Immunogenic potential and protective efficacy of formalin inactivated circulating Indian strain of West Nile virus
Pritom Chowdhury, Siraj Ahmed Khan, Rashmee Topno, Prafulla Dutta, R.N.S. Yadav, Jagadish Mahanta
1Regional Medical Research Centre, ICMR (NE Region), Dibrugarh, Assam, India
2Centre for studies in Biotechnology, Dibrugarh University, Dibrugarh-786004, Assam, India
Immunogenic potential and protective efficacy of formalin inactivated circulating Indian strain of West Nile virus
Pritom Chowdhury1,2, Siraj Ahmed Khan1*, Rashmee Topno1,2, Prafulla Dutta1, R.N.S. Yadav2, Jagadish Mahanta1
1Regional Medical Research Centre, ICMR (NE Region), Dibrugarh, Assam, India
2Centre for studies in Biotechnology, Dibrugarh University, Dibrugarh-786004, Assam, India
Objective:To assess the best suitable condition for virus inactivation, and to study the immunogenic potential and protective efficacy of a circulating West Nile virus (WNV) strain in Assam.Methods:Bulk preparation of circulating WNV: WNIRGC07 (GeneBank ID: HQ246154), was undertaken in a bioreactor using cytodex-1. Virus Inactivation was done in three different conditions; 22 ℃, 4 ℃ and room temperature. The virus preparations were evaluated for antigenicity by ELISA and toxicity by cell proliferation kit. Virus efficacy was done in-vivo on swiss albino mice against standard Indian WNV and Japanese encephalitis virus (JEV) strain. Humoral and cell mediated immune response was evaluated in mice sera by ELISA and neutralization assay.Results:Inactivation at 22 ℃ was found to be more suitable in terms of less toxicity and high antigenicity. The same was selected to study the immune response and efficacy in mice. It induced neutralizing antibody titre of 1: 625 and high IgG response. In vivo experiment showed 100% protective efficacy against WNV and 20.8% cross protective efficacy against JEV. Further assessment of cellular immunity through immunized mice revealed augmentation of high levels of pro-inflammatory cytokines and moderate levels of anti-cytokines indicating a mixed balance of Th1 and Th2 response.Conclusions:Findings suggest that formalin inactivated Indian WNV strain has a good immunogenic potential. This is the first study on assessment of immunogenic potential of a lineage 5 strain of WNV. Our study reveals that it would be a promising and effective candidate for vaccine studies which warrants further evaluation.
ARTICLE INFO
Article history:
Received 10 September 2014
Received in revised form 15 October 2014
Accepted 15 November 2014
Available online 20 December 2014
West Nile virus
Assam
Immunization
Efficacy
Neutralizing antibody
Cytokine
1. Introduction
West Nile virus (WNV), a pathogenic arbovirus belongs to genusflavivirus, familyFlaviviridae. Its natural transmission cycle involves reservoir hostsviz. wild and domestic birds. Mosquitoes, generallyCulexspecies act as principal vectors, humans are dead end hosts[1,2]. Most of the human WNV infections remain subclinical. Febrile illness and neuroinvasive disease develop in ~20% and <1% of the infected patients respectively[3]. Initially, the virus was distributed in Africa, Asia and Europe where it caused infrequent and unpredictable epidemics of mild systemic disease[4]. However, the virus has spread rapidly to new regions like Romania (1996) and the United States (1999) and recently in Greece and Italy (2010) resulting in hundreds of neurological and fatal cases worldwide[4-6]. In India, antibodies against WNV were first detected in human sera from Bombay (1952)[7]. Since then, febrile illness in epidemic form and clinically overt encephalitis cases have been observed from southern, central and western India[8]. During 2006, WNV was detected in Assam as another etiological agent causing acute encephalitis syndrome in addition to the endemic Japanese encephalitis virus (JEV)[9]. Control of WNV is difficult due to availability of abundant breeding habitats of potential vectors and role of frequentlyvisiting migratory birds which act as reservoir hosts in the virus transmission cycle. Evidence of nonvector transmission like intrauterine and mother-to-child transmission of WNV via breast milk further suggest a complex epidemiology of the disease[10]. Therefore, the development of a safe and effective vaccine attains an urgent priority.
Currently, there are five inactivated and chimeric vaccines licensed for veterinary use[11]. However, no human vaccine is available though several candidate vaccines are in clinical trial. A Chimeric vaccine Chimerivax-WN02 in phase Ⅱclinical trial is found to be safe and highly immunogenic in adults and elderly persons[12]. One important factor to consider when evaluating flavivirus vaccine candidates is the possibility of cross protection due to similarities among viral epitopes. Studies in experimentally infected bonnet macaques have shown that inoculation with inactivated WNV results in limited protection against a JEV challenge, while inactivated JEV immunization offers full protection against a WNV challenge[13]. A study in mice showed that formalin inactivated West Nile (WN) vaccine candidateviz. WN-VAX is a safe and effective vaccine that offered 100% protection against lethal WNV challenge[11]. Consequently, there is considerable concern and scientific interest regarding invasion of WNV into a JEV endemic region. In Assam, a JE endemic belt; subsequent invasion of WN in 2006 has led to newer public health concerns. It will be important to study the immune response of circulating WNV strain and to assess its cross protective role. WNV strains are classified into at least 8 putative genetic lineages[14], in which, all the Indian isolates from 1950s to 1980 fall under lineage V, with two exceptions; one from a human patient (1967) and another from a bat (1968)-both closely related to the lineage 1 strain[15]. To our knowledge, no study has been undertaken to study the immune response and efficacy of WNV isolates from India.
In the present study, a Baby Hamster kidney 21 (BHK-21) cell culture derived, formalin inactivated WNV isolated from human clinical sample in Assam during 2007 was assessed for its immunogenicity and efficacy. WNV was inactivated at different temperatures to screen the best condition for inactivation in terms of toxicity and antigenicity. It was further assessed for cross neutralization and immunogenic potential in augmenting humoral and cellular immune response in Swiss albino mice model.
2. Materials and methods
2.1. Virus
A circulating strain of WNV: WNIRGC07, isolated in 2007 from human acute encephalitis syndrome patient (GeneBank ID: HQ246154) from Assam, India, was used. Phylogenetic analysis placed it under lineage V (RMRC, Dibrugarh unpublished data). Four virus passages in infant Swiss albino mice and subsequently two passages in BHK-21 cell line were done to increase adaptability and virus titer. An Indian prototype strain of JEV, P20778 (Isolated in 1985, human brain: AF080251) was used to check for cross protection and an Indian WNV prototype strain G22886 (isolated in 1980,Culex vishnui: DQ256376) was used as control. Virus strains were obtained from the virus repository of National Institute of Virology, Pune, India.
2.2. Cells and cell propagation
BHK-21cell line was used to propagate the virus. The cell line was obtained from National Centre for Cell Science, Pune, India, and maintained in Eagles Minimal Essential Medium (EMEM, Sigma) supplemented with 10% heat inactivated fetal bovine serum, 7.5% sodium bicarbonate (Sigma) and 2 mM L-glutamine.
2.3. Virus production
BHK-21 cells were grown on Cytodex-1 microcarrier (Sigma) prepared according to manufacturer’s instructions in 5 000 mL capacity bioreactor (Bioflow 110, New Brunswick Scientific). Cytodex-1 beads were suspended into 9×103BHK-21 cells/mL culture medium at a concentration of 2 g/L. Cells were then allowed to attach on microcarriers for 3 h at 37 ℃, with intermittent stirring. Volume of the media was made up to 3 000 mL. Bioreactor was then incubated at 37 ℃under SPM and stirred at 40 rpm. After 4 d the confluent beads were infected with virus inoculums added at a multiplicity of infection of 0.1. It took 4 d for the cytopathic effect (CPE) to become pronounced and at this stage BHK-21 cells were detached from the cytodex beads. The culture supernatant was harvested and virus titers were determined by CPE assay[16].
2.4. Formalin inactivation
The virus infected culture supernatant was clarified by centrifugation at 10 000 rpm for 1 h at 4 ℃. Formalin (37% formaldehyde, Merck) was diluted 1:40 in PBS (the final pH 7.2-7.4) and was filtered through a 0.22 μm nitrocellulose filter unit. A final concentration of 5 mM formaldehyde was added to the suspensions for inactivating the virus at 3 different temperaturesviz.22 ℃, 4 ℃ and room temperature for 10 d. The suspensions were centrifuged at10 000 rpm for 1 h at 4 ℃. The supernatant was treated with protamine sulphate (1 μg/mL) and kept at 4 ℃ for 30 min. The suspension was centrifuged at 5 000 rpm for 20 min. Inactivated virus preparations were tested for residual virus infectivity by intra-cerebral inoculation of neonatal mice and on to monolayer of BHK-21 cell line.
2.5. Inactivated virus antigenicity
The antigenicity of virus preparations were determined by indirect antigen capture ELISA by following standard method[17]. Nunc maxisorp microtitre plates were coated with 50 μL/well of Flavivirus specific monoclonal antibody (HX-B) at a dilution of 1:50 in coating buffer. Biotinylated HX-B was used as detector antibody.
2.6. In vitro microcytotoxicity assay
Cell toxicity assay of inactivated virus preparations were evaluated for cell cytotoxicity by using 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-tetrazolium Bromide (MTT) cell Proliferation kit (Roche) with slight modification. Briefly, 10 μL of inactivated virus preparation was inoculated into a 96 well plate pre-seeded with 3.5×105cells/mL. After the incubation period, the treated cells were evaluated for cell cytotoxicity by adding 10 μL of MTT labeling reagent and incubated for 4 h in incubator. A total of 100 μL of solubilization reagent (Roche) was added into the treated wells and left overnight in incubator. The percentage of cytotoxicity was calculated as [(A-B)/A×100] where A and B are the absorbances of control and treated cells respectively[18].
2.7. Reverse transcriptase (RT)-PCR
The viral RNA was extracted from 140 μL of inactivated virus and an untreated WNV pool as a positive control using QIAamp viral RNA mini kit (Qiagen, Germany), according to manufacturer’s instruction. RT-PCR was done targeting the NS5 region of WNV using the primer sequence Forward 5’-GCTCCGCTGTCCCTGTGA-3’ and reverse 5’-CACTCTCCTCCTGCATGGATG-3’[19]. Briefly, two steps RTPCR was carried out; cDNA was prepared using MMLV reverse transcriptase enzyme. PCR was done with 25 μL reaction volume containing 12.5 μL of Promega 2×PCR master mix, 2 μL of 10 μm primers. The thermal profile was set at 94 ℃ for 3 min, 35 cycles of 94 ℃ for 30 sec, 59 ℃for 1 min, 72 ℃ for 1 min and final extension at 72 ℃ for 5 min. Amplified product was run in Bioanalyzer (Agilent) for screening of any WNV nucleic acid.
2.8. Animal immunization and virus challenge
2.8.1. Mice immunization
For immunogen preparation and efficacy test, the best suitable inactivated virus preparation was mixed with equal volume of alhydrogel (2% alum, Sigma) and incubated at room temperature for 30 min and stored at 4 ℃. A total of 12 groups (n= 8 each) of 3 to 4 week old Swiss albino mice were immunized subcutaneously with 50 μg of immunogen. Three groups were injected with PBS as control. Booster injections with same formulation were given on 14 and 28 d after first immunization.
2.8.2. Efficacy tests
One week after administration of the second booster dose, out of 12 groups immunized, 9 groups of mice were challenged with 7×105PFU/mL of WNIRGC07, WNV G22886 strain and JEV P20778 (3 groups each) and a control group challenged with PBS. All animal studies were conducted in accordance with experimental protocol approved by Animal Welfare and Animal Care Committee of RMRC, Dibrugarh.
2.9. Determination of humoral immune response
Neutralizing antibodies against G22886, WNIRGC07 and P20778 virus strains were measured for the immune sera collected at 7 days post immunization by following standard method[15]. On day 7, post second booster administrations, 2 groups of immunized mice and 1 control group were bled and serum was separated. They were pooled separately for immunized and control groups.
2.10. Assessment of cell mediated immune response
On day 2 post second booster administrations, 1 group of immunized mice with and 1 control group were bled and serum was separated. They were pooled separately for immunized and control groups. Mice were sacrificed according to guidelines of laboratory animal handling. The cytokine profiling were done for interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10) and tumor necrosis factor (TNF-α) by using BD biosciences ELISA kit as per manufacturer’s guidelines.
2.11. Statistical analysis
Ag capture ELISA data was determined as end point titer (log10) ±SE against respective three temperature conditions. Survival rates of the immunized mice were expressed as the proportions of mice mortality at the end of the observation period.Chi-square test was used to determine significantdifferences between the groups, P-value <0.05 was considered to be significant.
3. Results
3.1. Growth of WNV in BHK-21 cells using microcarriers
BHK-21 cells grown on microcarrier (cytodex-1) beads were harvested 96 h post infection (pi) after appearance of a pronounced CPE. The virus titer on BHK-21 cell monolayer was found to be 107TCID50for the WNV cultured in BHK-21 using micro carriers.
3.2. Inactivation of WNV
WNV inactivation was confirmed after repeated passages into infant mice and BHK-21 cell monolayer. No mortality was observed in infant mice inoculated intracranially, whereas, infants inoculated with live virus died after 3-4 d of inoculation. CPE was not observed in cell monolayer in all the three conditions. Quantification of inactivated viral PCR amplicons in Bioanalyzer showed no sign of viral nucleic acid. ELISA generated absorbance in three conditions-22 ℃, 4 ℃ and RT are log10(1.34±0.02), log10(1.25±0.01) and log10(1.22±0.01). Antigenicity of the formalin inactivated virus preparation stored at 22 ℃ was found to be higher with than those stored at 4 ℃ and RT.
3.3. Cell Toxicity evaluation by MTT assay
Cytotoxicity of inactivated WNV at three different storage temperature conditions was quantitatively determined by MTT assay. It was observed that the formalin treated virus stored at 4 ℃ was most toxic (induced cell death in 21% of cells). However, the batch stored at RT was less toxic (inducing 6.78% cell death). The best result was shown by the batch stored at 22 ℃ which produced only 2.99% cell toxicity. Thus, inactivation at 22 ℃ was found to be the best suitable condition to study efficacy and immune response in mice and inin-vitromodels.
3.4. Efficacy test
Mice were observed for 4 weeks following the virus challenge; any symptom of sickness and total survival were recorded. The immunized mice offered complete protection against the WNV’s challenge [WNIRGC07-100% (P<0.05) and G22886-100% (P<0.05)] and partial protection [20.8% (P>0.05)] against JEV. Infant mice inoculated with JEV showed hind limb paralysis followed by death 8-10 d post-inoculation.
3.5. Assessment of humoral immune response
Anti-WNV neutralizing antibody of the immunized group of mice was assayed by a neutralization test. Neutralizing antibody titer of 1:625 was observed in the group administered with the second booster dose of the inactivated WNV antigen. This result suggest strong protective efficacy of the vaccine candidate inin vitrosystem. However, the post-immunized WNV specific mice sera showed minimal cross protective neutralizing antibodies against JEV with a titre of 1:25 (Figure 1).
3.6. Assessment of cellular immune response
Further characterization of immune response in mice was carried out by investigating specific cytokine response to immunization. Compared to un-immunized mice sera, the immunized mice sera produced high level of proinflammatory cytokines IL-6 and TNF-α and moderate levels of anti-inflammatory cytokines IL-10 and IL-4 (Table 1).

Figure 1. Microseroneutralization test performed using WNV (G22886) and serial fivefold dilution of immunized mice sera (WNVIRGC 07, G22886, P20778) collected after 7 d of second booster dose.
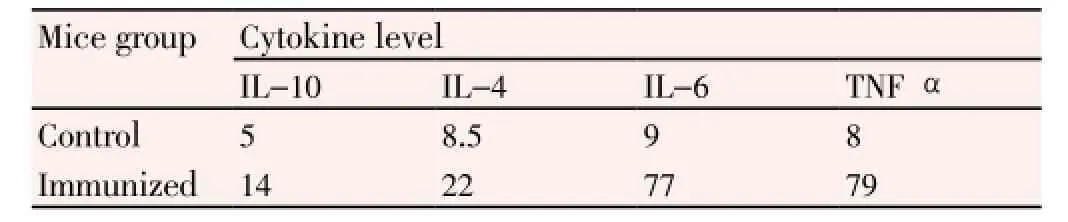
Table 1 Concentrations of cytokines in immunized mice after 48 h of second booster dose with inactivated WNV (pg/mL).
4. Discussion
WNV invasion continues to expand its geographic distribution. This has a significant impact on both human and veterinary concerns. Currently, there is no approved commercially available vaccine for prevention of WNV infection in humans and case management is limited to symptomatic and supportive medication; therefore development of an effective vaccine against it attains a high priority. Immunogenicity and virulence of different strains should be characterized to assess their immunogenic properties. One of the main advantages of inactivated virusvaccines is that they are safe and stable. They often do not require refrigeration which is an important selection criterion for its use in many developing countries. However, a disadvantage is the requirement of multiple booster doses to elicit and sustain an effective immune response. Further, in few cases the immune response may be imbalanced, leading to subsequent elicitation of the disease[20,21]. A recent study suggested a model for vaccination in JEV endemic areas where a single dose of WNV vaccination will be sufficient to elicit protection against JEV and WNV[22].
In the present study, a BHK-21 adapted Indian isolate of locally circulating WNV was used to study immune response and efficacy for a probable vaccine candidate. The WHO recommends the use of BHK-21 cells in the quality testing of JE vaccines such as the virus-inactivation test and potency estimation[23]. To exploit the adherent properties of BHK-21 cells, we adopted the microcarrier technology in the controlled condition of bioreactor[24]. High WNV titer was achieved by using microcarrier beads. Out of three temperature conditions employed for storing the virus during the inactivation process, 22 ℃ was found to be best suitable in account of higher antigenicity and less toxicity. Similar studies on JEV report inactivation at 22 ℃are more immunogenic than that of inactivation at 4 ℃[25]. Although, the exact mechanism of RNA degradation through formalin inactivation is not known, it may be due to the fact that formaldehyde reacts with RNA forming an N-methylol (N-CH2OH) followed by an electrophilic attack to form a methylene bridge between amino groups resulting in cross-linkage between nucleic acids and proteins. This cross-linking inhibits reverse transcription of the extracted RNA and interferes in cDNA synthesis[26]. Similar complete inactivation of JEV and Chikungunya virus at 22 ℃for 10 d with repeated filtration has been reported[27,28]. The immunogenic potential of BHK-21 adapted WNV Indian isolate was assessed through determination of humoral as well as cell-mediated immune response. The immune response of post-immunized mice sera was accomplished through determination of neutralizing antibodies. Studies carried out in humans and in animal model have indicated the importance of an effective humoral response in preventing flavivirus infection both in the periphery and within the central nervous system[29,30]. Successful vaccination against WNV requires induction of both neutralizing antibodies and cell-mediated immune responses. The role of cell mediated immunity in currently used vaccines (that have T-cell dependent antigens) is mainly by supporting antibody protection. T-cell independent antigens (eg., polysaccharides) do not stimulate cell mediated immunity and therefore do not produce durable immunity. Generally, killed vaccines elicit humoral immune response but poor inducer of cell-mediated immune responses (CMI). However, there is evidence of protective cell-mediated immune responses following immunization with killed viral vaccines against HIV, Sendai virus and chikungunya virus[28,31,32]. Since CMI is induced and regulated by cytokines, we studied the profile of four major cytokines. In present study, formalin inactivated WNV elicitated comparable amounts of TH1 response (IL-6 and TNF-α) and elicited minute level of TH2 response (IL-10 and IL-4). Activated TH1 cells produce a number of cytokines that defend against viruses either directly or indirectly.
To test the efficacy of inactivated WNV against a lethal challenge, Swiss albino mice model was used. The efficacy experiment showed that immunized mice were 100%protected against a lethal WNV G22886 challenge but they showed partial protection against JEV P20778. Our finding is in agreement with a study done on WN-VAX and JEV-VAX[22]. In our study higher levels of neutralizing antibodies against WNV and lower levels of crossneutralizing antibodies to JEV were found. There are studies which evaluated the efficacy of a formalin-inactivated WNV vaccine candidate of a lineage 1 New York strain (WNVNY99) and observed 100% protection in mice against WNV challenge[11]. In contrast we had performed our study in lineage V WNV strain circulating in India and observed full protection against WNV strain and partial protection against JEV in mice model which is important in geographic regions where WNV and JEV co-circulate. Moreover, we had studied TH1 response and TH2 response.
In conclusion, this study revealed that BHK-21 cells adapted microcarrier technology can support production of high titer of WNV. The formalin inactivated WNV elicited both humoral and cell-mediated immune response in mice. The protective efficacy was established throughin vitroneutralizing antibody titer. These findings clearly suggest that Indian strain of WNV would be a promising and effective candidate for vaccine studies with high protective immune response.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis 2002; 2: 519-529.
[2] Hubalek Z, Halouzka J. West Nile fever-a reemerging mosquitoborne viral disease in Europe. Emerg Infect Dis 1999; 5: 643-650.
[3] Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis 2005; 11: 1174-1179.
[4] Murge B, Zeller H, Deubel V. The ecological and epidemiology of West Nile Virus in Africa, Europe and Asia. Curr Trop Microbial Immunol 2002; 267: 195-221.
[5] Papa A, Perperidou P, Tzouli A, Castilletti C. West Nile virusneutralizing antibodies in humans in Greece. Vector Borne Zoonotic Dis 2010; 10: 655-658.
[6] Angelina P, Tamba M, Finarelli AC, Bellini R, Albieri A, Bonilauri P, et al. West Nile virus circulation in Emilia-Romagna, Italy: The intregrated surveillance system 2009. Eurosurveillance 2010; 15: 1-5.
[7] Banker DD. Preliminary observations on antibody patterns against certain viruses among inhabitants of Bombay city. Indian J Med Sci 1952; 6: 733-766.
[8] Paramasivan R, Mishra AC, Mourya DT. West Nile virus: the Indian scenario. Indian J Med Res 2003; 118: 101-108.
[9] Khan SA, Dutta P, Khan AM, Chowdhury P, Borah J, Doloi P, et al. West Nile Virus Infection Assam, India. Emerg Infect Dis 2011; 7: 947-948.
[10] Gould LH, Fikrig E. West Nile virus: a growing concern? J Clin Invest 2004; 113: 1102-1107.
[11] Herrera GP, Inoue S, Fuke I, Muraki Y, Mupua CA, Khan AH, et al. Development and evaluation of a formalin inactivated West Nile virus vaccine (WN-VAX) for a human vaccine candidate. Vaccine 2010; 28: 7939-7946.
[12] Biedenbender R, Bevilacqua J, Greqq AM, Watson M, Dayan G. PhaseⅡ, randomized double blind, placebo-controlled , multicellular study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J Infec Dis 2011; 203: 75-84.
[13] Goverdhan MK, Kulkarni AB, Gupta AK, Tupe CD, Rodrigues JJ. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol 1992; 36: 277-283.
[14] Papa A, Bakonyi T, Xanthopoulou K, Vazquez A, Tenori, A, Nowotny N. Genetic characterization of West Nile virus Lineage 2, Greece, 2010. Emerg Infect Dis 2011; 17: 920-922.
[15] Bondre VP, Jadi RS, Mishra AC, Yergolkar PN, Arankalle VA. West Nile virus isolates from India: evidence for a distinct genetic lineage. J Gen Virol 2007; 88: 875-884.
[16] Cui J, Counor D, Shen Di, Sun G, Hongxuan H, Vincent D et al. Detection of Japanese encephalitis virus antibodies in Bats in southern China. American J Trop Med Hyg 2012; 78: 1007-1011.
[17] Gajanana A, Rajendran R, Thenmozhi V, Samuel P, Tsai TF, Reuben R. Comparative evaluation of bioassay and ELISA for detection of Japanese encephalitis virus in field collected mosquitoes. South East Asian J Med Pub Health 1995; 26: 91-97.
[18] Fröhner CRA, Antonio RV, Pasa-Creczynski TB, Barardi CRM, Simões CMO. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz, Rio de Janeiro 2003; 98: 843-848.
[19] Briese T, Glass WG, Lipkin WI. Detection of West Nile virus sequences in Cerebrospinal fluid. Lancet 2000; 355: 1614-1615.
[20] Murphy BR, Chanock RM. Immunization against viral diseases. In: Knipe DM, Howley PM, Griffin DE, Martin MA, Lamba RA, et al. Fields virology. Philadelphia: Lippincott Williams and Wilkins; 2001, p. 435-467.
[21] Watson JC, Peter G. General immunization practices. In: Plotkin SA, Orenstein WA, editor. Vaccine. Philadelphia: WB Saunders Company; 1999, p. 47-73.
[22] Lim CK, Takasaki T, Kotaki A, Kurane I. Vero cell-derived inactivated West Nile (WN) vaccine induces protective immunity against lethal WN virus infection in mice and shows a facilitated neutralizing antibody response in mice previously immunized with Japanese encephalitis vaccine. Virology 2008; 374: 60-70.
[23] WHO. Technical Report Series. Requirement for JE vaccine (Inactivated) for human use. Geneva: WHO; 1998, p. 771, 131-151.
[24] VanWezel AL. Growth of cell strain and primary cells on microcarriers in homogeneous culture. Nature 1967; 216: 64-65.
[25] Appaiahari MB, Vrati S. Immunogenicity and protective efficacy in mice of a formaldehyde inactivated Indian strain of Japanese encephalitis virus grown in Vero cells. Vaccine 2004; 22: 3669-75.
[26] Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res 1999; 27: 4436-4443.
[27] Srivastava AK, Putnak JR, Lee SH, Hong SP, Moon SB, Barvir DA, et al. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine 2001; 19: 4557-4565.
[28] Tiwari M, Parida M, Santhosh SR, Khan M, Dash PB, Rao PVL. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine 2009; 27: 2513-2523.
[29] Roehrig JT, Staudinger LA, Hunt AR, Matthews JH, Blair CD. Antibody prophylaxis and therapy for flaviviral encephalitis infections. Ann N Y Acad Sci 2001; 951: 286-297.
[30] Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, RagerZisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile infection in mice. J Infect Dis 2003; 188: 5-12.
[31] Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol 2003; 77: 2578-2586.
[32] Burton DR, Moor JP. Why do we not have an HIV vaccine and how can we make one? Nat Med 1998; 4: 495-508.
ment heading
10.1016/S1995-7645(14)60167-X
*Corresponding authors: Dr Siraj A Khan, Scientist D, RMRC, ICMR (NE Region), Dibrugarh-786001, Post Box-105, Assam, India.
E-mail: sirajkhanicmr@gmail.com
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Chikungunya virus, epidemiology, clinics and phylogenesis: A review
- Induction of deletion mutation on ompR gene of Salmonella enterica serovar Typhi isolates from asymptomatic typhoid carriers to evolve attenuated strains for vaccine development
- Evaluation of protective effect of IL-22 and IL-12 on cutaneous leishmaniasis in BALB/c mice
- Genetic diversity and gene structure of mitochondrial region of Anopheles minimus (Diptera: Culicidae) - major malaria vector of North east India
- Fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. capitatum (L.)
- Antioxidant activity and free radical scavenging activities of Streptomyces sp. strain MJM 10778
