Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
Thirunavukkarasu Santhoshkumar, Abdul Abdul Rahuman*, Chidambaram Jayaseelan, Govindasamy Rajakumar, Sampath Marimuthu, Arivarasan Vishnu Kirthi, Kanayairam Velayutham, John Thomas, Jayachandran Venkatesan, Se-Kwon Kim
1Unit of Nanotechnology and Bioactive Natural Products, Post Graduate and Research Department of Zoology, C.Abdul Hakeem College, Melvisharam - 632 509, Vellore District, Tamil Nadu, India
2Centre for Nanobiotechnology, VIT University, Vellore 632 014. Tamil Nadu, India
3Department of Chemistry, Pukyong National University, Busan - 608 737, Republic of Korea
Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
Thirunavukkarasu Santhoshkumar1, Abdul Abdul Rahuman1*, Chidambaram Jayaseelan1, Govindasamy Rajakumar1, Sampath Marimuthu1, Arivarasan Vishnu Kirthi1, Kanayairam Velayutham1, John Thomas2, Jayachandran Venkatesan3, Se-Kwon Kim3
1Unit of Nanotechnology and Bioactive Natural Products, Post Graduate and Research Department of Zoology, C.Abdul Hakeem College, Melvisharam - 632 509, Vellore District, Tamil Nadu, India
2Centre for Nanobiotechnology, VIT University, Vellore 632 014. Tamil Nadu, India
3Department of Chemistry, Pukyong National University, Busan - 608 737, Republic of Korea
Objective:To determine the efficacies of antibacterial and antioxidant activities of aqueous leaf extract of Psidium guajava mediated biosynthesis of titanium dioxide nanoparticles (TiO2NPs).
Methods:Synthesized TiO2NPs were tested by disc diffusion method against against human pathogenic bacteria. The total antioxidant activity and phenolic content (Folin-Ciocalteau method) of synthesized TiO2NPs and aqueous plant extract were determined. The scavenging radicals were estimated by DPPH method. The synthesized TiO2NPs were characterized by XRD, FTIR, FESEM and EDX.Results:FTIR spectra of synthesized TiO2NPs exhibited prominent peaks at 3 410 cm-1(alkynes), 1 578 cm-1, 1 451 cm-1(alkanes), and 1 123 cm-1(C-O absorption). The morphological characterization of synthesized TiO2NPs was analysed by FESEM which showed spherical shape and clusters with an average size of 32.58 nm. The maximum zone of inhibition was observed in the synthesized TiO2NPs (20 μg/mL) against Staphylococcus aureus (25 mm) and Escherichia coli (23 mm). The synthesized TiO2NPs showed more antibacterial activity than the standard antibiotic disk, tetracycline which drastically reduces the chances for the development of antibiotics resistance of bacterial species. The plant aqueous extract and synthesized TiO2NPs were found to possess maximum antioxidant activity when compared with ascorbic acid. The content of phenolic compounds (mg/g) in leaf aqueous extract and synthesized TiO2NPs were found to be 85.4 and 18.3 mgTA/g, respectively.Conclusions:Green synthesized TiO2NPs provides a promising approach can satisfy the requirement of large-scale industrial production bearing the advantage of low-cost, eco-friendly and reproducible.
ARTICLE INFO
Article history:
Received 10 July 2013
Received in revised form 15 August 2014
Accepted 15 September 2014
Available online 20 December2014
TiO2nanoparticles
Psidium guajava
FTIR
Electron microscopy
Antibacterial activity
Antioxidant activity
1. Introduction
An important area of research in nanotechnology deals with the synthesis of nanoparticles of different chemical compositions, dimension and controlled monodispersity. Nanotechnology is emerging as a rapidly growing field with its application in science and technology for the purpose of manufacturing new materials at the nanoscale level[1]. Nanotechnology has gained massive applications in the fields of biology and pharmacology[2].
Nanomaterials are part of a commercial revolution that has resulted in an explosion of hundreds of new products due to their diverse physico-chemical properties, enabling their usage in a wide range of innovative applications[3,4]. To avoid the use of toxic organic solvents and severe reaction conditions (temperature, pressure, and long refluxingtime) for the preparation of nanomaterials, researchers recently have been exploring the possibilities of preparing nanomaterials in aqueous medium with the help of stabilizing or capping agents[5].
In recent years, titanium dioxide (TiO2) has been extensively used as an environmentally harmonious and clean photocatalyst, because of its optical properties, high chemical stability and nontoxicity[6,7]. Titanium dioxide nanoparticles (TiO2NPs) are one of the most important materials for cosmetics, pharmaceuticals[8], skin care wareses, particularly to protect skin from UV rays, whiteness, opacity to products such as paints, plastics, papers, inks, food colorants and toothpastes[9]. The current interest in the researchers due to the growing microbial resistance against metal ions, antibiotics and the development of resistant strains[10] and the TiO2NPs have demonstrated significant antibacterial activity[11]. Milleret al[12] reported that the TiO2generates reactive oxygen species when exposed to ultraviolet radiation, nanoparticulate TiO2used in antibacterial coatings and wastewater disinfection has been investigated as an anti-cancer agent. The biocidal polymer-functionalized TiO2NPs showed improved inhibition of bacterial growth againstEscherichia coli(E. coli), andStaphylococcus aureus(S. aureus) in comparison to the pristine TiO2NPs[13]. Zhang and Chen[14] reported that small Ag cluster size and the unique structure of TiO2NPs supporting highly dispersed to be the sources of superior bactericidal performance of the room temperature ionic liquids derived Ag/TiO2. A multifunctional multilayered film containing TiO2NPs as contact active antibacterial agent and nanosilver as a release-active antibacterial agent was fabricated via layer-by-layer assembly[15]. Marcianoet al[16] investigated the bactericidal activity of diamond like carbon films containing TiO2NPs and its action by oxidative damage to the bacteria wall, a decrease in the interfacial energy of bacteria adhesion causes an increase in the chemical interaction betweenE. coliand the films, which is an additional factor for increasing bactericidal activity. Rajakumaret al[17] reported that the biosynthesis of TiO2NPs was achieved usingAspergillus flavusextract as a reducing and capping agent which proved to be a good antibacterial material againstE. coli. Hassanet al[18] reported the synthesis and characterization of titania nanorods by sol-gel electrospinning technique and discussed the antibacterial activity and interaction mechanism againstS. aureus,E. coli,Salmonella typhimuriumandKlebsiella pneumoniae.
Antioxidant plays a crucial rule in terminating the oxidative rancidity in food by scavenging the free radical which is generated during oxidation process[19,20]. Generation of free radicals or reactive oxygen species during metabolism and other activities beyond the antioxidant capacity of a biological system gives rise to oxidative stress[21]. Oxidative stress plays a role in heart diseases, neurodegenerative diseases, cancer and in the aging process[22]. Oxidative stress is an emerging, general mechanism underlying nanoparticle toxicity[23,24]. Huet al[25] indicated that TiO2and zinc oxide nanoparticles could induce significant damage to earthworms due to their antioxidant effects. Crystalline, polyhedral rutile TiO2NPs were synthesized and reported for their reduction in cell viability, morphological alterations, compromised antioxidant system, intracellular reactive oxygen species production, significant DNA damage and potential of these NPs to induce cyto-and genotoxicity in cultured human amnion epithelial cells[26]. Nanocarbon black, C60 fullerene, nanoTiO2and nanosilica increased the activity of the antioxidant enzyme catalase and also stimulated glutathione transferase inMytilus galloprovincialis[27]. Daset al[28] reported the efficient antioxidant and bactericidal effect againstE. coliandPseudomonas aeruginosa(P. aeruginosa) by copper oxide nanoparticles.
In recent years, the biosynthetic method using plant extracts has received more attention than chemical and physical methods and even than the use of microbes, for the nano-scale metal synthesis due to the absence of any requirement to maintain an aseptic environment. New strategies are therefore needed to identify and develop the next generation of drugs or agents to control bacterial infections. Earlier authors reported that the TiO2NPs were synthesized fromAnnona squamosapeel extract[29],Catharanthus roseusleaf aqueous extract[30] andBacillus subtilis[31].
Guava is the common name of fruits ofPsidium guajava(P. guajava) (Myrtaceae) and spread to various parts of the tropical and subtropical areas.P. guajavaleaves are commonly used as popular medicine for diarrhoea which is also used for wounds, ulcers, rheumatic pain, and while they are chewed to relieve toothache[32,33].P. guajavahas been reported to have antidiarrheal[34], antibacterial[35], anti-inflammatory[36] and anticancer[37] activities. Galactose specific lectin isolated fromP. guajavafruit ripe was shown to bind withE. coli, preventing its adhesion to the intestinal wall and thus preventing infection resulting diarrhea[38]. Quercetin is a major flavonoid present inP. guajavaleaves and reported to have antidiarrhoeal activity[39].
Green synthesis of silver nanoparticles (Ag NPs) usingP. guajavaleaf extract showed better antibacterial properties than their chemical counterparts even though there was not much difference between their morphologies. FTIR analysis suggested the possible reduction of Ag+by the water soluble ingredients like tannins, eugenol and flavonoids in guava leaf[40]. Bashaet al[41] have reported that the synthesis of gold nanoparticles with guavanoic acid, a phytochemical ofP. guajavaand exhibited remarkable protein tyrosine phosphatase 1B inhibitory activity. Raghunandanet al[42,43] observed that the flavonoids isolated fromP. guajavaleaves were responsible for the biosynthesis of gold and Ag NPs. The antimicrobial activity of Ag NPs synthesized fromP. guajavashowed good activity againstE. coli,Bacillus cereusandCandida tropicalis[44].
The aim of the present study was to investigate theantibacterial activity of synthesized TiO2NPs using aqueous leaf extract ofP. guajavaagainst human pathogens. Hence, this process could be suitable for developing a biological process for mass scale production of nanoparticles. The synthesis of TiO2NPs was carried via biological reduction which provides suitable capping agent for the stability and viability of the synthesized nanoparticles and reported excellent antibacterial activity.
2. Materials and methods
2.1. Materials
Leaves ofP. guajavawere collected in and around the areas of Melvisharam, Vellore district, Tamil Nadu, India. TiO(OH)2was purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India.Aeromonas hydrophila(A. hydrophila) (MTCC-1739),Proteus mirabilis(P. mirabilis) (MTCC-442),E. coli(MTCC-1677),S. aureus(MTCC-3160) andP. aeruginosa(MTCC-4030) were obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India. 2, 2-diphenyl-1-picrylhydrazyl hydrate (DPPH), propyl gallate, sulphuric acid, sodium phosphate, ammonium molybdate, Folinciocalteau reagent, tannic acid, methanol, ascorbic acid were purchased from Merck, Mumbai, India. All the chemicals and reagents were used as received without further purification.
2.2. Synthesis of TiO2NPs using leaf aqueous extract of P. guajava
Aqueous leaf extract ofP. guajavawas prepared using freshly amassed leaves (20 g). They were surface cleaned with running tap water, followed by distilled water and boiled with 250 mL of double distilled water at 60 ℃ for 15 min. This extract was filtered through nylon mesh (spectrum), followed by millipore hydrophilic filter (0.22 μm) and used for further experiments. For synthesis of TiO2NPs, the Erlenmeyer flask containing 100 mL of TiO(OH)2(0.1 mM) was stirred for 2 h. Twenty mL of the aqueous extract ofP. guajavawas added in 80 mL of TiO(OH)2at room temperature under stirred condition for 24 h. The pure TiO(OH)2and aqueous leaf extract ofP. guajavadidn’t show any color change and there was no proof for the formation of nanoparticles. After the reaction ofP. guajavaextract with TiO(OH)2, the synthesized nanoparticles turned light green in color.
2.3. Characterization of synthesized TiO2NPs
X-ray diffraction (XRD) measurements of theP. guajavaleaf broth reduced TiO2NPs were carried out at 2θ in the range of 20-80 ℃ using Phillips®PW 1830 instrument (CuK α radiation, λ=1.540 6 Å), operated at 40 kV and 30 mA. FTIR analysis of the dried powder of synthesized TiO2NPs using Perkin elmer spectrum one spectrometer in attenuated total reflection mode and using spectral range of 4 000-400 cm-1with a resolution of 4 cm-1. For electron microscopic studies, 25 μL of sample was sputter coated on copper stub, and the images of nanoparticles were studied using FESEM (JSM-6700, JEOL, Japan). The fixed samples were coated with carbon and analyzed by energy dispersive X-ray (RONTEC’s EDX system, Model QuanTax 200, Germany).
2.4. Antibacterial test
The bacterial culture samples were lyophilized and suspended in nutrient broth with 0.5% sodium chloride at 37 ℃ for 24 h into the viable culture source. All these strains were grown in tryptic soy broth. The strains were grown aerobically at 37 ℃, with 10 mL of medium in 18-150 mm borosilicate glass culture tubes with shaking at 200 rpm under normal laboratory lighting conditions unless specified. Bacterial inoculums were prepared by growing a single colony overnight in nutrient broth and adjusting the turbidity to 0.5 McFarland standards. Mueller-Hinton agar plates were inoculated with this bacterial suspension; synthesized TiO2NPs (20 μg/mL) were added to a center well with a diameter of 8 mm. These plates were incubated for 15 min at 4 ℃ (to allow diffusion) and later on at 37 ℃for 24 h for the bacterial cultures. Positive test results were scored when a zone of inhibition was observed around the well after the incubation period. The zone of inhibition was measured by subtracting the well diameter from the total inhibition zone diameter[45]. Positive test results were scored when a zone of inhibition was observed around the well after the incubation period.
2.5. Total antioxidant activity
Total antioxidant activities of the samples of synthesized TiO2NPs and aqueous leaf extract were analysed according to the method of Prietoet al[46]. 100 mg of the synthesized TiO2NPs were taken into reaction vial and mixed with 0.05% DMSO. In brief, 0.1 mL aliquot of the sample was mixed with 1 mL of the reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and then incubated at 95 ℃ for 90 min. After samples were cooled to 25 ℃, the absorbance was measured at 695 nm against a blank. The blank contained 1 mL of the reagent solution without the sample. The total antioxidant activity was expressed as the absorbance of the sample. The higher absorbance value indicates the higher antioxidant activity. Ascorbic acid was also assayed for comparison.
2.6. Determination of total phenolics
The total phenolic content was determined by the Folin-Ciocalteau method with some modifications[47]. 1 g/10 mL of sample was filtered with Whatman no.1 paper. 0.5 mL of the sample was added to 2.5 mL of 0.2 N Folin-Ciocalteau reagents and placed for 5 min. 2 mL of 75 g/L of Na2CO3was then added to the total volume made upto 25 mL using distilled water. The above solution was then kept for incubation at room temperature for 2 h. Absorbance was measured at 760 nm using 1 cm cuvette in a perkin-elmer UV-vis lambda spectrophotometer. Tannic acid (0-800 mg/ L) was used to produce standard calibration curve. The total phenolic content was expressed in mg of tannic acid equivalents (TAE)/g of extract.
2.7. DPPH radical scavenging assay
The DPPH free radical scavenging assay was carried out by the method of Liyana-Pathiranan and Shahidi[48]. 1mL of each of different concentrations (100-500 mg in methanol) of the synthesized TiO2NPs and aqueous leaf extract was added to 1 mL of 0.135 mM DPPH in methanol solution. The reaction mixture left in the dark room at 30 min of room temperature. The absorbance of the mixture was measured spectrophotometrically at 517 nm.
3. Results
3.1. X- ray diffraction (XRD)
XRD pattern of the synthesized TiO2NPs showed the presence of both anatase and rutile forms which can be denoted at 2θ peaks at 27.57°, 36.21°, 41.37°, 54.45°, 56.76° and 69.12°, which were found to be (110), (101), (111), (211), (220) and (112) reflections, respectively and confirmed the nanocrystalline nature of the synthesized particles. The XRD sample showed a dominant peak at 2θ = 27.57° and 41.37° which proved the (110) crystallographic plane of anatase and (111) rutile form of TiO2NPs, respectively. The particles size estimation was performed by the Scherrer’s formula:
d=0.9λ/β Cos θ
where d is the mean diameter of the nanoparticles, λ is wavelength of X-ray radiation source, β is the angular FWHM of the XRD peak at the diffraction angle θ and the data obtained was matched with the data base of Joint Committee on Powder Diffraction Standards file No. 89-4202. The plant synthesized TiO2NPs were quite polydisperse calculated average size of 32.58 nm (Figure 1A and B).
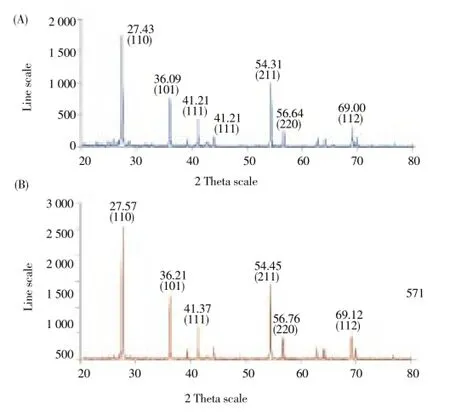
Figure 1. X-ray diffraction patterns of (A) Titanium dioxide, (B) Synthesized TiO2NPs.
3.2. Fourier transform infrared spectroscopy (FTIR)
FTIR peaks showed three spectrums namely TiO2control, synthesized TiO2NPs andP. guajavaleaf aqueous extract (Figure 2 A, B and C). The peaks were given along with the functional groups responsible for the synthesis of the TiO2NPs, which could be depicted to be 3 420-1alcohols (free OH), 3 410-1intramolecular bonded (weak), 3 425-1intramolecular bonded (strong), 2 922-1alkenes, 2 917-1carboxylic acids, 1 659-1nitro compounds (symmetrical stretch), 1 621-1and 1 618-1nitro compounds (asymmetrical stretch), 1 368-1and 1 384-1CH3 umbrella deformation, 1 078-1mononuclear aromatics and 1 065-1-aromatic (Aryl-O-CH2)[49].
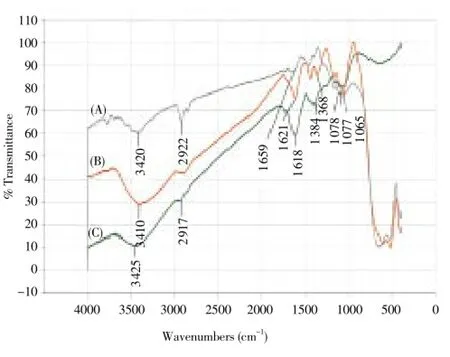
Figure 2. FTIR peaks of (A) Titanium dioxide, (B) Synthesized TiO2NPs, (C) P. guajava aqueous leaf extract.
Table 5 Effect of KV and CQ on enzymic and non-enzymic antioxidant profiles of P. berghei-infected mice.
3.3. Field emission scanning electron microscope (FESEM)
FESEM images were measured and topographical analysis was performed based upon the surface study. Synthesized TiO2NPs were smooth and spherical in shape. The images showed the synthesized nanoparticles in various magnifications 15 000×, 30 000× and 50 000× which clearly gives physical morphology, particle size and aspect ratio (Figure 3 A, B and C). The energy dispersive X-ray analysis study (EDX) proves that the particles are crystalline in nature and indeed metallic TiO2NPs (Figure 3D). The presence of carbon, oxygen, magnesium and chlorine indicate that the extracellular organic moieties are adsorbed on the surface of the metallic nanoparticles.
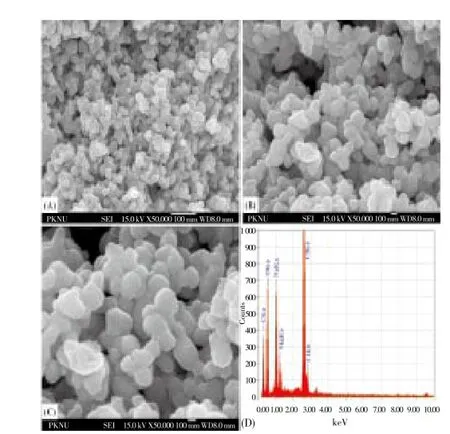
Figure 3. FESEM images of synthesized TiO2NPs at different resolution (A) 15 000× (B) 30 000× (C) 50 000× (D) EDX showing the chemical composition.
3.4. Antibacterial activity
The disk diffusion method was performed against theA. hydrophila,P. mirabilis,E. coli,S. aureusandP. aeruginosa. The synthesized TiO2NPs displayed antibacterial activity of pathogenic strains ofA. hydrophila(17 mm),P. mirabilis(20 mm),E. coli(23 mm),S. aureus(25 mm),P. aeruginosa(19 mm) at 20 μg/mL, respectively. The maximum zone of inhibition was observed in the TiO2NPs againstS. aureusandE. coli(Figure 4).
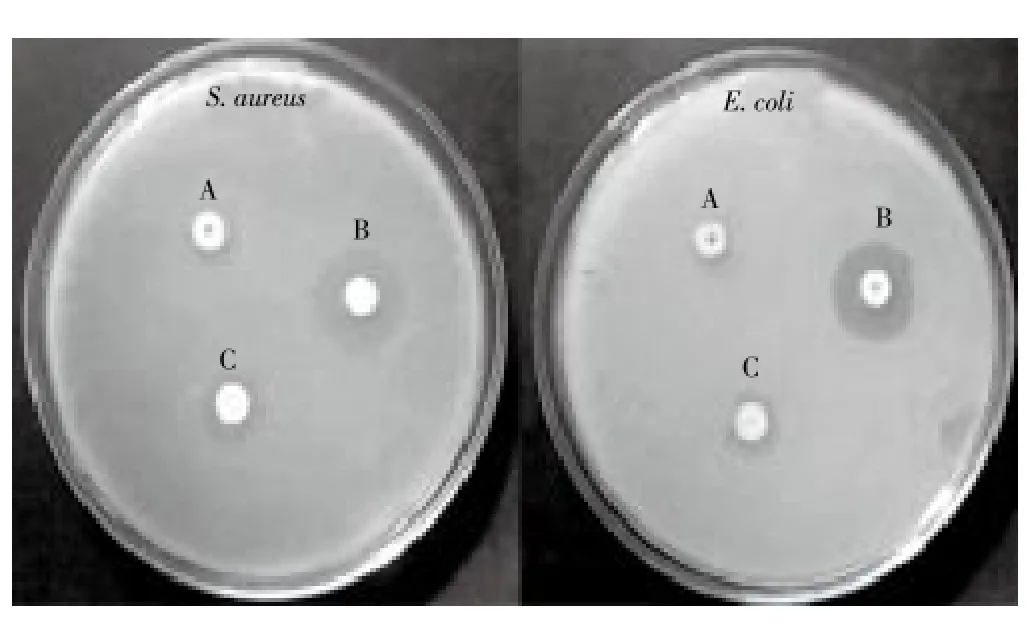
Figure 4. Zone of Inhibition observed against S. aureus and E. coli. (A) Titanium dioxide-bulk (B) Synthesized TiO2NPs (C) Tetracycline.
3.5. Antioxidant activity
In this study, antioxidant activity of the aqueous leaf extract ofP. guajavaand synthesized TiO2NPs were investigated and the TiO2NPs were found to be effective antioxidants compared with the aqueous leaf extract ofP. guajava. The antioxidant activity determined using this method differed according to the sample analysed (Figure 5 A). When the absorbance of the extract was compared with that of standard ascorbic acid, the extract was found to possess a higher level of antioxidant activity than ascorbic acid with the highest activity noted in the synthesized TiO2NPs. The content of phenolic compounds (mg/g) in leaf aqueous extract and synthesized TiO2NPs were found as 85.4 and 18.3 mg TA/g.
The DPPH scavenging assay exhibited effective inhibition activity of both aqueous leaf extract ofP. guajavaand synthesized TiO2NPs when compared with the standard, ascorbic acid (Figure 5 B). The DPPH activity of the nanoparticles was found to increase in a dose-dependent manner. However, the synthesized TiO2NPs exhibited more inhibition with more than 85% scavenging activity of DPPH than aqueous leaf extract ofP. guajava. The DPPH free radical scavenging assay showed that synthesized TiO2NPs have higher free radical scavenging activity compared to aqueous leaf extract alone.
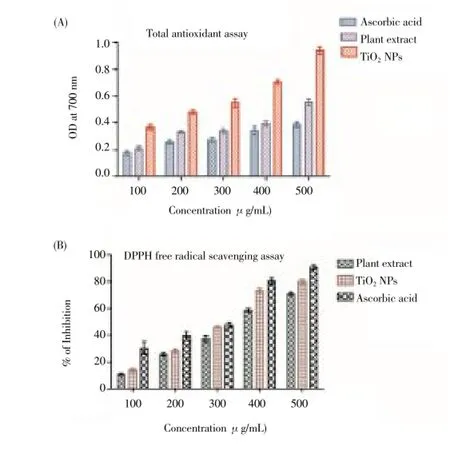
Figure 5. Antioxidant activity of the synthesised TiO2NPs.(A) Total antioxidant activity; (B) DPPH radical scavenging assay.
4. Discussion
The use of the highly structured physical and biosynthetic activities of biological cells for the synthesis of nanosized materials has recently emerged as a novel approach for the synthesis of metal nanoparticles. The positions of principal peaks in XRD were found to be in agreement with the literature[50]. This pattern reflects the shape of the wave functions of the electronic states of the Ti-O-Ti-O chain on the TiO2(110)/H2O interface[51]. Rajakumaret al[52] reported the band intensities of the FTIR spectrum for the synthesized TiO2NPs fromEclipta prostrateleaf aqueous extract are 3 410-1, 1 621-1, 1 368-1, 1 077-1and 1 065 cm-1. These results indicated that alcohols (OH), asymmetrical stretch, primary amines, aromatics and aliphatic amines inP. guajavamay have been participated in the process of nanoparticle synthesis. Functional groups associated with these were the cause for the bioreduction of TiO(OH)2to TiO2NPs and the FESEM showed poorly dispersed with spherical clusters in shape.
Anaset al[53] reported the antibacterial activity of aqueous and organic extracts ofP. guajavaleaves were evaluated against multidrug resistant clinical isolates ofS. aureusstrain. In several studies, the methanolic extract ofP. guajavashowed significant antibacterial activity againstStaphylococcus,Shigella,Salmonella,Bacillus,E. coli,ClostridiumandPseudomonasand the aqueous extract was more effective againstE. coliandP. aeruginosa[54]. The chemical synthesis may still lead to the presence of some toxic chemical species adsorbed on the surface that may have adverse effects in medical applications. The antibacterial activity of TiO2nanocomposites was investigated qualitatively and quantitatively[55]. Most of the recent researches on the inhibition of bacterial cell growth have been studied by using the suspended-TiO2in solution[56-58]. The ability of TiO2NPs to produce reactive oxygen species, their toxicity[59], and their applicability[60] has received considerable attention. The antibacterial activity of TiO2was related to reactive oxygen species production, especially hydroxyl free radicals and peroxide formed under UV- irradiation via oxidative and reductive pathways, respectively[61]. In suspension, TiO2NPs were trapped onto the bacteria surface resulting in the adsorption of TiO2particles on the bacteria surface, which could lead to the inactivation of bacteria in couple with the photocatalytic oxidation reaction. There are several possible mechanisms to explain the bactericidal effect of TiO2particles. TiO2exhibits antimicrobial activity due to its strong oxidizing property when exposed to sunlight or UV-light. The microbial surface was the primary target of the initial oxidative attack when irradiated TiO2particles come into contact with microbes[62,63]. The antibacterial test was conducted againstS. aureusandE. colibacterium using the synthesized sulfated β-cyclodextrin treated fabric TiO2NPs[64]. Earlier studies indicated that the antibacterial activity of TiO2-incorporated polyethylene films should be due to the killing effect property of TiO2nanoparticles againstS. aureusand the TiO2-incorporated polyethylene film exhibited more effective antibacterial activity[65]. This evidence supports the fact the generation of H2O2at the TiO2-biofilm interfaces resulting in the destruction of the bacteria within biofilm. The amount of H2O2generated on TiO2particles has also been reported to achieve antibacterial activity against various bacterial species[62,66].
Phenols are very important plant constituents becauseof their scavenging ability owing to their hydroxyl groups[67]. Phenolic compounds from plants are known to be good natural antioxidants and the activity of synthetic antioxidants was observed to be higher than that of natural antioxidants[68]. The total antioxidant capacity of the aqueous leaf extract ofP. guajavaand synthesized TiO2NPs were based on the phosphomolebdnum method where the reduction of Mo (Ⅵ) to Mo (Ⅴ) by the antioxidant compound and the formation of a green phosphate/Mo (Ⅴ) complex[69]. The synthesized TiO2NPs were found to have very high total antioxidant capacity as compared to aqueous leaf extract ofP. guajava.The synthesized TiO2NPs showed free radical scavenging activity up to the IC50value of 21.4 μg/mL which is relatively higher in comparison to leaf aqueous extract ofP. guajava. The TiO2and nanosilver have been shown to activate oxidative stress, DNA and mitochondrial damage bio-chemical pathways[70].
In conclusion, as the technological benefits of nanotechnology begin to rapidly move from laboratory to large-scale industrial production, the nanomaterials are used in all biomedical applications. In conclusion, the present novel method is capable of reducing TiO(OH)2to TiO2NPs usingP. guajavaleaf aqueous extract. The synthesized TiO2nanoparticles were characterized by using XRD, FTIR, FESEM, EDX and the biological route of synthesis for the TiO2, provides a fast, purest form of producing nanoparticles. This biological reduction of metal would be boon for the development of clean, nontoxic and environmentally acceptable "green approach" to produce metal nanoparticles, involving organisms even ranging higher plants.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Abdelrahim SI, Almagboul AZ, Omer ME, Elegami A. Antimicrobial activity of Psidium guajava L. Fitoterapia 2002; 73: 713-715.
[2] Wang Y, Wang J, Wu M, Deng X, Wen T, Chen C, et al. Internalization, translocation and biotransformation of silicacoated titanium dioxide nanoparticles in neural stem cells. J Nanosci Nanotechnol 2010; 10(11): 7121-7125.
[3] Salata O. Application of nanoparticles in biology and medicine. J Nanobiotechnol 2004; 2: 3-6.
[4] Gwinn MR, Vallyathan V. Nanoparticles: Health effects-pros and Cons. Environ Health Perspect 2006; 114: 1818-1825.
[5] Shervani Z, Yamamoto Y. Size and morphology controlled synthesis of gold nanoparticles in green solvent: Effect of reducing agents. Mat Lett 2011; 65: 92-95.
[6] Hoffmann MR, Martin ST, Choi WY, Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chem Rev 1995; 95: 69-96.
[7] Fujishima A, Rao TN, Truk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C: Photochem 2000; 1: 1-21.
[8] Gelis C, Girard S, Mavon A, Delverdier M, Pailous N, Vicendo P. Assessment of the skin photo protective capacities of an organomineral broad spectrum sunblock on two ex vivo skin models. Photodermatol Photoimmunol Photomed 2003; 19: 242-253.
[9] Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 2009; 69: 8784-8789.
[10] Gong P, Li H, He X, Wang K, Hu J, Zhang S, et al. Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology 2007; 18: 604-611.
[11] Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Fut Microbiol 2011; 8: 933-940.
[12] Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS. TiO2nanoparticles are phototoxic to marine phytoplankton. PLOS Biol 2012; 7:1-7.
[13] Kong H, Song J, Jang J. Photocatalytic antibacterial capabilities of TiO2-biocidal polymer nanocomposites synthesized by a surfaceinitiated photopolymerization. Environ Sci Technol 2010; 44(14): 5672-5676.
[14] Zhang H, Chen G. Potent antibacterial activities of Ag/TiO2nanocomposite powders synthesized by a one-Pot sol-gel method. Environ Sci Technol 2009; 43(8): 2905-2910.
[15] Yuan W, Ji J, Fu J, Shen J. A facile method to construct hybrid multilayered films as a strong and multifunctional antibacterial coating. J Biomed Mater Res B Appl Biomater 2008; 85(2): 556-563.
[16] Marciano FR, Lima-Oliveira DA, Da-Silva NS, Diniz AV, Corat EJ, Trava-Airoldi VJ. Antibacterial activity of DLC films containing TiO2nanoparticles. J Colloid Interface Sci 2009; 340: 87-92.
[17] Rajakumar G, Rahuman AA, Roopan SM, Khanna VG, Elango G, Kamaraj C, et al. Fungus-mediated biosynthesis and characterization of TiO2nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta A Mol Biomol Spectrosc 2012; 91: 23-29.
[18] Hassan MS, Amna T, Mishra A, Yun SI, Kim HC, Kim HY, et al. Fabrication, characterization and antibacterial effect of novel electrospun TiO2nanorods on a panel of pathogenic bacteria. JBiomed Nanotechnol 2012; 8(3): 394-404.
[19] Coppin EA, Pike OA. Oil stability index correlated with sensory determination of oxidative stability in light-exposed soybean oil. J Am Oil Chem Soc 2001; 78: 13-18.
[20] Beltran E, Pla R, Yuste J, Mor-Mur M. Use of antioxidants to minimize rancidity in pressurized and cooked chicken slurries. Meat Sci 2004; 66(3): 719-725.
[21] Zima TS, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, et al. Oxidative stress, metabolism of ethanol and alcoholrelated diseases. J Biomed Sci 2001; 8: 59-70.
[22] Astley SB. Dietary antioxidants-past, present and future? Trends Food Sci Technol 2003; 14: 93-98.
[23] Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science 2006; 311(5761): 622-627.
[24] Mocan T, Clichici S, Agoston-Coldea L, Mocan L, Simon S, Ilie IR, et al. Implications of oxidative stress mechanisms in toxicity of nanoparticles. Acta Physiol Hung 2010; 97(3): 247-255.
[25] Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY. Toxicological effects of TiO2and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem 2010; 42: 586-591.
[26] Saquib Q, Al-Khedhairy AA, Siddiqui MA, Abou-Tarboush FM, Azam A, Musarrat J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol in Vitro 2012; 26(2): 351-361.
[27] Canesi L, Fabbri R, Gallo G, Vallotto D, Marcomini A, Pojana G. Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nanoparticles (Nano carbon black, C60 fullerene, Nano-TiO2, Nano-SiO2). Aquat Toxicol 2010; 100(2): 168-177.
[28] Das D, Nath BC, Phukon P, Dolui SK. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf B 2012; 101: 430-433.
[29] Roopan SM, Bharathi A, Prabhakarn A, Rahuman AA, Velayutham K, Rajakumar G, et al. Efficient phyto-synthesis and structuralcharacterization of rutile TiO2nanoparticles using Annona squamosa peel extract. Spectrochim Acta A Mol Biomol Spectrosc 2012; 98: 86-90.
[30] Velayutham K, Rahuman AA, Rajakumar G, Santhoshkumar T, Marimuthu S, Jayaseelan C, et al. Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol Res 2011; 111(6): 2329-2337.
[31] Kirthi AV, Rahuman AA, Rajakumar G, Marimuthu S, Santhoshkumar T, Jayaseelan C, et al. Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mat Lett 2011; 65: 2745-2747.
[32] Teixeira RS, Camparoto ML, Mantovani MS, Vicentini VEP. Asessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L., in in vitro and in vivo assays. Genet Mol Biol 2003; 26: 234-239.
[33] Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: healersconsensus and cultural importance. Soc Sci Med 1998; 47: 1859-1871.
[34] Ojewole JA, Awe EO, Chiwororo WD. Antidiarrhoeal activity of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rodents. J Smooth Muscle Res 2008; 44(6): 195-207.
[35] Neviton RS, Aparicio DGC, Simone MS, Vataru CN, Prado BF. An evaluation of antibacterial activity of Psidium guajava (L). Braz Arch Biol Technol 2005; 8(3): 429-436.
[36] Ojewolw JA. Antiinflammatory and analgesic effects of Psidium guajava Linn (Myrtaceae) leaf aqueous extracts in rats and mice. Meth Find Exp Clin Pharmacol 2006; 28(7): 441-446.
[37] Sang-Bang L, Hae-Ryong P. Anticancer activity of guava (Psidium guajava L.) branch extract against HT-29 human coloncancer cells. J Med Plant Res 2010; 4(10): 891-896.
[38] Coutino-Rodriguez R, Hernandez-Cruz P, Giles-Rios H. Lectins in fruits having gastrointestinal activity: their participation in the hem agglutinating property of Escherichia coli 0157:H7. Arch Med Res 2001; 32: 251-257.
[39] Gutiérrez RM, Mitchell S, Solis RV. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2008; 117(1): 1-27.
[40] Parashar UK, Kumar V, Bera T, Saxena PS, Nath G, Srivastava SK, et al. Study of mechanism of enhanced antibacterial activity by green synthesis of silver nanoparticles. Nanotechnology 2011; 22(41): 415104.
[41] Basha SK, Govindaraju K, Manikandan R, Ahn JS, Bae EY, Singaravelu G. Phytochemical mediated gold nanoparticles and their PTP 1B inhibitory activity. Colloids Surf B 2010; 75(2): 405-409.
[42] Raghunandan D, Basavaraja S, Mahesh B, Balaji S, Manjunath SY, Venkataraman A. Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. J Nanobiotechnol 2009; 5: 34-41.
[43] Raghunandan D, Mahesh BD, Basavaraja S, Balaji SD, Manjunath SY, Venkataraman A. Microwave-assisted rapid extracellular synthesis of stable bio-functionalized silver nanoparticles from guava (Psidium guajava) leaf extract. J Nanopart Res 2011; 13: 2021-2028.
[44] Prasad TNVKV, Elumalai EK, Khateeja S. Evaluation of the antimicrobial efficacy of phytogenic silver nanoparticles. Asian Pac J Trop Biomed 2011; 1(1): 82-85.
[45] Kora AJ, Manjusha R, Arunachalam J. Superior bactericidal activity of SDS capped silver nanoparticles: Synthesis and characterization. Mater Sci Eng C 2009; 29: 2104-2109.
[46] Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to thedetermination of vitamin E. Anal Biochem 1999; 269: 337-341.
[47] Amin I, Norazaidah Y, Hainida KIE. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem 2006; 94: 47-52.
[48] Liyana-Pathiranan CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agricul Food Chem 2005; 53: 2433-2440.
[49] Silverstein RM, Webster FX, Kiemle D. Spectrometric identification of organic compounds. 7th edition. John Wiley&Sons; 2005, p. 502.
[50] Wang C, Deng ZX, Li Y. The synthesis of nanocrystalline anatase and rutile titania in mixed organic media. Inorg Chem 2001; 40(20): 5210-5214.
[51] Kobayashi E, Matsuda K, Mizutani G, Ushioda S. SHG observation of rutile TiO2(110)/H2O interface under UV light illumination. Surf Sci 1999; 294: 427-428.
[52] Rajakumar G, Rahuman AA, Priyamvada B, Khanna VG, Kumar D K, Sujin PJ. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mat Lett 2012; 68: 115-117.
[53] Anas K, Jayasree PR, Vijayakumar T, Kumar PRM. In vitro antibacterial activity of Psidium guajava Linn. Leaf extract on clinical isolates of multidrug-resistant Staphylococcus aureus. Indian J Exp Biol 2008; 46: 41-46.
[54] Abdelrahim SI, Almagboul AZ, Omer MEA, Elegami A. Antimicrobial activity of Psidium guajava L. Fitoterapia 2002; 73: 713-715.
[55] Senapati S, Ahmad A, Khan MI, Sastry M, Kumar R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2005; 1: 517-520.
[56] Gumy D, Morais C, Bowen P, Pulgarin C, Giraldo S, Hajdu R, et al. Catalytic activity of commercial of TiO2powders for the abatement of the bacteria (E. coli) under solar simulated light: Influence of the isoelectric point. Appl Catal B Environ 2006; 63: 76-84.
[57] Gumy D, Rincon AG, Hajdu R, Pulgarin C. Solar photocatalysis for detoxification and disinfection of water: Different types of suspended and fixed TiO2catalysts study. Sol Ener 2006; 80(10): 1376-1381.
[58] Verran J, Sandoval G, Allen NS, Edge M, Stratton J. Variables affecting the antibacterial properties of nano and pigmentary titania particles in suspension. Dyes Pigm 2007; 73: 298-304.
[59] Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol 2006; 40: 4346-4352.
[60] Badireddy AR, Hotze EM, Chellam S, Alvarez P, Wiesner MR. Inactivation of bacteriophages via photosensitization of fullerol nanoparticles. Environ Sci Technol 2007; 41: 6627-6632.
[61] Kikuchi Y, Sunada K, Iyoda T, Hashimoto K, Fujishima A. Photocatalytic bactericidal effect of TiO2thin films: dynamic view of the active oxygen species responsible for the effect. J Photochem Photobiol A: Chem 1997; 106: 51-56.
[62] Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol 1999; 65: 4094-4098.
[63] Foster AH, Sheel WD, Sheel P, Evans P, Varghese S, Rutschke N, et al. Antimicrobial activity of titania/silver and titania/copper films prepared by CVD. J Photochem Photobiol A 2010; 216: 283-289.
[64] Selvam S, Gandhi RR, Suresh J, Gowri S, Ravikumar S, Sundrarajan M. Antibacterial effect of novel synthesized sulfated β-cyclodextrincrosslinked cotton fabric and its improved antibacterial activities with ZnO, TiO2and Ag nanoparticles coating. Int J Pharm 2012; 434(2): 366-374.
[65] Xing Y, Li X, Zhang L, Xu Q, Che Z, Li W, et al. Effect of TiO2nanoparticles on the antibacterial and physical properties of polyethylene-based film. Prog Org Coat 2012; 73: 219-224.
[66] Howard A, Foster IB, Ditta S, Varghese AS. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. App Microbiol Biotechnol 2011; 90: 1847-1868.
[67] Hatano T, Edamatsu R, Mori A, Fujita Y, Yasukara T, Yoshida T. Effects of the interaction of tannins with co-existing substances.Ⅵ: Effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull 1989; 37: 2016-2021.
[68] Ningappa MB, Dinesha R, Srinivas L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem 2008; 106: 720-728.
[69] Narendhirakannan RT, Smeera T. In vitro anti-oxidant studies on ethanolic extracts of leaves and stems of nyctanthes arbor-tristis. l (night-flowering jasmine). Int J Biol Med Res 2010; 1(4): 88-192.
[70] Jin C, Tang Y, Yang FG, Li XL, Xu S, Fan XY, et al. Cellular toxicity of TiO2nanoparticles in anatase and rutile crystal phase. Biol Trace Elem Res 2011; 141: 3-15.
ment heading
10.1016/S1995-7645(14)60171-1
*Corresponding authors: Dr. A. Abdul Rahuman, Unit of Nanotechnology and Bioactive Natural Products, Post Graduate and Research Department of Zoology, C. Abdul Hakeem College, Melvisharam - 632 509, Vellore District, Tamil Nadu, India. Tel.: +91 94423 10155; +91 04172 269009
Fax: +91 04172 269487.
E-mail: abdulrahuman6@hotmail.com
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Assessment of traumatic brain injury degree in animal model
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
