor (RF) joint detection for rheumatoid arthritis. Gansu Yiyao 2014; 33(3): 212-214
[24] Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 2010; 8(6): 471-483.
ment heading
10.1016/S1995-7645(14)60175-9
*Corresponding author: Hong-Mei Liu, M.S., Associate Chief Physician, Tangshan Clinic Medicine College of Hebei Medical University, Tangshan, Hebei, 063000, China.
Tel: 13363206788
E-mail: liuhmt1972@163.com.
Foundation project: It is supported by Science and Technology Research and Development Program of Tangshan City, Grant Number: 07130233d.
Rheumatoid arthritis
TNF-α
Anti-itnflammatory effect
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Assessment of traumatic brain injury degree in animal model
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
- Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
