Assessment of traumatic brain injury degree in animal model
Jian-Qiang Chen, Cheng-Cheng Zhang, Hong Lu*, Wei Wang
1Department of Radiology, Haikou Hospital Affiliated to Xiangya School of Medicine, Central South University, Haikou 570208, China
2Department of Radiology, Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, China
Assessment of traumatic brain injury degree in animal model
Jian-Qiang Chen1,2, Cheng-Cheng Zhang1, Hong Lu1*, Wei Wang2*
1Department of Radiology, Haikou Hospital Affiliated to Xiangya School of Medicine, Central South University, Haikou 570208, China
2Department of Radiology, Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, China
Objective:To establish stable and controllable brain injury with accurate degree and good repeatability in rat model.Methods:Controlled cortical impact (CCI) device was used to prepare for the rat brain injury model by the impact head of different model (Group A No. 4, Group B No. 5, Group C No. 6) and the impact depth (Group A: 1.5-2.0 mm, Group B: 2.5-3.0 mm, Group C: 3.5-4.0 mm) with impact time of 0.1 s and impact velocity of 2.5 m/s. Twelve rats with three months of age were used in each group (the impact depth of every two rats was added 1 mm respectively). After modeling for 1 h, magnetic resonance imaging (MRI) was received and brain histopathology was observed to assess degree of injury by model parameters of three groups.Results:After modeling of Group A, MRI showed that the cortex structure was damaged with a small amount of bleeding in center and mild edema around, and the total volume of injury was (28.69±4.94) mm3. Pathology revealed the injury was confined to the superficial cortical with mild edema of nerve cell, which was assessed as mild cerebral contusion. While after modeling, MRI of Group B showed that the structure of cortex and medulla were damaged simultaneously and extended to cerebral nuclei zone, with 4 cases of hematoma in the center and larger edema range around, and the total volume of injury was (78.38±9.28) mm3. Pathology revealed the injury range was reached nuclei zone, with swell of nerve cell and mitochondria, which was assessed to moderate cerebral contusion. After modeling of Group C, MRI showed that extensive tissue injury was appeared in cortex and medulla and deep nuclei, with 9 cases of hematoma and large edema signal of surrounding tissue T2WI, while in 5 cases, lateral nucleus of injury signal was increased, and the total volume of injury was (135.89±24.80) mm3. Pathology revealed the deep cerebral nuclei was damaged, with the disappearance of neuronal structure and vacuolization of mitochondria, which was assessed as severe cerebral contusion. MRI changes were consistent with pathological changes in three groups of model, and the injury range was significantly different (P<0.01).Conclusions:Application of CCI can make stable quantitative traumatic brain injury model, which overcomes the randomness in previous injury model and possesses highly unity in iconography and pathology changes. This can provide quantitative modeling reference for clinical research.
ARTICLE INFO
Article history:
Received 24 September 2014
Received in revised form 10 October 2014
Accepted 15 November 2014
Available online 20 December 2014
CCI
1. Introduction
Traumatic brain injury (TBI) is the leading causes of human death and disability in modern industrialized society[1]. The injury degree of brain function, the risk of complication, prognosis and the selection of clinical treatment are all closely related to the degree of trauma. Therefore, basic research of different degree of brain trauma is very important. Currently, there are many methods to produce traumatic brain model, inducing free-fall method[2], negative pressure wound therapy[3], impact method of acceleration[4] and controlled cortical impact (CCI)[5],etc. However, it is still not unified to establish the quantitative criteria of different degree of trauma in animal model. Hence it brought the differences of research, so it is very necessary to establish quantitative and normative degree of injury in animal model. CCI has advantages of accuratequantitative, small elastic injury, high repeatability and is less affected by Individual difference factors. The present study aims to establish the rat brain injury model of different degree and to further assess its reliability.
2. Materials and methods
2.1. Experimental animal and grouping
Healthy SD rats (n=36) aged 30 weeks of either gender (weighting 220-250 g) were provided by Laboratory Animal Center of Sichuan University [animal license SCXK (Chuan) 2008-24). Rats were feed in separate cages at 22-25 ℃ in 12 h: 12 h light: dark cycle with food and water ad libitum. Rats were randomly divided into three groups (Groups A, B and C) of 12 each for model production.
2.2. Model production of traumatic brain injury
Rats were anesthetic by intraperitoneal injection of 3% chloral hydrate (3 mL/kg) and head hair was shaved by electricity push knife. Head was placed in standard stereotaxic frame, fixed by portals and incisor, and cut 10 mm near the midline to expose bregma and lambda. The skin and fascia was cut to perform craniectomy at right temporal and parietal cortex by electric rotary drill bit (0.75 mm Bits). Then it was washed by normal saline with intervals to decrease the heat produced in the process of the bone drill. When close to skull, operation was performed carefully to avoid the injury of endocranium. The impact head and dura mater of rats were impacted in the angle to of 15° (considering the degree of curvature of the rat skull), and was aligned at the impact of the right edge and midline seam, while the back edge was aligned at horizontal component of lambda. The impact location moved 3 mm forward and move 2.7 mm to the right. CCI device (Hatteras, USA) was controlled by computer and equipped solenoid drive impactor with the steel tip of 3.0 mm diameter to install on stereotaxic apparatus (electric pulse produced by the contact of impact head and endocranium). Impact parameter was shown in Table 1. Then the incision was sewn up with silk 4.0. All the surgeries were performed under aseptic conditions. After surgery, rats were placed on constant temperature heating pad to maintain body temperature until fully awake.
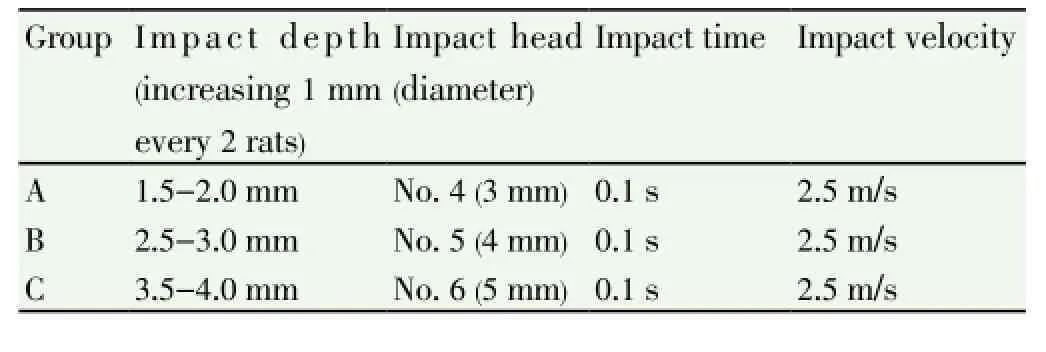
Table 1 Impact parameter.
2.3. MR examination
Respiratory monitoring pad (SA Instruments, Inc., Stony Brook, NY) was put in rat’s abdomen to monitor respiratory status. Constant temperature air at 37 ℃ was blew through MR aperture to keep warm. Imaging device was used Bruker 7T BioSpin MR Spectrometer (Bruker, Germany), with the surface pulse coil of 24 lanes. The imaging sequence were T2WI:TR:1 200.0 ms, TE: 50.0 ms, matrix: 256×256, number of excitation: 4, depth of stratum 1.0 mm, interlamellar spacing 0.00 mm, FOV: 3.6 cm×3.6 cm.
2.4. Calculation of injury volume of brain tissue after CCI modeling for 1 h
Three models were received MRI examination before 1 h of biopsy, and injury volume was calculated by MRI T2WI image. Calculating method: workstation was processed after T2WI data were transferred, with axial view manual painting high signal edge (offside and deep nucleus injury were also included). Injury area was generated automatically by system, multiplied by the depth of stratum, calculated volume of each depth of stratum in order from top to bottom (abnormal signal from beginning to end), and then the total injury volume was calculated: Total volume=∑depth of stratum×S (area).
2.5. Observation of pathology
In order to verify whether the pathologic change of brain tissue conformed to the mild, moderate and severe brain injury in injury model of three groups, after 1 h of modeling, 2 rats of mild, moderate and severe injury were randomly performed with deep anesthesia, respectively. 0.9% sodium chloride for animals (containing 1 000 U/L heparin) and 4% paraformaldehyde (pH 7.4) were used for heart perfusion. Then rats were decapitated and whole brains were collected. Coronary slice of 30 μm thickness from olfactory bulb to the medulla oblongata were obtained by frozen section. The brain slices were put with cryoprotectant in freezer at -20 ℃, then stained with H&E. Optical microscope (OM) and transmission electron microscopy (TEM, EX-2000) were used to observe the structural and morphological changes of nerve cell and organelle, respectively.
3. Results
3.1. General data
After surgery, all the rats of Group C occurred apnea with different degrees (2-3 s), while Group B occurred 2 cases, and no apnea was found in Group A. Three cases of Group C died (2 were dead within 24 h, and 1 was dead within 72 h). In Group B, 1 was dead within 24 h, while others weresurvived to 30 days of follow-up period. Injury incidence of Groups A-C was 100.00%, 91.67%, 75.00%, respectively.
3.2. MRI manifestation and mild, moderate and severe injury of gross specimen in three groups of model
MRI imaging manifestation is shown in Figure 1. T2WI image of three groups of model showed that different degrees of high signal was appeared in injury area, with the damage volume of Group C>Group B> Group A (P<0.001). After 1 h, Group A showed the injury of cortical tissue structure with 1 case (1/12) of a small amount of bleeding and mild edema signal around the lesion area. Group B showed that the structure of cortex and medulla were damaged simultaneously and extended to cerebral nuclei zone with 4 cases (4/12) of bleeding, hematoma and moderate edema signal around the lesion area, 3 cases having edema signal in deep nuclei. Group C expressed that extensive tissue injury was appeared in cortex and medulla and deep nuclei, with 9 cases (9/12) of hematoma, clear signal around the lesion area, and different degree of edema in deep nuclei, while different range of edema was appeared in 6 cases of contralateral injury, among which 4 were combined with contralateral damaged area along the cortex fibre. The range and extent of damage of MRI in three groups of model complied with mild, moderate and severe brain injury change.
Gross specimen manifestation from the naked eye is shown in Figure 1. In Group A, fracture and bleeding were present in superficial brain tissue, while brain parenchyma was present as subdural hemorrhage with plate shaped. In Group B, the injury area was increased with the cortex of large splinter hemorrhage. In Group C, brain tissue was swelling with limitation, and part of medulla showed ectropion with obvious bleeding where the injury surface was crimson.
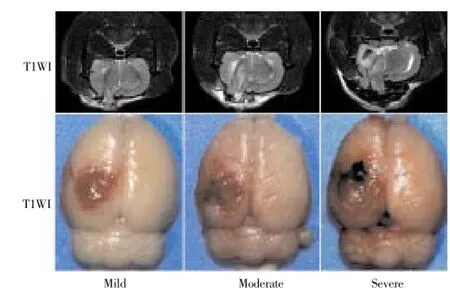
Figure 1. Gross specimen manifestation from the naked eye.
3.3. Changes in histopathology
In Group A, lesion was confined to the superficial cortex of brain injury, which showed mild subdural hemorrhage and a small amount of subarachnoid hemorrhage. Electron microscope showed swell of vascular endothelial cell and mitochondria, conforming to change of mild damage. In Group B, lesion was widely distributed with cerebral hemisphere being mainly damaged in which the injury degree exceeded cerebral cortex. So it is clear that subdural and subarachnoid hemorrhage were obvious. Electron microscopy showed the swell of nerve cell and mitochondria and the vacuolation of mitochondria, conforming to change of moderate damage. While in Group C, lesion was spread to contralateral brain tissue in which injury degree could reached deep structures such as basal ganglia and hippocampus. Nuclei were darker, nucleolus was blurred, and lesion of deep brain issue was obvious. Electron microscope showed that nerve cell in cerebral cortex and nucleus area was swelling, protuberance was increased, neurogliocyte, endothelial cells and pericyte were all swelling, nerve cell was almost lost structure, mitochondria were all in vacuolation, and ribosome lost severely, that was conformed to change of mild damage (Figures 2 and 3).

Figure 2. Pathology observation of H &E.
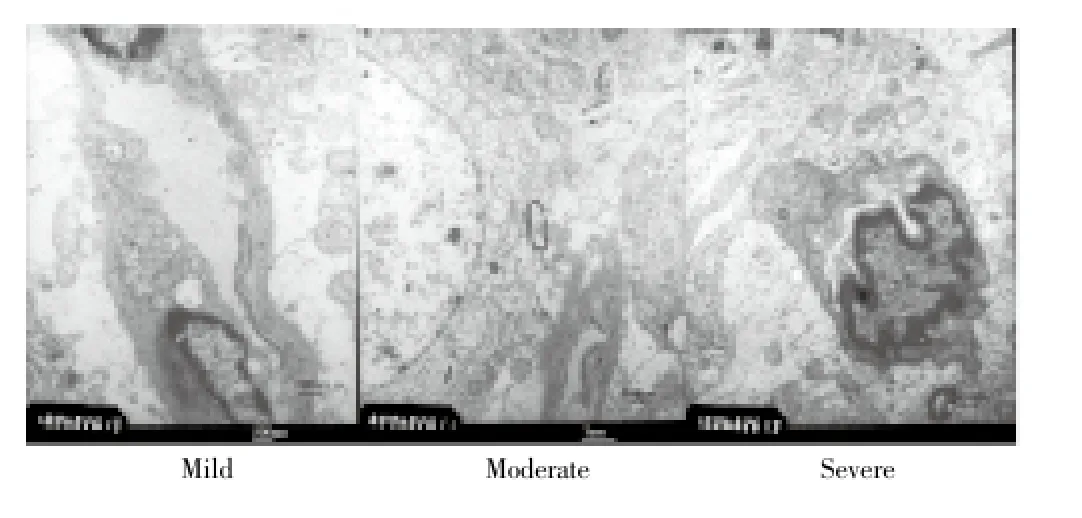
Figure 3. Changes in histopathologyMild: Swell of vascular endothelial cell and mitochondria; Moderate: swell of nerve cell and mitochondria, and vacuolation of mitochondria; Severe: endothelial cells and pericyte were swelling, nerve cell was almost lost structure, mitochondria were all in vacuolation, and ribosome lost severely-necrosis.
4. Discussion
The present study established three groups of model (Groups A, B and C) with different degree of injury by CCI. Through MRI observation and pathology certification, it was found that three groups were conformed to mild (Group A),moderate (Group B) and severe (Group C) respectively in injury model of rats
Currently, CCI device is widely used as a tool for production of animal injury model. It is an important tool for quantization of different injury degree in animals by changing impact depth, impact range and impact velocity of impact head, which is widely used in production of animal injury model among rodents[5-11]. It has been reported that impact velocity will cause the change of injury at different levels and types. However, in the clinical work, it is hard to control the injury accurately related to velocity and action time, and relatively injury depth and range are more intuitional in clinical diagnosis and treatment and research since injury depth and range can be measured accurately through all kinds of instruments and equipment (including CT , MRI and so on). Hence, it is more valuable to produce degree models with different injury depth and range for clinical targeted therapy. Nevertheless, there is no normalized parameter of damage degree for reference at present time. The injury parameter was single in previous studies[8], which ignored the errors caused by parameter change and individual differences. Therefore, this study established a degree model divided by impact depth and impact range through change of the impact head size and change of impact depth in random interval, and assessed its feasibility for reference in correlated clinical research.
In addition to the advantages of being quantifiable and good repeatability, a good brain injury degree model should satisfy the reliability of degree differences and the demand of clinical relevant research. Hence, this study has carried out relevant assessment by quantifiable establishment of the damage degree model. Radioactive medicine is the important foundation in rapid development of modern neurosurgery medicine, and is the important tool of diagnosis and treatment and follow-up visit for TBI patients, which can provide the important information including brain injury range, degree and prognostic evaluation,etc[12-16]. CT is most widely applied in brain injury, but its resolution is less than MRI[15,16]. MRI technology has the characteristics of sensitive for brain hemorrhage, peripheral edema and less obvious injury zone. Therefore, animal degree model of brain injury was included to the assessment indicators in the present study, of which T2WI in brain damage range, degree of encephaledema and if combing acute bleeding were relatively sensitive[17]. Through T2WI imaging results, MRI image in the three groups of model showed that the damage range increased obviously with the increase of damage degree, and acute bleeding range and probability also increased obviously. This is connected with the increase of CCI probe area and the increase of the damage probability of cortical feeding artery by impact depth. And the data showed that after modeling of 1 h, encephaledema around the area in the three groups of model can express significant difference, which was consistent with the results of our previous work that early injury can form clearer angioedema[18]. It is worth noting that MRI can also reflect the brain issue area which is not directly damaged (we call it the indirect injury area). In the present study, indirect injury area increased with the increase of damage degree in the three groups of model, and the indirect injury area often reflected some rules, that was indirect injury area of severe injury group was often in ipsilateral deep nuclei (6/12) and contralateral cortex (7/12), small part was in contralateral deep nuclei (3/12), moderate injury group was only in contralateral cortex, and mild injury group was not found in which signal limitations were increased in MRI. It may be due to ipsilateral deep nuclei caused contrecoup, and right side cortex of contralateral cortex was damaged by traction, while the reason of contralateral deep nuclei needed further study. Hence, it was confirmed by MRI that three groups of model was conformed to the change of mild, moderate and severe brain injury model in imaging change.
Subsequently, we observed the change differences of the three groups of model by pathology. The results showed that pathological change was highly consistent with image feature, and visible difference was formed in damage degree. The injury of Group A was mainly confined to pallium structure, which showed mild subdural hemorrhage and small amount of subarachnoid hemorrhage. Vascular endothelial cell and mitochondria were swelling under the electron microscope, showing that the injury area was relatively limited only with cortex involvement, and degree of nerve cell injury was mild, thus accorded with change of mild damage. The lesion range of Group B was obviously increased compared to mild group. The damage degree exceeded cerebral cortex and arrived in deep nuclei. Subdural and subarachnoid hemorrhage was evident. The electron microscope showed the swell of nerve cell and mitochondria and the vacuolation of mitochondria, which indicated the damage degree involved in deep brain and part of the nerve cells were damaged clearly, thus accorded with change of moderate damage. While in Group C, in addition to the obvious increase of the ipsilateral injury range, injury was also involved in lateral brain issue to some degree. The damage depth can arrive in deep nuclei where lesion of deep brain issue was obvious. The electron microscope showed that cerebral cortex, neurogliocyte of deep nuclei, endothelial cells and pericyte were all swellingobviously, and cleft around nervous tissue, almost loss of nerve cell structure, vacuolation of whole mitochondria, and severe loss of ribosome illustrated the damage range of brain tissue was wide, contrecoup was found in contralateral brain tissue, and extensive irreversible injury and structural injury were appeared in nerve cells, thus accorded with change of severe damage.
In conclusion, the present study established a quantifiable three-dimensional injury model (mild, moderate and severe) by changing the size of impact probe and impact depth, and the image and pathological observations were carried out. The results showed that the three-dimensional injury model had significant difference in image features and pathological change. The damage degree was highly consistent with image change and pathological damage degree, thus illustrating the established scheme of the three groups of model is accurate and reliable and can be applied in clinically relevant experimental study.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Reilly P. The impact of neurotrauma on society: an international perspective. Prog Brain Res 2007; 161: 3-9.
[2] Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury I. Methodology and local effects of contusions in the rat. Brain Res 1981; 211(1):67-77.
[3] Mahmood A, Lu D, Lu M, Wang L, Li Y, Lu M, et al. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurg 2003; 53(3): 697-702.
[4] Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Path physiology and biomechanics. Neurosurg 1994; 80(2): 291-300.
[5] Lighthall JW. Controlled cortical impact: a new experimental brain injury model. Neurotrauma 1988; 5(1): 1-15.
[6] Watson WD, Buonora JE, Yarnell AM, Lucky JJ, D’Acchille MI. Impaired cortical mitochondrial function following TBI precedes behavioral changes. Front Neuroenergetics 2014; 5: 12.
[7] Geddes RI, Sribnick EA, Sayeed I, Stein DG. Progesterone treatment shows benefit in a pediatric model of moderate to severe bilateral brain injury. PLoS One 2014; 9(1): e87252.
[8] Yu S, Kaneko Y, Bae E. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res 2009; 1287: 157-163.
[9] Baskaya MK, Dogan A. Application of 2,3,5-triphenyltetrazolium chloride staining to evaluate injury volume after controlled cortical impact brain injury: role of brain edema in evolution of injury volume.J Neurotrauma 2000; 17(1): 93-99.
[10] Takuji I, Igarashi T, Matthew B. Injury severity determines Purkinje cell loss and microglial activation in the cerebellum after cortical contusion injury. Exp Neurol 2007; 203(1): 258-268.
[11] Patricia M, Washington PM. The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J Neurotrauma 2012; 29(13): 2283-2296.
[12] Bigler ED, Ryser, DK, Gandhi P, Kimball J, Wilde EA. Dayof-injury computerized tomography, rehabilitation status, and development of cerebral atrophy in persons with traumatic brain injury. Am J Phys Med Rehabil 2006; 85: 793-806.
[13] Furlow B. Computed tomography imaging of traumatic brain injury. Radiol Technol 2013; 84(3): 273CT-290CT; quiz p.291CT-294CT.
[14] Ichise M, Chung DG, Wang P, Wortzman G, Gray BG, Franks W. Technetium-99m-HMPAO SPECT, CT and MRI in the evaluation of patients with chronic traumatic brain injury: a correlation with neuropsychological performance. J Nucl Med 1994; 35(2): 217-226.
[15] Metting Z, Rodiger LA, Stewart RE, Oudkerk M, De Keyser J. Perfusion computed tomography in the acute phase of mild head injury: regional dysfunction and prognostic value. Ann Neurol 2009; 66(6): 809-816.
[16] Jones DK, Dardis R, Ervine M, Horsfield MA, Jeffree M, Simmons A, et al. Cluster analysis of diffusion tensor magnetic resonance images in human head injury. Neuroradiology 2000; 47(2): 306-309.
[17] Ding GL, Chopp M, Poulsen DJ, Li L, Qu C, Li Q. MRI of neuronal recovery after low-dose methamphetamine treatment of traumatic brain injury in rats. PLoS One 2013; 8(4): e61241.
[18] Lu H, Lei XY, Hu H. Relationship between AQP4 expression and structural damage to the blood-brain barrier at early stages of traumatic brain injury in rats. Chin Med J (Engl) 2013; 126(22): 4316-4321.
ment heading
10.1016/S1995-7645(14)60174-7
*Corresponding author: Hong Lu, Department of Radiology, Haikou Hospital Affiliated to Xiangya School of Medicine, Central South University, Haikou 570208, China.
Tel: 18976256293
E-mail: 471739847@qq.com
Wei Wang, Department of Radiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, China.
Tel: 13508480956
E-mail: cjr.wangwei@vip.163.com Foundation project: It is supported by funding project of Natural Science Foundation of China (Grant No. 81160181) and major project of Haikou City (Grant No. 2014-079).
Traumatic brain injury
MRI
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
- Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
