Fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. capitatum (L.)
Abbas Khani, Monireh Heydarian
Department of Plant Protection, Faculty of Agriculture, University of Zabol, Zabol, Iran
Fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. capitatum (L.)
Abbas Khani*, Monireh Heydarian
Department of Plant Protection, Faculty of Agriculture, University of Zabol, Zabol, Iran
Objective:To test fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. capitatum (L.).Methods:The fumigant toxicity test was performed at (27±1) ℃, (65±5)% relative humidity, and under darkness condition and 24 h exposure time. The chemical composition of the isolated oils was examined by gas chromatographymass spectrometry.Results:The major compounds were α-cadinol (46.2%), caryophyllene oxide (25.9%), α muurolol epi (8.1%), cadalene (3.7%) and longiverbenone (2.9%). In all cases, considerable differences in mortality of insect to essential oil vapor were observed in different concentrations and exposure times. Callosobruchus maculatus (C. maculates) (LC50=148.9 μL/ L air) was more susceptible to the tested plant product than Teucrium castaneum (T. castaneum) (LC50=360.2 μL/L air) based on LC50values. In the present investigation, the concentration of 3 μL /mL acetone showed 60% and 52% repellency against T. casteneum and C. maculatus adults, respectively.Conclusions:The results suggests that sesquiterpene-rich essential oils from the tested plant could be used as a potential control agent for stored-product insects.
ARTICLE INFO
Article history:
Received 10 July 2014
Received in revised form 15 August 2014
Accepted 15 October 2014
Available online 20 December 2014
Callosobruchus maculatus
Fumigant toxicit
Gas chromatography-mass
spectrometry
Repellency
Terpenoids
Tribolium castaneum
1. Introduction
The invasion of food products by insects contribute greatly to the loss of quality and quantity. The cowpea weevil,Callosobruchus maculatus(C. maculatus) F. (Coleoptera: Bruchidae), and the rust red flour beetle,Tribolium castaneum(T. castaneum) (Herbst) (Coleoptera: Tenebrionidae), are among the most widespread and destructive stored product pests throughout the world. They cause significant losses of stored products, particularly in tropical and warm temperate regions[1].
Chemicals and fumigants play a vital role in controlling this problem but they have been known to cause serious toxicological and environmental problems. Persistent development of resistance is a serious threat to the future use of these materials, and consequently, there is an urgent need to develop affordable, safe and sound pest control agents and techniques[2]. Researchers are now focused on finding alternative insecticides which will be effective, environmentally friendly, biodegradable, safer and cheaper than existing ones. Plant oils, as an important natural resource of insecticides, are generally considered broadspectrum and safe for the environment because the array of compounds they contain quickly biodegrade in the soil[2-4]. Their lipophilic nature facilitates their interference with basic metabolic, biochemical, physiological and behavioral functions of insects[4].
The genusTeucriumbelongs to the family Lamiaceae (Labiatae), and consists of about 300 species, most of which are found in the dry and stony places of the hills and deserts of Mediterranean countries, South Western Asia, Europe and North Africa[5].Teucrium polium(T. polium) subsp.
capitatumL. is a perennial, pubescent, aromatic plant, 20-50 cm high, white or grey hairs on stems, with greengrayish leaves and white flowers.T. polium(locally called kalpooreh) is one of the wild-growing flowering species and is abundantly found in Iran. Various biological activities have been reported forT. polium, such as antiphytoviral[6], antibacterial[7], antifungal[8], repellent[9,10] and insecticidal properties[11,12].
Phytochemical investigations have shown thatTeucriumgenus contains various compounds such as terpenoids, flavonoids and iridoids[13]. The results of Bezicet al[6] showing that the major compounds inT. poliumessential oil were the sesquiterpene hydrocarbons β-caryophyllene and germacrene D. However, there have been some variations in the constituents of this oil from different countries, and a chemo-geographical variation has been observed in essential oil composition of this species[14].
In the present study, the chemical components of essential oils fromT. poliumsubsp.capitatum(Lamiaceae) aerial parts was determined, and the repellent and insecticidal activity of it was tested against the adult stages of the stored-products pests,T. castaneumHerbst (Coleoptera: Tenebrionidae) andC. maculatusF. (Coleoptera: Bruchidae).
2. Materials and methods
2.1. Insect material
C. maculatusandT. castaneumwere reared in plastic containers (20 cm length, 14 cm width, and 8 cm height, covered by a fine mesh cloth for ventilation) containing bean grain and wheat flour mixed with yeast (10:1, w/w), respectively. The culture was maintained in the dark, in a growth chamber set at (27±1) ℃ and (65±5)% relative humidity. All experiments were carried out under the same environmental conditions.
2.2. Plants and essential oils
Aerial parts (leaves and flowers) ofT. poliumwere collected in Kashmar (35o14’ N, 58o28’ E; 1 100 m a.s.l.) located in Khorasan Razavi province, Iran, from May to July 2010. Essential oil extraction was done following the method described by khaniet al[15]. The extracted oil was dehydrated with anhydrous sodium sulphate (10 min) and immediately stored in airtight glassware in refrigerator at 4 ℃).
2.3. Gas chromatography (GC)-mass spectrometry
The essential oils was analyzed on a gas chromatograph mass spectrometer (GC-mass) (Shimadzu-17A-QP5050, Japan) following the method described by khaniet al[15]. The GC column was DB-5 (30 m×0.25 mmid., 0.25 μm film thickness). The column oven temperature was set at 60 ℃for 3 min, and then increased to 260 ℃ at a rate of 5 ℃/min. Injector and detector temperatures were 230 ℃ and 245 ℃, respectively. The GC mass analysis was carried out with the same characteristics as used in GC. The ionization energy was 70 ev with a scan time of 1s and mass range of 40-500 amu.
2.4. Fumigant toxicity
Fumigant toxicity of the oil on adults of each species (1-7 days old of undefined sex) determined following the method described by khaniet al[15]. Lethal effect assessed in glass vials (60 mL) at concentrations of 166.66, 250, 333.33, 416.67 and 450 μL/L air forT. castaneumand concentrations of 5, 50, 83.33, 166.66, 250 and 416.67 μL/L air forC. maculatus. Each concentration was replicated four or eight times with ten individuals per each replicate. Mortality was determined 24 h after exposure. When no signs of leg or antennal movement were observed, insects were considered dead. The treatment bottles were monitored for 48 h after recording the data, and no affected insect recovered.
To evaluation 50% lethal doses (LC50) after an exposure time of 24 h, the concentrations of the essential oils causing 10% to 90% mortality were used. Data obtained from each dose response bioassay were subjected to probit analysis. LC50values were determined by log-probit regression using SPSS 16.0 for Windows[16].
2.5. Repellency bioassay
Repellency was assessed as described by Fieldset al[17]. The repellency tests were consisted of two clear plastic chambers (65 mL volume) joined to either side of a central main chamber with the same size by a small tubing (2 cm long and 3 mm in diameter). Concentrations of the test solutions were 0.2, 0.5, 1 and 3 μL of essential oil in 1 mL acetone. One milliliter of each solution was applied on 40 seeds of wheat forT. castaneumand 20 seeds of bean forC. maculatus. In the control, the food was treated with 1 mL acetone only. The treated and control seeds were kept for 20 min to evaporate the solvent completely, and placed in the center of treated and control chambers respectively. Three replications were used for each concentration. Fifty nonsexed adults of each species (1-7 days old) were introduced into the center of each main chamber. Central chamber was covered by plastic screen but the treated and control chambers were covered by lids and the whole setup was left in darkness. After 24 h, the number of beetles at each chamber was counted and the percentage repellency (R) values were computed using the formula of Liuet al[18]:
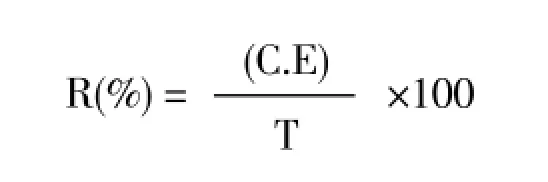
where C is the number of insects in control, E is the number of insects in oil treated chamber and T is the number of total released insects.
2.6. Statistical analysis
Statistical analysis of the mortality and repellency data was performed by one-way analysis of variance (ANOVA) with apost-hocTukey test using SPSS version 16.00. Normality of data was tested using, Kolmogorov-Smirnov test, a nonparametric test. The results were expressed as mean±SE, and considered significantly different at P< 0.05.
3. Results
3.1. Chemical composition of essential oils
The chemical composition of the essential oils ofT. poliumsubsp.capitatumaerial parts (leaves and flowering heads) is presented in Table 1. The major compounds were α -cadinol (46.2%), caryophyllene oxide (25.9%), α muurolol epi (8.1%), cadalene (3.7%), longiverbenone (2.9%), carotol (2.7%) and diethyl phthalate (2.5%) in the essential oils ofT. poliumsubsp.capitatumaerial parts.
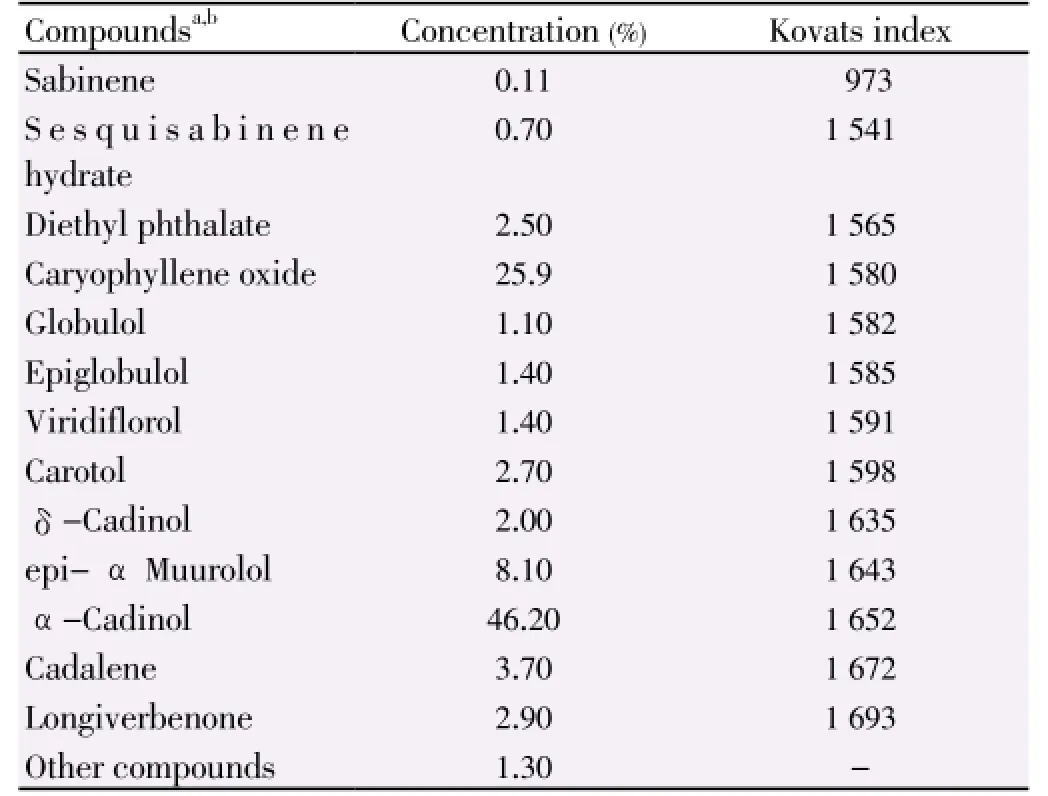
Table 1 Chemical composition of essential oils of T. polium aerial parts (leaves and flowering heads).
3.2. Fumigant toxicity
The mortality increased significantly with rising concentrations (Figure 1) (F4,27=24.16; P=0 forT. castaneumand F5,35=30.13; P=0 forC. maculatus). Results indicated that the oil of tested plant was significantly more toxic againstC. maculatusthanT. castaneum, as inferred by the confidence intervals of LC50(Table 2). Based on LC50(Table 2) and fumigant toxicity experiments (Figure 1), the oil ofM. communisdisplayed strong insecticidal activity againstC. maculatus(LC50=148.9 μL/L air), but revealed fewer activity againstT. castaneum(LC50=360.2 μL/L air) (Figure 2, Table 2).
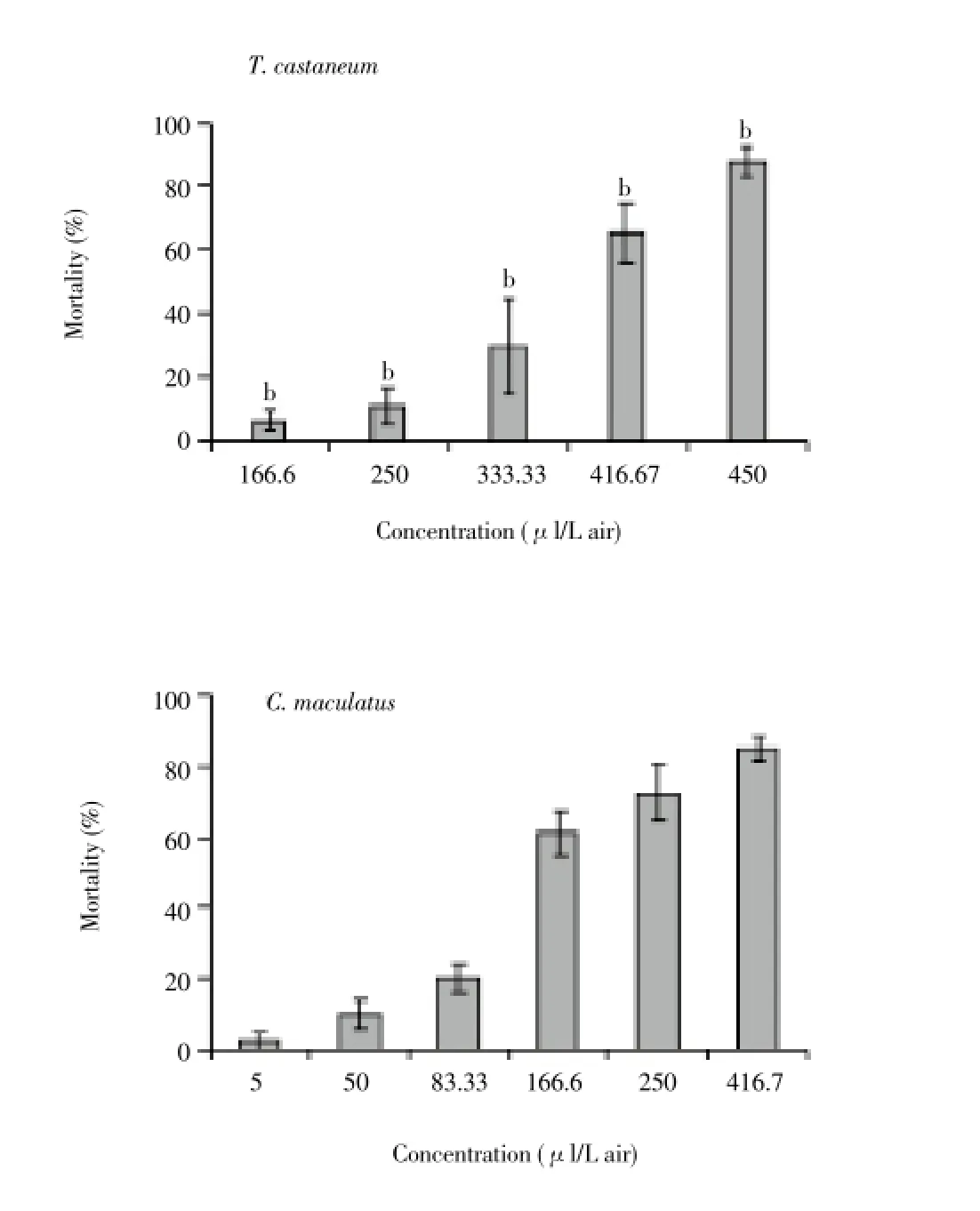
Figure 1. Lethal effect of essential oil from T. polium aerial parts on T. castaneum and C. maculatus after 24 h.

Table 2 Efficiency of essential oil extracted from T. polium against T. castaneum and C. maculatus adults.
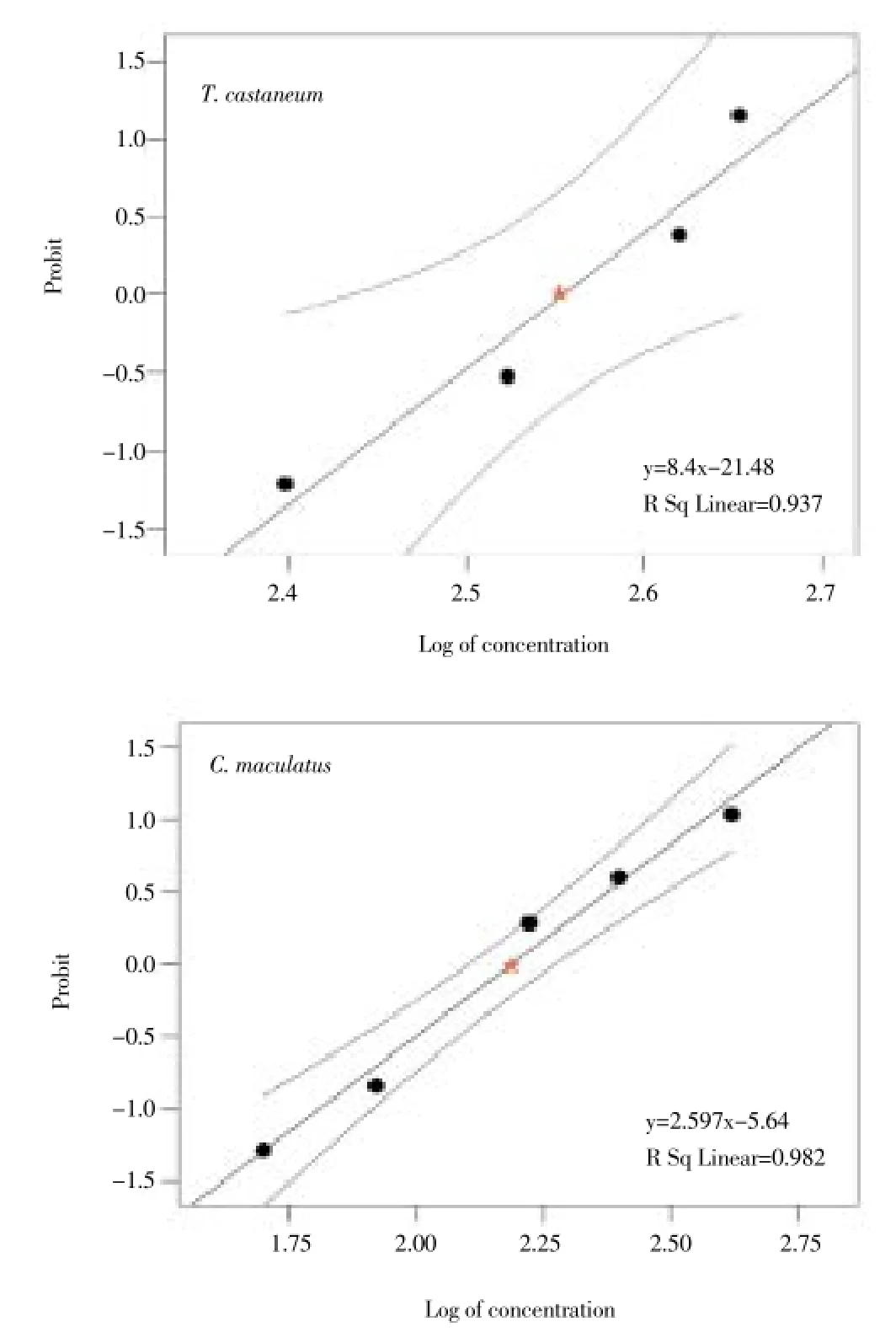
Figure 2. Mortality probit line and 95% confidence limits for T. castaneum and C. maculatus after 24 h exposure to essential oil extracted from T. polium aerial parts. LC50point is shown by the star sign.
3.3. Repellent activity
T. poliumessential oil moderately repelledT. castaneumandC. maculatusin the tested concentrations and the repellency for these two insects was not statistically different in all concentrations (t=-0.8,df= 22,P=0.43) (Table 3). The test showed that the repellency was concentrationdependent and the repellency increased significantly with increasing concentration of essential oil in all cases. In the present investigation, the concentration of 3 μL/mL acetone showed 60% repellency againstT. casteneumbeetle while it was 24.7% repellency at lower concentration 0.2 μL/mL acetone (F3,11=52.38; P=0). Also the repellency forC. maculatuswas obvious when compared to the lowest concentration and the highest one (20% and 52% respectively) (F3,11= 5.03;P=0.3).
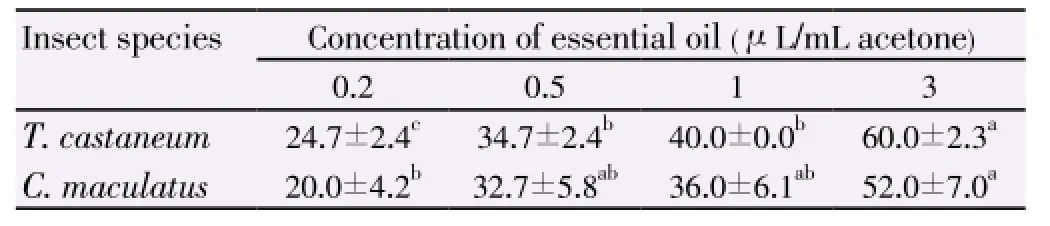
Table 3 Percent repellency (mean±SE) of the essential oil from T. polium on T. castaneum and C. maculatus adults.
4. Discussion
Essential oils ofT. poliumssp.capitatumhave been the subject of many studies. According to the plant origin studied, the amounts of main constituents differed notably[19]. Important differences were found with regard to the major constituents of the essential oils of five populations ofT. capitatumgrown in Portugal. τ-cadinol (24.1%) and α-cadinol (9.8%) were the major compounds in the oil from one population, whereas δ-cadinene (9.8%) and E-caryophyllene (4.6%) or α-muurolol (6.0%) was the major constituents in the other population[20]. Conversely α-pinene (7.7%), sabinene (11.2%) and β-pinene (10.3%) were the main compounds in the other sample[20]. α -pinene (28.8%), β-pinene (7.2%) and p-cymene (7.0%) were the main components of the essential oil ofT. poliumssp.capitatumfrom Corsica, France[21]. Menichiniet al[22] reported carvacrol (10.1%), (E)-β-caryophyllene (9.8%), α -cadinol (4.5%) and verbenone (3.7%) were the most abundant components inT. poliumssp.capitatumfrom Greece. In the oil ofT. poliumssp.capitatumfrom Crete, Italy, the most abundant compounds were (E)-β-caryophyllene (10.1%) and the monoterpene, carvacrol (9.6%)[23]. The dominant constituents of the aerial parts ofT. poliumssp.capitatumcollected during the flowering period from Serbia were sesquiterpenes, germacrene D (31.8%), transcaryophyllene (8.8%) and bicyclogermacrene (6.2%), and monoterpenes, linalool (14.0%) and β- pinene (10.7%)[24]. While the essential oil from Bulgaria was characterized by a high percentage of monoterpenes, β-pinene (26.8%), α -pinene (9.3%) and limonene (6.4%), and the sesquiterpene, germacrene D (17.7%)[24].
It is noticeable that the chemical composition of IranianT. poliumsubsp.capitatumstudied here was to some extent in accordance with some populations ofT. capitatumgrown in Portugal[20]. Furthermore, it is the first time that globulol and Longiverbenone (vulgarone B) have been characterized inT. poliumoil. These discrepancies might account for geographical variation and explain the probability of the existence of several chemical races within the species.
Results indicated that the oil of tested plant was more toxic but not significantly againstC. maculatusthanT. castaneum, as inferred by the confidence intervals of LC50. A difference in the response of the insect species to the essential oils haspreviously been reported for stored-product insects[15,25,26]. It has been reported that the oil and extract of the tested plant had insecticidal activity against other insects. For example, An LC50of 80 ppm was estimated with the larvae ofMusca domesticain bioassays when the essential oil ofT. poliumwas admixed with artificial diet. Furthermore a reduction of carbohydrase activity of α-amylase, α -glucosidase and β-glucosidase were detected in larval midgut extract[12]. The contact LD50ofT. poliumessential oil against house fly larvae was 0.028 μL/larvae[27]. The LC50of essential oil ofT. poliumfor adults ofC. maculatusandTribolium confusumwere 9.6 μL/L and 35.5 μL/L, respectively[27].
Terpenes have been well documented as active fumigants, repellents, and insecticides toward stored-product insects[4]. The insecticidal activity of the essential oils investigated in our study may be attributed to their having major terpenoids components. There are numerous reports on the toxicity of the essential oils from various plants species with major components similar toT. poliumin our study, such as α -cadinol[28], caryophyllene oxide[29], Longiverbenone (vulgarone B)[30,31], viridiflorol[32-34] which are classified as active toxic compounds.
Furthermore, insecticidal activity of major constitutes of the tested oil were before reported. α-cadinol possessed the strong antitermitic activity with 70% mortality ofC. formosanusShiraki at 10 mg/g dosage for 14 days[35]. In addition, α-cadinol possessed the strong antimite activities.Dermatophagoides pteronyssinusandDermatophagoides farinae, the dominant species of house dust mites, were all killed at a low concentration (6.3 mg/cm2) dosage of α -cadinol after 24 h of exposure[36]. Caryophyllene oxide showed high insecticidal activity (LC50=0.18 mg/L) againstT. castaneum(Coleoptera: Tenebrionidae) adults[37]. LC50value of caryophyllene oxide inCinnamomum osmophloeumleaf essential oil against mosquito species,Aedes albopictuslarvae was 65.6 μg/mL[29]. Caryophyllene oxide inhibited the mitochondrial electron transport chain in mammalian cells[38].
The essential oil assayed was not only active insecticide againstT. castaneumandC. maculatus, but it was also effective repellent. Based on our results, adults of these two beetles were found to be repelled by essential oil tested even at very low concentration. Our results are in accordance with those of Heydarzadeet al[9] who reported the mean repellency percentage ofT. poliumat the highest concentration (3 μL/mL acetone) was 84.9% and 76.1% on males and females ofC. maculatus, respectively. Other study has shown that 35% effective repellent doses ofT. poliumessential oil against adults ofC. maculatusandTribolium confusumwere 6.45 and 2.91 μL/mL acetone, respectively[10]. The strong repellent activity of the tested oil could be due to caryophyllene oxide, one of the main constituent of this oil (>25%). In previous research, this compound has shown to be a powerful repellent towardsT.castaneumwith complete repellency at 0.03 mg/cm2[37].
The essential oil from the plant could become a viable alternative to conventional chemical control strategies. However, further studies need to be conducted in order to evaluate the safety of the oil before practical use in storedproduct insect control.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by the University of Zabol, which is greatly appreciated.
[1] Hill DS. Pests of stored products and their control. London: Belhaven Press; 1990, p. 8-55.
[2] Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 2006; 51: 45-66.
[3] Rajendran S, Sriranjini V. Plant products as fumigant for storedproduct insect control. J Stored Prod Res 2008; 44: 126-135.
[4] Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological efects of essential oils- A review. Food Chem Toxicol 2008; 46: 446-475.
[5] El Oualidi J, Verneau O, Puech S, Dubuisson JY. Utility of rDNA ITS sequences in the systematics of Teucrium section Polium (Lamiaceae). Plant Syst Evol 1999; 215: 49-70.
[6] Bezic N, Vuko E, Dunkic V, Rušcic M, Blaževic I, Burcul F. Antiphytoviral activity of sesquiterpene rich essential oils from four Croatian Teucrium species. Molecules 2011; 16: 8119-8129. doi: 10.3390/molecules16098119.
[7] Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol 2000; 70: 343-349.
[8] Hashem M. Antifungal properties of crude extracts of five egyptian medicinal plants against dermatophytes and emerging fungi. Mycopath 2011; 172: 37-46. DOI 10.1007/s11046-010-9390-6.
[9] Heydarzade A, Moravvej G, Golestani Z, Sarbaz S. Evaluation of repellent activity of essential oils from Foeniculum vulgare, Teucrium polium and Satureja hortensis against Callosobruchus maculatus adults (Coleoptera: Bruchidae). 2nd Iranian Pest Management Conference, 2011, Kerman, Iran, pp. 34. (With english abstr.)
[10] Nabavi B, Talebi-Jahromi Kh, Goldansaz SH. Investigation of repellent effect of poly-Germander Teucrium polium essential oil against cowpea weevil Callosobruchus maculatus and confused flour beetle Tribolium confusum. Processings of the 19th Iranian Plant Protection Congress, 2010, Tehran, Iran, pp. 305. (With english abstr.)
[11] Pavela R. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 2008; 102: 555-559.
[12] Bigham M, Hosseininaveh V, Nabavi B, Talebi Kh, Esmaeilzadeh NS. Effects of essential oil from Teucrium polium on some digestive enzyme activities of Musca domestica. Entomol Res 2010; 40: 37-45.
[13] Piozzi F, Bruno M, Rosselli S, Maggio A. Advances on the chemistry of furano-diterpenoids from Teucrium genus. Heterocycles 2005; 65: 1221-1234.
[14] Bahramikia S, Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae). Phytother Res 2012; DOI: 10.1002/ptr.4617.
[15] Khani A, Basavand F, Rakhshani E. Chemical composition and insecticide activity of Lemon verbena essential oil. J Crop Prot 2012; 1(4): 313-320.
[16] George D, Mallery P. SPSS for Windows Step by Step: A simple guide and reference,16.0 Update (9th Edition). London: Allyn & Bacon, 2008.
[17] Fields PG, Xie YS, Hou X. Repellent effect of pea (Pisum sativum) fractions against stored-product insects. J Stored Prod Res 2001; 37: 359-370.
[18] Liu CH, Mishra AK, Tan RX, Tang C, Yang H, Shen YF. Repellent and insecticidal activities of essential oils from Artemisia princes and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Biores Tech 2006; 97: 1969-1973.
[19] Djabou N, Muselli A, Allali H, Dib Mel A, Tabti B, Varesi L, et al. Chemical and genetic diversity of two Mediterranean subspecies of Teucrium polium L. Phytochemistry 2012; 83: 51-62.
[20] Antunes T, Sevinate-Pinto I, Barroso JG, Cavaleiro C, Salgueiro LR. Micromorphology of trichomes and composition of essential oil of Teucrium capitatum. Flav Fragr J 2004; 19(4): 336-340.
[21] Cozzani S, Muselli A, Desjobert JM, Bernardini AF, Tomi F, Casanova J. Chemical composition of essential oil of Teucrium polium subsp.capitatum (L.) from Corsica. Flav Fragr J 2005; 20: 436-441.
[22] Menichini F, Conforti F, Rigano D, Formisano C, Piozzi F, Senatore F. Phytochemical composition, anti-inflammatory and antitumour activities of four Teucrium essential oils from Greece. Food Chem 2009; 115: 679-686.
[23] De Martino L, Formisano C, Mancini E, DeFeo V, Piozzi F, Rigano D, et al. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat Prod Commun 2010; 5: 1969-1976.
[24] Mitic V, Jovanovic O, Stankov-Jovanovic V, Zlatkovic B, Stojanovic G. Analysis of the essential oil of Teucrium polium ssp. capitatum from the Balkan Peninsula. Nat Prod Commun 2012; 7(1): 83-86.
[25] Lee S, Peterson CJ, Coats JR. Fumigation toxicity of monoterpenoids to several stored product insects. J Stored Prod Res 2003; 39: 77-85.
[26] Khani A, Asghari J. Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum and the cowpea weevil, Callosobruchus maculatus. J Insect Sci 2012; 12(73). [Online]Available from: insectscience.org/12.73.
[27] Nabavi B. Repellent and insecticidal effect of essential oil from Salvia sclarea and Teucrium polium against several insects. Master's thesis. University of Tehran, Iran; 2009, p.14. (In Persian, English abstr.)
[28] El-Shazly AM, Hussein KT. Chemical analysis and biological activities of the essential oil of Teucrium leucocladum Boiss. (Lamiaceae). Biochem System Ecol 2004; 32: 665-674.
[29] Cheng SS, Liu JY, Huang CG, Hsui YR, Chen WJ, Chang ST. Insecticidal activities of leaf essential oils from Cinnamomum osmophloeum against three mosquito species. Biores Technol 2009; 100: 457-464.
[30] Meepagala KM, Sturtz G, Mischke CC, Wise D, Duke SO. Molluscicidal activity of vulgarone B against ram's horn snail (Planorbella trivolvis). Pest Manag Sci 2004; 60: 479-482.
[31] Joshi RC, Meepagala KM, Sturtz G, Cagauan AG, Mendoza CO, Dayan FE, et al. Molluscicidal activity of vulgarone B from Artemisia douglasiana (Besser) against the invasive, alien, mollusc pest, Pomacea canaliculata (Lamarck). Int J Pest Manag 2005; 51: 175-180.
[32] Gonzalez-Coloma A, Martin-Benito D, Mohamed N, Garcia-Vallejo MC, Soria AC. Antifeedant effects and chemical composition of essential oils from different populations of Lavandula luisieri L. Biochem System Ecol 2006; 34: 609-616.
[33] De Morais SM, Facundo VA, Bertini LM, Cavalcanti ESB, Junior JFd, Ferreira SA, et al. Chemical composition and larvicidal activity of essential oils from Piper species. Biochem System Ecol 2007; 35: 670-675.
[34] Mossi AJ, Astolfi V, Kubiak G Lerin L, Zanella C, Toniazzo G, et al. Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais motschulsky (Coleoptera, Curculionidae). J Sci Food Agric 2011; 91(2): 273-277.
[35] Chang ST, Cheng S, Wang S. Antitermitic activity of essential oils and components from Taiwania (Taiwania cryptomerioides). J Chem Ecol 2001; 4: 717-724.
[36] Chang ST, Chen PF, Wang SY, Wu HH. Antimite activity of essential oils and their constituents from Taiwania cryptomerioides. J Med Entomol 2001; 38: 455-457.
[37] Kim SI, Yoon JS, Jung JW, Hong KB, Ahn YJ, Kwon HW. Toxicity and repellency of origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J Asia-Pac Entomol 2010; 13: 369-373.
[38] Monzote L, Stamberg W, Staniek K, Gille L. Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicol Appl Pharmacol 2009; 240: 337-347. DOI: 10.1016/ j.taap.2009.08.001
ment heading
10.1016/S1995-7645(14)60169-3
*Corresponding authors: Abbas Khani, Department of Plant Protection, Faculty of Agriculture, University of Zabol, Zabol, Iran.
Tel & Fax: +98-542-2242501
E-mail: abbkhani@yahoo.com or abbkhani@uoz.ac.ir
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Chikungunya virus, epidemiology, clinics and phylogenesis: A review
- Induction of deletion mutation on ompR gene of Salmonella enterica serovar Typhi isolates from asymptomatic typhoid carriers to evolve attenuated strains for vaccine development
- Evaluation of protective effect of IL-22 and IL-12 on cutaneous leishmaniasis in BALB/c mice
- Immunogenic potential and protective efficacy of formalin inactivated circulating Indian strain of West Nile virus
- Genetic diversity and gene structure of mitochondrial region of Anopheles minimus (Diptera: Culicidae) - major malaria vector of North east India
- Antioxidant activity and free radical scavenging activities of Streptomyces sp. strain MJM 10778
