Evaluation of protective effect of IL-22 and IL-12 on cutaneous leishmaniasis in BALB/c mice
Hajar Ziaei Hezarjaribi, Fatemeh Ghaffarifar, Abdolhossein Dalimi, Zohreh Sharifi
1Department of Parasitology and Mycology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
2Parasitology and Entomology Department, Faculty of Medical Sciences, Tarbiat Modares University, P.O. Box: 14115-331, Tehran I.R. Iran
3Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
Evaluation of protective effect of IL-22 and IL-12 on cutaneous leishmaniasis in BALB/c mice
Hajar Ziaei Hezarjaribi1, Fatemeh Ghaffarifar2*, Abdolhossein Dalimi2, Zohreh Sharifi3
1Department of Parasitology and Mycology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
2Parasitology and Entomology Department, Faculty of Medical Sciences, Tarbiat Modares University, P.O. Box: 14115-331, Tehran I.R. Iran
3Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
Objective:To investigate the protective effect of IL-22 and IL-12 on cutaneous leishmaniasisin BALB/c mice.Methods:The protective effect of IL-22 and IL-12 on cutaneous leishmanias in BALB/c mice was evaluated by measurement of IL-4, INF-γ, total IgG, IgG1 and IgG2a after challenge with Leishamania major. Clinical evaluations were performed by measurement of lesion diameter, and survival rate of the mice.Results:In week 27 post infection, the mortality rates for control groups were 100%. While the survival rates for the IL-12, IL-12 + IL-22, and IL-22(5 ng/ g) groups were 100%. The size of lesions decreased in the presence IL-22 (5 ng/g) of mice weight, which was statistically significant in comparison with other groups (P<0.05). Mean of total IgG, IgG1 and IgG2a for IL-22 (5 ng/g) group was more than other groups. In IL-22 group (5 ng/g), INF-γ production was significantly higher than other groups and IL-4 was significantly lower than other groups.Conclusions:The results obtained indicate the effectiveness of IL-22 and its effect on IL-12 in protection of cutaneous leishmaniasis.
ARTICLE INFO
Article history:
Received 24 September 2014
Received in revised form 10 October 2014
Accepted 15 November 2014
Available online 20 December 2014
Leishmania major
IL-22
IL-12
Protection
Mice
1. Introduction
Leishmaniasis is a widespread parasitic disease, reported from 98 countries of the world. So far, there is not any effective vaccine or drug to control this disease, or appropriate chemical method for definite fight against the vector available. The annual new cases of leishmaniasis are estimated to be one to two million cases. Leishmaniasis globally threatens 350 millions of people throughout the world. Visceral leishmaniasis is responsible for 20 000 to 40 000 deaths annually[1-3].
Using the currently available treatment methods, it is not possible to treat cutaneous leishmaniasis completely. Moreover, the treatments are associated with the infection recurrence, secondary bacterial infections, high costs, and being opportunistic in immunocompromised people. Considering these facts and also the high prevalence rate of the disease and the disease distribution, evaluation of novel effective methods for prevention and treatment of cutaneous leishmaniasis is indicated[3,4].
Some plasmids encoding exogenous cytokines (such as IL-6, IL-7, or IL-12) can potentially induce the cellular and humoral immune responses. Particularly when application of these plasmids is accompanied by DNA vaccines, they provide a robust method for enhancement or shift of the immune response against the antigen encoded by the DNA[5-7].
IL-12 is involved in stimulation of adaptive and natural immune responses, and plays an important role in induction of the Th1 response and suppression of the Th2 response. This cytokine increases the cytotoxic activity and production of INF-γ by dendritic cells (DCs). Moreover, IL-12 enhances INF-γ production and Th1 evolution through the signaling pathways increasing the STAT-4 activity. Therefore, the cytokines have been introduced as crucial inducers in evolution of T-helper subpopulations[8, 9].
Studies have shown that IL-12 production is suppressed in contaminated macrophages. Promastigotes and amastigotes of different Leishmania strains suppress IL-12 production in contaminated cells. IL-12 is produced by non-contaminated macrophages and dendritic cells that are not suppressed. The mechanism of suppressionis not thoroughly understood. In this respect, it is known that lipophosphoglycans involve in suppression of IL-12 production. However, other molecules are also effective in the process. Binding of CR3 receptors would decrease IL-12 production. Since Leishmania employs this receptor to enter the cell, inhibition of IL-12 production may be explained by this mechanism[10, 11].
IL-22, which is also called interleukin-10-related T cellderived inducible factor (IL-TIF), is secreted by Th-17, natural killer cells, Th22 and T cells (Th1) immediately after activation of these cells by IL-4. Moreover, the cytokine is secreted by thymic and brain mast cells following their activation by Con-A. This is one of the few cytokines, which has been confirmed to be present in non-mammalian animals such as fish[12-16].
It has been confirmed that cutaneous keratinocytes have receptors for IL-22. In the skin, IL-22 induces antimicrobial peptides, enhances the proliferation and inhibits the differentiation of keratinocytes, and plays a role in wound healing and innate immunity mechanisms. The role of IL-22 in skin disorders such as atopic eczema and allergic contact dermatitis is unknown[17]. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion and activation of macrophages[18].
In a previous study it was shown that resistance to human Kala-azar is related to levels of IL-22[19]. The present study was fulfilled to evaluate the effect of IL-22 administration solely and accompanied by the IL-12-encoding plasmid on cutaneous leishmaniasis caused byLeishmania major(L. major) in BALB/c mice.
2. Materials and methods
2.1. Experimental mice
Since BALB/c mice are susceptible to promastigotes ofL. major, we bought 60 white female inbred BALB/c mice at the age of eight weeks from the Razi Vaccine and Serum Research Institute (Hesarak, Karaj). The mice were kept under standard condition and received standard food and water. The mice were categorized according to the treatments they received. The sixty mice were divided into six groups, each including 10 mice.
2.2. Recombinant plasmid pCAGGS-IL-12
Recombinant IL-12 plasmid containing a part of the gene expressing IL-12, which was constructed in the form of pCAGGS-IL-12, was kindly donated by Prof. Masanori Matsui (University of Tokyo, Japan).
Primary and mass culture of the plasmid containing IL-12 was performed in TG1 bacterium by LB broth culture medium (Merck). The plasmid was extracted by the endofree plasmid purification Mega Kit (Qiagen). Then, the OD values of the plasmid extraction products were read and elution was performed according to the injection of 100 μg to each mouse[7].
2.3. Preparation and injection of IL-22
Recombinant IL-22 (rIL-22) protein of murine (Cat. Number 582-ML; R&D) was prepared according to the manufacturer’s protocol. Single doses of rIL-22 5 or 10 ng per g body weight (ng/g) in the volume of 100 μL were injected intramuscular before the challenge with promastigotes ofL. major[20].
2.4. Cytokine injection
In this study, we divided 60 female BALB/c mice of eightweek age into six groups of 10 mice. One group of 10 mice received PBS (100 μL) and another group of 10 mice received eukaryotic empty plasmid pCAGGS (50 μg) as control groups. Twenty mice received the plasmid encoding IL-12 gene (pCAGGS-IL-12) (50 μg in 100 μL, eluted in PBS), in three doses with three-week intervals. Among the mice that received pCAGGS-IL-12, a group of 10 mice also received rIL-22 (5 ng/g, IM). The remaining two groups received single dose of rIL-22 (5 and 10 ng/g in the volume of 100 μL) in the quadriceps muscle using 30-gauge insulin syringes[7, 20].
Classification of the mice with regard to the treatment they received and the follow up. Based on the treatment received, mice were divided in 6 groups (n=10 each group) as follow: Control groups treated with PBS (100 μL) and empty pCAGGS (100μg), rIL-22 treated group with 5 ng/g of body weight, rIL-22 treated group with 10 ng/g of body weight, IL-12 (100 μg) treated group and IL-12+ IL-22 (100 μg and 5 ng/g body weight respectively). All the mice were treated intra-muscular (IM) three times with three-week intervals. Further each group was divided in two sub-groups of 5 mice as follows:
(1) Seven weeks after applying the above-mentioned treatments, the animals were challenged byL. majorpromastigote of the MRHO/IR/75/ER strain and then seven weeks later the animals’ humoral and cellular responses were measured.
(2) Seven weeks after applying the treatments, the animals were challenged byL. majorpromastigote of the MRHO/ IR/75/ER strain and then lesion size and weight and survival rate of the mice were evaluated.
2.5. Challenge with promastigotes of L. major
Seven weeks after the last immunization, the BALB/c mice were infected withL. major. The mice were challenged with at least 2×106L. majorpromastigotes of the Iranian standard strain MRHO/IR/75/ER. The promastigotes were in the stationary phase, and were inoculated in the tail base by subcutaneous injection (100 μL). Three to four weeks after the injection, a nodule was developed at the injection site, and a lesion resulted from the growth of the parasite was formed in the tail base[20,21].
2.6. Evaluation of humoral immunity
The humoral immunity was evaluated by measurement of total IgG in the sera samples of the immunized and control groups using ELISA kits. Furthermore, the antibody subtypes (IgG1 and IgG2a) were measured by Mouse Monoclonal Antibody Isotyping Reagent Kit (Sigma).
To determine the antibody levels, optimized volumes of the antigen, serum concentration, and conjugated antibody concentration were determined using checker board titration as previously defined[21]. Then, all sera samples were examined by the ELISA method. Then, 100 μL of the crude soluble Leishmania antigen (SLA) at the concentration of 20 μg/mL in the carbonate-bicarbonate buffer was prepared. Then, 100 μL of the sera eluted in PBS buffer (1:10) was used.
2.7. Extraction and culture of lymphocytes for cytokine evaluation
In the mice of sub-group 1, spleen was collected and meshed-up to extract the lymphocyte. 2.5×106/mL lymphocytes were plated in 24 well tissue culture plate with complete RPMI media (Gibco, BRL, Maryland, USA) containing 20% FCS (Gibco, BRL, Maryland, USA), stimulated with 40ug/ml SLA and cultured at 37 degree, 5% CO2for 72 hours to access the cytokine release. Seventy two hours later, the cellular supernatant was collected and kept at -70 ℃ until cytokine assay[22].
2.8. Cytokine assay
INF-γ and IL-4 levels in the cellular supernatant prepared from the spleen lymphocytes of immunized and control mice, which were kept at -70 ℃, were measured by the ELISA method[21], using U-CyTech biosciences (Netherlands). According to the standard measures included in the kit, the standard diagrams were drawn and the samples were evaluated.
2.9. Evaluation of number of parasites in spleen of the mice after the challenge
Seven weeks after challenge by the parasites, the treated and control group mice were sacrificed; the spleens were removed under sterile conditions and weighed and large volumes of the spleen cellular supernatant was used for lymphocyte extraction. Almost 60 μL of the spleen cellular suspension was cultured under sterile condition in 1.5 mL microtube containing 1 mL RPMI 1640 (containing 20% FCS, 100 unit/mL penicillin, and 100 μg/mL streptomycin). After eight days of incubation at 24-25 ℃, the presence or absence of mobile promastigotes and the number of promastigotes in each ml was microscopically evaluated[21].
2.10. Measurement of lesion size and body weight of mice
In subgroup 2, after the challenge and creation of the lesion, the lesions size were measured weekly by a digital caliper and considered as the lesionsize. To confirm the lesion resulted from Leishmania, we obtained samples from the lesions. The body weight of mice measured by a digital scale weekly. After the parasite challenge, the life span and survival rate of the immunized and control mice were weekly measured[21].
2.11. Statistical analysis
The results were analyzed using SPSS software. In this regards, INF-γ, IL-4, MTT, IgG and its subtypes, parasite loads in spleen, lesion size and survival rate of mice were analyzed. Non-parametric data were compared by Mann-Whitney test.
3. Results
3.1. Survival rate
In all groups, the survival rate was 100% up to week six. Mortality of the control groups started from week seven on (week seven: mortality 20%; survival rate 80%).
Mortality for the IL-12, IL-12+IL22, and IL-22-5 ng/g groups started from week 26 and 27.
The mortality rates for PBS and pCAGGS control groups were 100% in weeks 26 and 27 after the challenge withL. major. This is while at this point, the survival rates for IL-12, IL-12+IL-22, and IL-22-5 ng/g groups were 100%. The survival rate of 80% for IL-12 and IL-12+IL-22 groups was observed at week 28, while this was observed at week 26 for the IL-22-5 ng/g group (Figure 1).
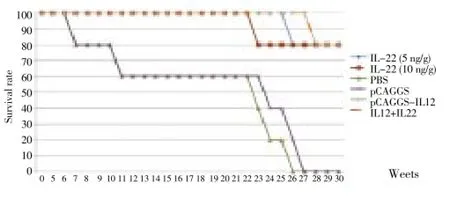
Figure 1. Survival rate of the mice that received IL-12, IL-22, and IL-12+IL-22 in comparison with the control groups during the 30 weeks of evaluation after the challenge with 2×106promastigotes of L. major.
3.2. Body weight of mice
The groups that received 5 and 10 ng of rIL-22 were not significantly different with regard to the weight of mice. However, rIL-22-5 ng/g was significantly different from the control groups (pCAGGS and PBS) in this respect (P<0.05)(Figure 2).
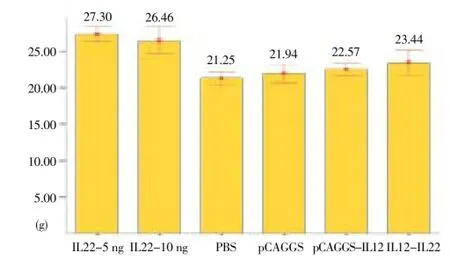
Figure 2. Mean and standard deviation of weight (g) in treated and control groups of BALB/c mice at week 7 after the challenge with 2 × 106L. major promastigotes.
Statistically significant differences were observed among the IL-12, IL-12+IL-22, and IL-22-5 ng/ggroups (P<0.05). The results did not show anysignificant difference between IL-12 and IL-12+IL-22 groups in the weight of mice (P>0.05).
3.3. Lesion status
The mean lesion size in the IL-22-5 ng/g was smaller than that in the IL-12 group, and as shown, the differences in different weeks of the study were statistically significant (P<0.05).
Furthermore, the lesion size in IL-12+IL-22 group was smaller than that in the IL-12 group. In different weeks of the study, both groups were significantly different in this respect (P<0.05) (Figure 3).
3.4. IgG total, IgG1 and IgG2
In the mice of sub-group 1 the humoral immunity was determined. Comparison between mean and SD of IgG total, IgG1 and IgG2 in different groups of mice is given in Table 1.3.5. INF-γ
The mean INF-γ level in the IL-22 (5 ng/g) was significantly higher than that in the IL-22 (10 ng/g). Also in IL-22 (5 ng/g) group, INF-γ production was significantly higher than other groups.
The mean level of INF-γ in the IL-22-10 ng/g was significantly different from the levels in the pCAGGS and PBS groups (P<0.05). The mean level of INF-γ in the IL-22-5 ng/g group was significantly higher than the values obtained for other groups (P<0.05) (Table 2).
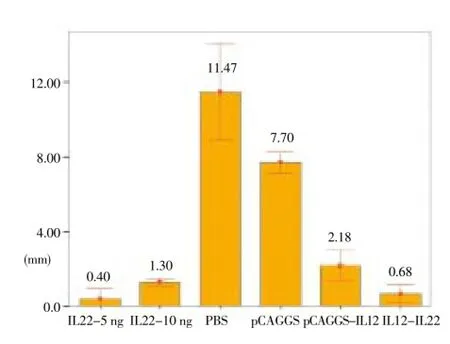
Figure 3. The mean and SD of lesion size (mm) in treated and control groups of BALB/c mice in three doses with three-week intervals, 7 weeks after the challenge with 2 × 106L. major promastigotes.
3.6. IL-4
The mean level of IL-4 showed a decreasing trend in the groups that received IL-22. These groups were significantly different from the other groups.
In IL-22 group (5 ng/g), IL-4 was significantly lower than other groups, whereas the IL-4 for IL-22 (10 ng/g) group was significantly lower only with control groups (Table 2).
3.7. Spleen weight and number of parasites in spleen after challenge
Enlargement of the spleen (as a lymphatic organ) and increase in its weight in the control mice are parameters indicating the increase in number and accumulation of immune cells in the organ. The accumulation could demonstrate the presence of the parasite in the spleen or exposure of the immune cells with infectious factors.
The data related to the spleen weight and parasite count in week 7 after the challenge byL. majordemonstratedthat the control groups were significantly different from the experiment groups in this respect (Table 3).
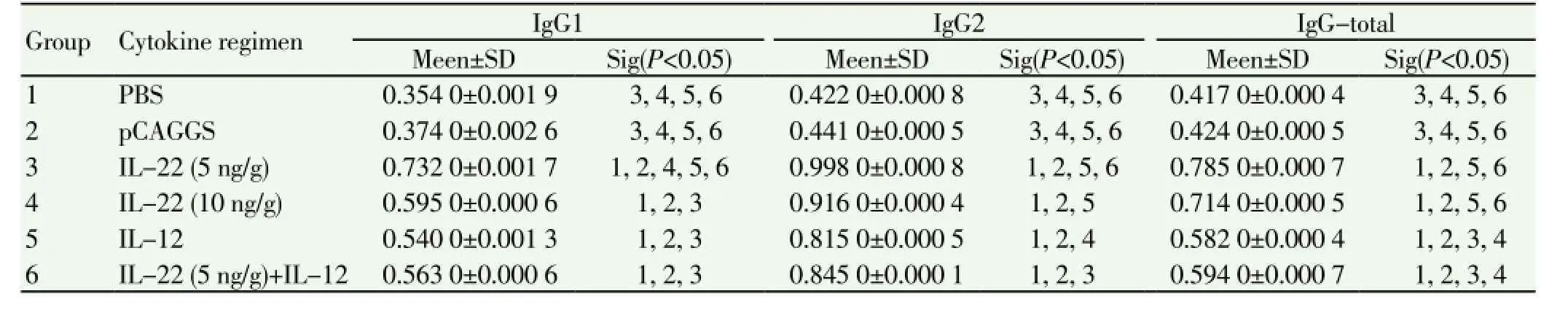
Table 1 Mean and SD of IgG total, IgG1 and IgG2 in different groupsof mice seven weeks afterchallenge with L. major promastigotes of the MRHO/IR/75/ ER strain.
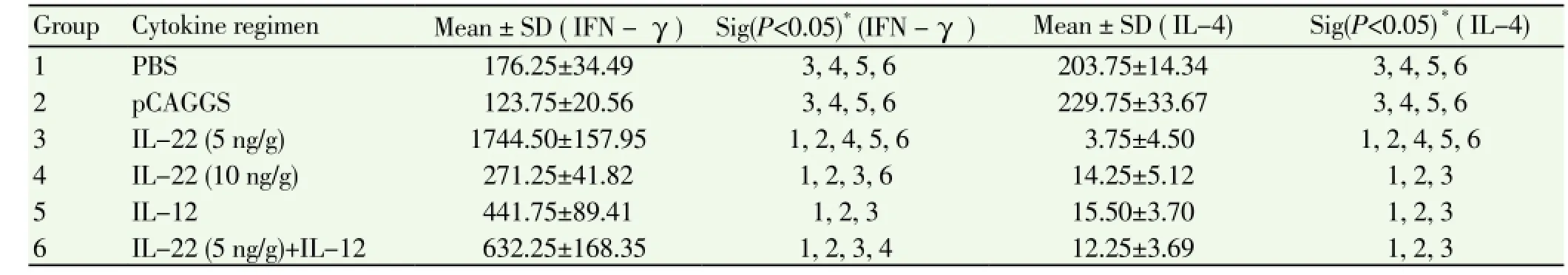
Table 2 Mean comparison of INF-γ and IL-4 levels (pg/mL) of the groups after the challenge.
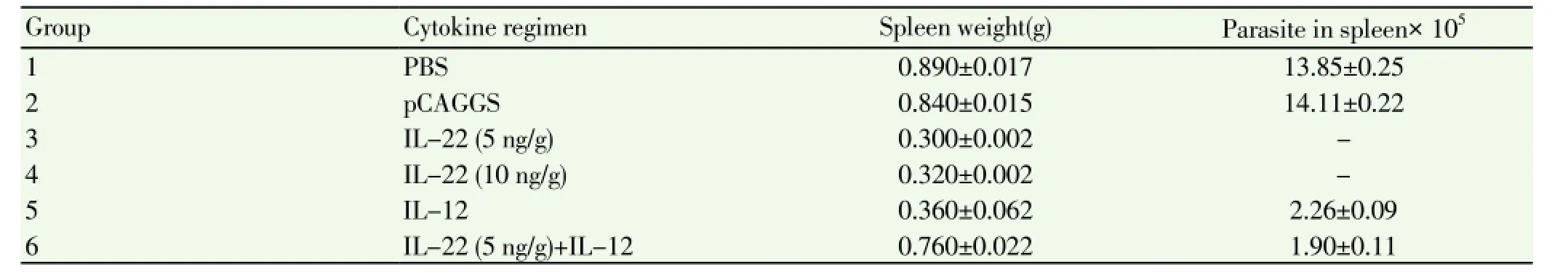
Table 3 Mean and standard deviation of spleen weight (g) and parasite count in week 7 after the challenge.
4. Discussion
In the current study, for the first time, we evaluated the effect of IL-22 together with IL-12 on cutaneous leishmaniasis caused byL. majorin BALB/c mice. The results indicated higher effectiveness of IL-22 in treatment of cutaneous leishmaniasis. This is supported by the lowest lesion size among the groups in the IL-22-5 ng/g group in week seven. Furthermore, mice in the IL-22-5 ng/g group had the highest weight and survival rate.
The results of INF-γ and IL-4, evaluations in BALB/ c mice demonstrated that IL-22 evidently increased the INF-γ production and reduced the IL-4 production after the challenge. Administration of IL-22 caused a statistically significant difference between this group and other groups in this respect. This shows that IL-22 resulted in development of Th1 cytokine responses and indicates that the cytokine is effective in treatment of cutaneous leishmaniasis. Coadministration of cytokines with other treatments is currently performed in treatment of many infectious and parasitic diseases to enhance the protective responses of T cell subpopulations[7, 18-20, 23-24]. In this study, it was observed that IL-22 has a dose-dependent effect; such as 5 ng/g produced better responses in comparison with 10 ng/g. At some doses, IL-22 has synergistic effect with other cytokines; for instance, IL-22 at 200 ng/mL together with IL-17A (20 ng/mL) is effective in treatment of psoriasis. This is caused by activation of S100A8 and S100A9 pathways through the synergistic effect of IL-17A and IL-22[25].
In some studies, long-term and high doses of IL-22 have been reported to be associated with adverse effects and there are some reports on the carcinogenicity of the cytokine[25]. However, such reports are not available for the permitted doses (2-20 ng/mL).
There is some evidence indicating that IL-17 and IL-22 play the complement role in natural immunity againstLeishmania donovani(L. donovani). This hypothesis is consistent with the effect of Th17 on monocytes and neutrophils and the ability of Th17 cells in infiltration of Th1 cells and formation of granulomas[19]. Furthermore, IL-17 and 22 hasten tissue regeneration and strengthen the epithelial cells; thus, contributing to the immunity and protection[25].
Mean lesion size in the groups that received IL-22 was significantly different from that in other groups. The lesion size in the group that received IL-12+IL-22 was close to the values obtained for IL-22 groups. Therefore, it could be probably stated that IL-22 performed better than IL-12 in reducing the lesion growth and wound healing in cutaneous leishmaniasis. It has been found that skin keratinocytes have receptors for IL-22. It was shown in a study that, IL-22 induces antimicrobial peptidesin skin, enhances proliferation of keratinocytes and inhibits their differentiation, and plays a role in skin regeneration in innate immunity mechanisms[17]. The protective role of IL-22 against human Kala-azar has been reported previously[19]. In our own previous study, we found that IL-22 as an adjuvant could show effective role in DNA vaccine with plasmid encoded LACK gene against cutaneous leishmaniasis[26]. The mean levels of IFN-γ in the groups that received IL-22 were significantly higher than those in other groups. This indicates that IL-22 resulted in development of Th1 cytokine response in mice suggesting its protective role in cutaneous leishmaniasis specially when used at 5 ng/g concentration.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was performed and supported by Tarbiat Modares University. The authors would like to thank Dr. Masanori Matsui (University of Tokyo, Japan).
[1] Reed SG. Leishmaniasis vaccination: targeting the source of infection. Exp Med 2001; 194(3): F7- F9.
[2] WHO. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010. WHO Technical Report No. 949. apps[Online]. Avaliable from: who.int/iris/bitstream/10665/44412/1/WHO_ TRS_949_eng.pdf [Accessed 10 March 2013].
[3] Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012;7(5): e35671. doi:10.1371/journal.pone.0035671.
[4] Wolff JA, Malone RW, Williams P, Ascadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990; 247(4949 pt 1): 1465-1468.
[5] Bhopale GM. Development of a vaccine for toxoplasmosis: current status. Microb Infect 2003; 5: 457-462.
[6] Kofta W, Wedrychowicz H. c-DNA vaccination against parasitic Infection: Advantages and disadvantages. Vet Parasitol 2001; 100: 3 -12.
[7] Hoseinian Khosroshahi K, Ghaffarifar F, Sharifi Z, D’Souza S, Dalimi A, Hassan Z, et al. Comparing the effect of IL-12 genetic adjuvant and alum non-genetic adjuvant on the efficiency of the cocktail DNA vaccine containing plasmids encoding SAG-1 and ROP-2 of Toxoplasma gondii. Parasitol Res 2012; 111: 403-411.
[8] Archambaud C, Sansoni A, mingueneau M, Devilard E, Delsol G, Malissen B, et al. STAT6 deletion converts the Th2 inflammatory pathology afflicting Lat (Y136F) mice into a lympho proliferative disorder involving Th1 and CD8 effector T cells. J Immunol 2009; 182(5): 2680-2689.
[9] Beraghella AM, Totaro R, Pellegrini P, Contasta I, Russo T, Carolei A, et al. Immunological study of IFN beta-1atreated and untreated multiple sclerosis patients: clatifying IFN beta mechanisms and establishing specific dendritic cell immunotherapy. Neuroimmunomodulation 2005; 12(1): 29-44.
[10] Muller I, Titus R, Caldumbide I, Louis JA. T cell responses in resistance and susceptibility to experimental infection with Leishmania major. In: Adam M, Al KE (eds). Frontiers of infectious diseases “New strategies in parasitology. London: Churchill Livingston; 1989, p. 158-175.
[11] Louis JA, Muller I. Experimental infection of mice with Leishmania major: Analysis of the role of T cells in resistance and susceptibility. In: Melchers FEA (ed). Progress in immunology. Berlin: Springer Verlag; 1989, p. 971-978.
[12] Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol 2001; 167(7): 3545-3549.
[13] Conti P, Kempuraj D, Frydas S, KandereK, Boucher W, Letourneau R, et al. IL-10 subfamily members: IL-19, IL-20, IL-22, IL-24 and IL-26. Immunol Lett 2003; 88(3):171-174.
[14] Igwa D, Sakai M, Savan R. An unexpected discovery of two interferon gamma-like genes along with interleukin (IL) 22 and 26 from teleost: IL-22 and -26 genes have been described for the first time outside mammals. Mol Immunol 2006; 43(7): 999-1009. [15] Dumoutier L, Van RE, Ameye G, Michaux L, Renauld JC. ILTIF/IL-22: genomic organization and mapping of the human and mouse gene. Genes Immun 2000; 1(8): 488-494.
[16] Npcddlrjoceal AR. Crystal structure of recombinant human interleukin-22. Stucture 2002:10: 1051-1062.
[17] Boniface K. IL22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005; 174: 3695-3702.
[18] Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, et al. IL22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 2009; 183: 6639-6645.
[19] Maira G, Pitta R, Romano A, Cabantous S, Sandrine H, Hammad A, et al. IL17 and IL22 are associated with protection against human Kala azar caused by Leishmania donovani. J Clin Invest 2009; 119: 2379-2387.
[20] Ziaee Hezarjarib H, Ghaffarifar F, Dalimi A, Sharifi Z. The survey of the effect of cytokine IL22 on the ulcer originated from L. major in BALB/c mice. J Mazand Univ Med Sci 2012; 12: 285-294.
[21] Ghaffarifar F, Jorjani O, Sharifi Z, Dalimi A, Hassan Z, Tabatabaie F, et al. Enhancement of immune response induced by DNA vaccine cocktail expressing complete LACK and TSA genes against Leishmania major. APMIS 2013;121(4):290-298. doi: 10.1111/j.1600-0463.2012.02968.x.
[22] Farnandez-Botran R, Vetyickaz V. Methods in cellular immunology. 2nd ed. Portland: CRF Press LNC; 2001.
[23] Tapia E, Perez-Jimenez E. The combination of DNA vectors expressing IL-12+IL-18 elicits high protective immune response against cutaneous leishmaniasis after priming with DNA-p36/ LACK and the cytokines, followed by a booster with a vaccinia virus recombinant expressing p36/LACK. Microbes Infect 2003; 5(2): 73-84.
[24] Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22(IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor hepatocytes via STAT3 activation. Hepatology 2004; 39(5): 1332-1342.
[25] Aujla SJ, Kolls JK. IL-22: A critical mediator in mucosal host defense. J Mol Med 2009; 87: 451-454.
[26] Ziaee Hezarjaribi H, Ghaffarifar F, Dalimi A, Sharifi Z, Jorjani O. Effect of IL-22 on DNA vaccine encoding LACK gene of Leishmania major in BALB/c mice. Exp Parasitol 2013; 134: 141-148.
ment heading
10.1016/S1995-7645(14)60166-8
*Corresponding author: Fatemeh Ghaffarifar, Parasitology and Entomology Department, Faculty of Medical Sciences, Tarbiat Modares University, P.O. Box: 14115-331, Tehran I.R. Iran.
Tel: +982182884553
Fax: +982182884555
E-mail: ghafarif@modares.ac.ir
 Asian Pacific Journal of Tropical Medicine2014年12期
Asian Pacific Journal of Tropical Medicine2014年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Serum inflammatory factor and cytokines in AECOPD
- miR-218 expression in osteosarcoma tissues and its effect on cell growth in osteosarcoma cells
- Therapeutic effect of Captopril on rheumatoid arthritis in rats
- Assessment of traumatic brain injury degree in animal model
- Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease
- Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting
