miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
Hong-Tai Wang, Ai-Gang Liu, Dao-Shu Luo, Zhang-Nan Zhou, Hong-Guang Lin, Rong-Zi Chen, Jin-Shui He, Kun Chen*
1Department of Orthopaedics, PLA 180 Hospital, Quanzhou 362000, China
2Basic Medical College, Fujian Medical University, Fuzhou 350108, China
3Department of Pediatrics, Zhangzhou Hospital Affiliated to Fujian Medical University, Zhangzhou 363000, China
miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
Hong-Tai Wang1, Ai-Gang Liu1, Dao-Shu Luo2, Zhang-Nan Zhou1, Hong-Guang Lin1, Rong-Zi Chen1, Jin-Shui He3, Kun Chen1*
1Department of Orthopaedics, PLA 180 Hospital, Quanzhou 362000, China
2Basic Medical College, Fujian Medical University, Fuzhou 350108, China
3Department of Pediatrics, Zhangzhou Hospital Affiliated to Fujian Medical University, Zhangzhou 363000, China
Objective:To investigate the expression of miR-218 and its clinical significance in osteosarcoma tissues and explore its effect on proliferation and apoptosis in osteosarcoma cells.Methods:miR-218 expression was detected in 76 samples of surgically resected osteosarcoma and matched normal tumor-adjacent tissues using quantitative reverse transcription polymerase chain reaction (qRT-PCR). MiR-218 was over-expressed by exogenous miR-218 plasmids in Saos-2 cells, and then BrdU cell proliferation assay and flow cytometry were used to determine cell proliferation and apoptosis.Results:The expression of miR-218 in osteosarcoma tissues was significantly lower than those in normal tumor-adjacent tissues (t=8.735, P<0.001). MiR-218 expression in tumor tissues was significantly correlated with tumor size(Χ2=5.380, P=0.020), clinical stage (Χ2=6.692, P=0.010) and distant metastasis (Χ2=4.180, P=0.041). MiR-218 was obviously overexpressed by exogenous miR-218 plasmids (t=19.42, P<0.001), and miR-218 overexpression significantly reduced cell proliferation (t=9.045, P<0.001) and induced apoptosis (t=12.38, P<0.001) in Saos-2 cells.Conclusions:The low-expression of miR-218 is correlated with the poor clinicopathological features in osteosarcoma. Moreover, miR-218 overexpression reduces cancer cell proliferation and induces apoptosis in Saos-2 cells, suggesting that miR-218 may play a key role in the progression of human osteosarcoma.
ARTICLE INFO
Article history:
Received 24 September 2014
Received in revised form 10 October 2014
Accepted 15 November 2014
Available online 20 December 2014
MiR-218
1. Introduction
Osteosarcoma is the most common primary malignant cancer in bone[1]. It has been reported that the incidence of osteosarcoma is obviously correlated with the age of patient[2]. The annual incidence of osteosarcoma is 0.017‰in children under 10 years old, while it is 0.082‰ in children among 10 to 19 years old[2]. The emerging evidence showed that major advances in diagnosis and treatment have improved the outlook for osteosarcoma patients, but it has not yet achieved satisfactory curative effect. Therefore, it is important to explore new biomarkers for predicting the prognosis and improving the outcome of osteosarcoma patients.
MicroRNAs (miRNAs) are an abundant group of endogenous non-coding single strand RNAs of ~22 nucleotides[3]. They regulate gene expression at post-transcriptional level by translational repression or degradation of target mRNA. In this manner, they participate in various biological process including cell development[4,5], cell cycle[6], and stem cell renewal[7]. Aberrant expression of miRNAs plays a key role in cancer development and progression through modulating oncogenic and tumor suppressor pathways. Using microarray or real-time PCR, miRNA expressing profileswhich can differentiate cancer and normal tissues have been identified[8]. Moreover, different types of cancers have varied expression pattern of miRNAs. Due to its high stability in serum, miRNAs are becoming promising diagnostic and prognostic biomarkers for patients with malignancy[9,10].
MiR-218 is proposed as a novel tumor-related miRNA and has been found to be significantly deregulated in tumors[11-17]. It was down-regulated in cervical[11], gastric[12], lung[13], colon[14], prostate[15], bladder cancer[16] and hepatocellular carcinoma[17]. Furthermore, low miR-218 expression was correlated with a significant worse survival of HCC patients[17]. Recently, Jin et al reported that reduced miR-218 expression was observed in osteosarcoma tissues[18]. Otherwise, miR-218 inhibited osteosarcoma cell migration and invasion by downregulating T-cell lymphoma invasion and metastasis 1 (TIAM1), matrix metalloproteinase2 (MMP2) and MMP9[18]. However, the clinical significance of miR-218 in osteosarcoma tissues and its role in cell growth remain poorly understood.
In this study, we detected miR-218 expression in human osteosarcoma and matched normal tumor-adjacent tissues using qRT-PCR. The correlation between miR-218 expression and clinicopathologic features of osteosarcoma were systemically analyzed. Furthermore, we explored the role of miR-218 in the proliferation and apoptosis of osteosarcoma cells to confirm the effect of miR-218 on the initiation and development of human osteosarcoma.
2. Materials and methods
2.1. Clinical samples
68 osteosarcoma samples were collected from patients including 40 males and 28 females, who underwent the resection of their primary osteosarcoma in the Department of Orthopaedics, PLA 180 Hospital during Jan 2004 to Dec 2008. All samples were used after obtaining informed consent. The demographic features and clinicopathologic date are shown in Table 1. All the patients did not receive preoperative blood transfusion, radiation therapy and chemotherapy. The Union for International Cancer Control (UICC) tumor-nodemetastases (TNM) Staging Classification (6th ed.) was used for the staging of the tumor. The Fujian Medical University Ethics Committee approved all protocols according to the Helsinki Declaration (as revised in Edinburgh 2000).
2.2. Real time quantitative reverse transcription-PCR (qRTPCR)
Total RNA was isolated using TRIzol® (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The PCR amplification for the quantification of the miR-218 and U6 was performed using TaqMan miRNA Reverse Transcription Kit (Applied Biosystms, Foster City, CA, USA) and TaqMan Human MiRNA Assay Kit (Applied Biosystems). The relative expression of miR-218 was shown as fold difference relative to U6.
2.3. Cell line and transfection
The human osteosarcoma cell line, Saos-2 (ATCC, Manassas, VA, USA), were cultured in complete Dulbecco’s modified Eagle medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Gibco) with 100 units/mL penicillin and 100 μg/mL streptomycin (Sigma, St-Louis, MO, USA) in a humidified containing of 5% CO2 incubator at 37 ℃.
MiRNA vectors, including miR-218 and the negative control for the miR-218 were purchased from Genecopoeia (Guangzhou, China). Cells were transfected with the vectors mentioned above using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA).
2.4. BrdU cell proliferation assay
Saos-2 cells that were transfected with miR-218 or control plasmids were seeded into 96-well plates at 5 000 cells per well for 24 hours and detected using a Cell Proliferation ELISA, BrdU (5-bromodeoxyuridine) (chemiluminescent) (Roche, Indianapolis, IN, USA), as previously described[19].
2.5. Cell apoptosis assay
Briefly, Saos-2 cells were transfected with miR-218 or control plasmids and 106 treated cells were collected for flow cytometry. The flow cytometry for the quantification of the cell apoptosis was performed using a BD FACS Canto II Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and an Annexin-V-FLUOS Staining Kit (Roche), as previous reported[20].
2.6. Statistical analysis
Results are expressed as Mean±SEM. Significance was established, with GraphPad Prism 5 software (GraphPadSoftware, Inc, San Diego, CA, USA), using a Pearson Chisquared test and a student’s t-test when appropriate. Difference were considered significant when P< 0.05.
3. Results
3.1. Levels of miR-218 expression in osteosarcoma and matched noncancerous tissues
To determine the expression of miR-218 in osteosarcoma specimens, we detected the levels of miR-218 expression in a retrospective cohort of 76 osteosarcoma and matched normal tumor-adjacent tissues using qRT-PCR. Quantitative analysis indicated that the level of miR-218 expression in osteosarcoma tissues was significantly lower as compared with that in matched nontumor tissues (0.31±0.04vs.0.63± 0.06,t=8.735, P<0.001).
3.2. Clinical significance of miR-218 in osteosarcoma cases
Since reduced miR-218 expression correlates with poor clinicopathologic features of human cancers[11-17]. To investigate the clinical significance of miR-218 in osteosarcoma, we analyzed the correlation between miR-218 expression and different clinicopathological parameters of osteosarcoma. The level of miR-218 was considered as either high (T/NT≥1) or low (T/NT<1) expression. As shown in Table1, Clinical association analysis using a Pearson chi-squared test indicated that low miR-218 expression was evidently correlated with large tumor size (Χ2=5.380, P=0.020), advanced clinical stage (Χ2=6.692, P=0.010) and distant metastasis (Χ2=4.180, P=0.041). Taken together, our data indicate that the level of miR-218 expression is downregulated in osteosarcoma and reduced miR-218 level is associated with poor clinicopathological characteristics.
3.3. Upregulation of miR-218 inhibits cell proliferation and induces apoptosis in Saos-2 cells
Recent researches suggested that miR-218 acts as a tumor suppressor by inhibiting cancer cell proliferation and inducing apoptosis[11,14,17]. To confirm the role of miR-218 in osteosarcoma, Saos-2 cells that were transduced with miR-218 or miR-control plasmids were subjected to BrdU assay and flow cytometry for cell proliferation and apoptosis. As measured by qRT-PCR, the level of miR-218 expression was significantly upregulated by exogenous miR-218 in Saos-2 cells (0.97±0.04vs.16.86±0.77,t=19.42, P<0.001). BrdU assays were performed to determine the effect of altering miR-218 levels on tumor cell proliferation. We found that upregulation of miR-218 resulted in a significant reduction of cell proliferation in Saos-2 cells (133.70±6.27 vs. 60.83 ±5.06,t=9.045, P<0.001). Furthermore, as determined by flow cytometer, the number of apoptotic Saos-2 cells was prominently increased after upregulation of miR-218 (5.67±0.76%vs.30.20±1.89%,t=12.38, P<0.001; Figure 1). Thus, miR-218 may exert an anti-growth effect by inhibiting cell proliferation and inducing apoptosis in osteosarcoma.
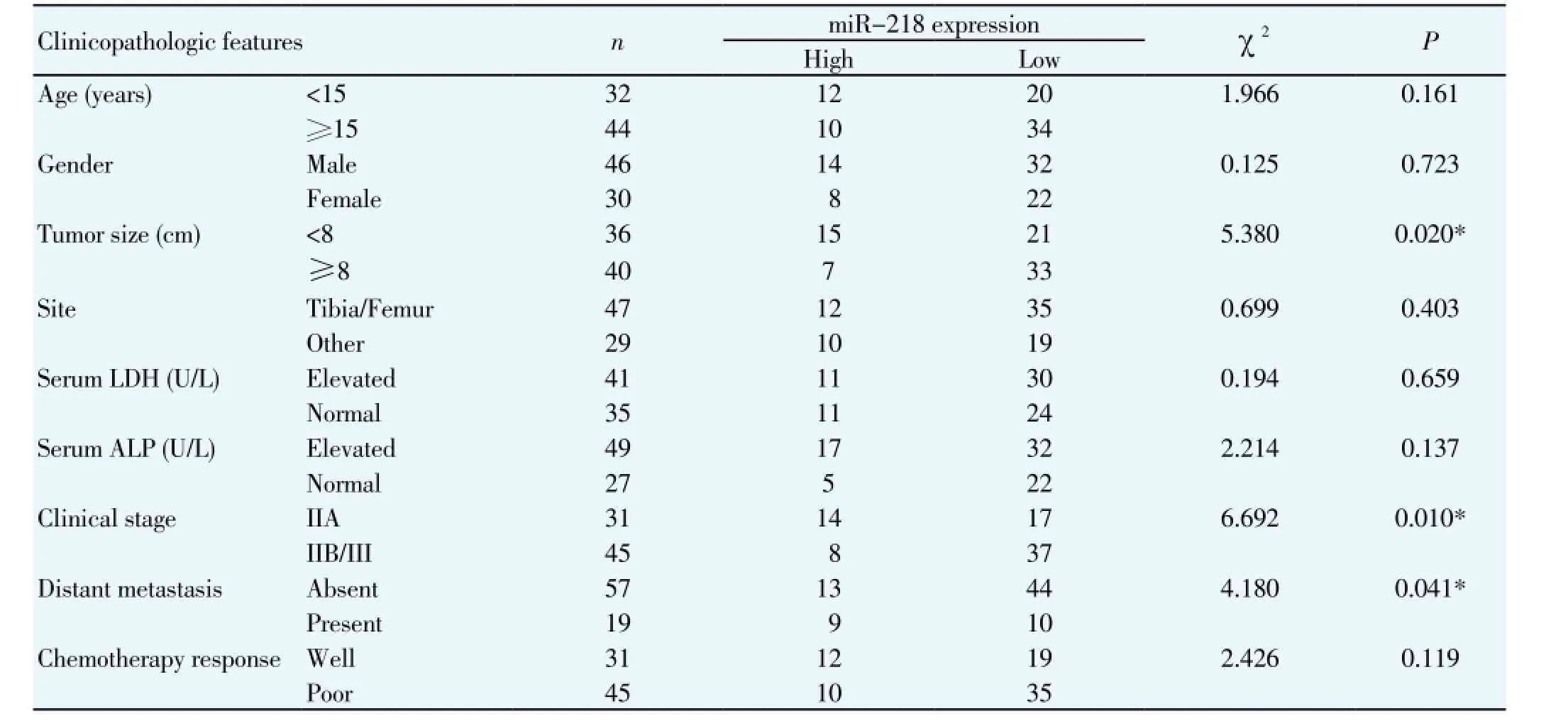
Table 1 Correlation between the clinicopathological characteristics and expression of miR-218 in the osteosarcoma patients (n=76).

Figure 1. Upregulation of miR-218 increased apoptotic osteosarcoma cells.Saos-2 cells that were transfected with miR-218 or miR-control were subjected to flow cytometry for cell apoptosis. Quantitative data revealed that upregulation of miR-218 led to a significant elevated number of apoptotic Saos-2 cells. n=6, * P<0.05 by t test.
4. Discussion
Recently, low miR-218 expression has been observed in various human tumor cells and tissues[11-17]. The level of miR-218 expression is down-regulated in cervical cancer tissues, and human papilloma virus 16 (HPV 16) lead to reduction of miR-218 in cervical cancer cells[11, 21]. In colon cancer, the expression of miR-218 in the cancer tissues is significantly lower than that in the matched noncancerous tissues[14]. The level of miR-218 is correlated with TNM stage, lymph node metastasis and histological differentiation. Furthermore, low miR-218 expression confers a worse survival of colon cancer patients[14]. Tu et al has reported that the expression of miR-218 is impaired in hepatocellular carcinoma tissues[17]. MiR-218 is expressed at significant lower level in patients with large tumor size and advanced TNM tumor stage[17]. In this study, the levels of miR-218 expression were detected in 76 pairs of osteosarcoma and matched nontumor tissues. We found that miR-218 expression in the osteosarcoma tissues was prominently lower than that in the matched noncancerous tissues, which was consistent with previous report[18]. Moreover, we showed that miR-218 expression was obviously correlated with tumor size, clinical stage and distant metastasis in osteosarcoma. Taken together, our results indicate that miR-218 may be a potential tumor suppressor and act as a prognostic biomarker for predicting survival of osteosarcoma patients.
Prior study reported that miR-218 inhibited cell cycle progression and promoted apoptosis through downregulating B lymphoma Mo-MLV insertion region 1 homolog (BMI-1) in colon cancer[14]. Venkataraman et al reported that miR-218 acted as a tumor suppressor by targeting several cancerrelated genes including cyclin dependent kinase (CDK6), Rictor and cathepsin B (CTSB) in medulloblastoma[22]. Researchers found that tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma[23]. The data from Liu ea al indicated that miR-218 inhibited oncogenic transcription factor lymphoid enhancer factor 1 (LEF1) and subsequently reversed high invasiveness of glioblastoma cells[24]. Recently, Alajez et al approved that miR-218 inhibited nasopharyngeal cancer progression by inversely regulating of survivin and the SLIT2-ROBO1 pathway[25]. All the researches above suggest that miR-218 functions as a potent tumor suppressor in multiple human cancers by targeting different genes. MiR-218 in osteosarcoma has been reported previously, Jie et al suggest that miR-218 inhibits osteosarcoma cell migration and invasion by down-regulating TIAM1, MMP2 and MMP9 expression[18]. However, the role of miR-218 in osteosarcoma cell proliferation and apoptosis remain poorly undetermined. In our study, miR-218 was over-expressed in Saos-2 cells via exogenous expression plasmids transfection. BrdU cell proliferation assays found that miR-218 overexpression led to a significant reduction of cell proliferation in Saos-2 cells. Otherwise, flow cytometer detection showed that miR-218 overexpression increased the percentage of apoptotic Saos-2 cells. Our data indicate that miR-218 may suppress tumor progression by inhibiting cell proliferation and inducing apoptosis in osteosarcoma.
In conclusion, we find that the expression of miR-218 is down-regulated in osteosarcoma tissues and its low expression is correlated with tumor size, distant metastasis and clinical stage. Furthermore, miR-218 overexpression leads to growth arrest and apoptosis in Saos-2 cells, suggesting that miR-218 may be a potential valuable biomarker and therapeutic target for human osteosarcoma.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol 2013; 25(4): 398-406.
[2] Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009; 152: 3-13.
[3] Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol 2010; 42(8): 1273-1281.
[4] Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136(2): 215-233.
[5] Liu Z, Tu K, Liu Q. Effects of microRNA-30a on migration, invasion and prognosis of hepatocellular carcinoma. FEBS Lett 2014; 588(17): 3089-3097.
[6] Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007; 318(5858): 1931-1934.
[7] Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005; 122(1): 6-7.
[8] Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev 2013; 23(1): 3-11.
[9] Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6(11): 857-866.
[10] Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol 2012; 6(6): 590-610.
[11] Li J, Ping Z, Ning H. MiR-218 Impairs Tumor Growth and Increases Chemo-Sensitivity to Cisplatin in Cervical Cancer. Int J Mol Sci 2012; 13(12): 16053-16064.
[12] Xin SY, Feng XS, Zhou LQ, Sun JJ, Gao XL, Yao GL. Reduced expression of circulating microRNA-218 in gastric cancer and correlation with tumor invasion and prognosis. World J Gastroenterol 2014; 20(22): 6906-6911.
[13] Davidson MR, Larsen JE, Yang IA, Hayward NK, Clarke BE, Duhig EE, et al. MicroRNA-218 is deleted and downregulated in lung squamous cell carcinoma. PLoS One 2010; 5(9): e12560.
[14] He X, Dong Y, Wu CW, Zhao Z, Ng SS, Chan FK, et al. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Mol Med 2013; 18: 1491-1498.
[15] Nishikawa R, Goto Y, Sakamoto S, Chiyomaru T, Enokida H, Kojima S, et al. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci 2014; 105(7): 802-811.
[16] Tatarano S, Chiyomaru T, Kawakami K, Enokida H, Yoshino H, Hidaka H, et al. miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int J Oncol 2011; 39(1): 13-21.
[17] Tu K, Li C, Zheng X, Yang W, Yao Y, Liu Q. Prognostic significance of miR-218 in human hepatocellular carcinoma and its role in cell growth. Oncol Rep 2014; 32(4): 1571-1577.
[18] Jin J, Cai L, Liu ZM, Zhou XS. miRNA-218 inhibits osteosarcoma c1ell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev 2013; 14(6): 3681-3684.
[19] Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, et al. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer 2014; 13(1): 110.
[20] Tu K, Zheng X, Zhou Z, Li C, Zhang J, Gao J, et al. Recombinant human adenovirus-p53 injection induced apoptosis in hepatocellular carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls c-Myc and cyclin E. PLoS One 2013; 8(7): e68574.
[21] Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene 2008; 27(18): 2575-2582.
[22] Venkataraman S, Birks DK, Balakrishnan I, Alimova I, Harris PS, Patel PR, et al. MicroRNA 218 acts as a tumor suppressor by targeting multiple cancer phenotype-associated genes in medulloblastoma. J Biol Chem 2013; 288(3): 1918-1928.
[23] Kinoshita T, Hanazawa T, Nohata N, Kikkawa N, Enokida H, Yoshino H, et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget 2012; 3(11): 1386-400.
[24] Liu Y, Yan W, Zhang W, Chen L, You G, Bao Z, et al. MiR-218 reverses high invasiveness of glioblastoma cells by targeting the oncogenic transcription factor LEF1. Oncol Rep 2012; 28(3): 1013-1021.
[25] Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Re 2011; 71(6): 2381-2391.
ment heading
10.1016/S1995-7645(14)60176-0
*Corresponding author: Kun Chen, M.D., Department of Orthopaedics, PLA 180 Hospital, Quanzhou 362000, China.
E-mail: chenkun2323@163.com
Foundation project: This study is supported by a grant from the Youth Scientific Research Subject of Fujian Province Health Department (2012-2-114).
Osteosarcoma
Proliferation
Apoptosis
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Assessment of traumatic brain injury degree in animal model
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
- Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties
