Aristolochia gehrtii inhibits liver toxicity and apoptosis in Schistosoma malayensis infection
Khaled M. M. Koriem,2*, Razmy E. Shahabudin, Rafiq Z. Jamaludin
1Advanced Medical and Dental Institute (AMDI), Universiti Sains Malaysia (USM), 13200 Kepala Batas, Pulau Pinang, Malaysia
2Department of Medical Physiology, National Research Centre, 12622 Dokki, Giza, Egypt
Aristolochia gehrtii inhibits liver toxicity and apoptosis in Schistosoma malayensis infection
Khaled M. M. Koriem1,2*, Razmy E. Shahabudin1, Rafiq Z. Jamaludin1
1Advanced Medical and Dental Institute (AMDI), Universiti Sains Malaysia (USM), 13200 Kepala Batas, Pulau Pinang, Malaysia
2Department of Medical Physiology, National Research Centre, 12622 Dokki, Giza, Egypt
Objective: To evaluate a protective effect of Aristolochia gehrtii (A. gehrtii) leaves to inhibit liver toxicity and apoptosis in Schistosoma malayensis (S. malayensis) infection. Methods: Forty male albino mice were divided into four equal groups: group 1 control including noninfected healthy mice and groups 2, 3 & 4 subcutaneously infected with S. malayensis cercariae where groups 3 & 4 pretreated with A. gehrtii leaves (200 mg/kg, bwt) & cinnamoylamide (250 mg/kg, bwt), respectively. Results: S. malayensis caused a significant increase in serum AST, ALT, ALP, MDA, NO, bilirubin, urea, creatinine, total cholesterol, LDL, triglycerides, and HDL levels. The pretreatment of A. gehrtii leaves and cinnamoylamide significantly inhibited that increase. On the other hand, S. malayensis induced a significant decrease in serum total protein, albumin, globulin, albumin/globulin ratio, blood SOD and GPx, while A. gehrtii leaves and cinnamoylamide pretreatment increased the above parameters. Treatment with A. gehrtii leaves and cinnamoylamide to S. malayensis infected mice increased p53 expression but decreased bcl-2 expression. These results were supported by histopathological investigations. Conclusions: A. gehrtii inhibits liver toxicity and apoptosis in S. malayensis infection and this effect is associated with the major cinnamoylamide ingredient of A. gehrtii leaves.
ARTICLE INFO
Article history:
Received 10 May 2014
Received in revised form 15 June 2014
Accepted 15 August 2014
Available online 20 September 2014
Schistosoma malayensis
Aristolochia gehrtii
Aristolochiaceae
Liver
Apoptosis
Mice
1. Introduction
Schistosomiasis remains one of the most prevalent parasitic infections in the world. It has been estimated that more than 207 million people are infected and 779 million people are at risk of infection[1]. Schistosomiasis, also known as bilharzia, is a parasitic disease that leads to chronic ill health. It is the major health risk in the rural areas of China and South East Asia and continues to rank high in other developing countries[2]. The disease is diagnosed either by the presence of blood in the urine or, in the case of intestinal schistosomiasis, by initially atypical symptoms which can lead to serious complications involving the liver[2]. Schistosomiasis, caused by various Schistosoma species, is estimated to cause infection in more than 200 million people in rural agricultural and peri-urban areas in tropical and subtropical developing countries particularly in China, Africa and South America[3,4]. In South East Asia,Schistosoma japonicumis prevalent in southern Philippines and Indonesia, whereas Schistosoma mekongi is endemic in the Mekong Delta[5,6]. In Malaysia, Schistosoma malayensis (S. malayensis) were detected among the Orang Asli in west Malaysia, while an evidence of Schistosoma was found in a monkey and in liver and rectal biopsies of immigrants from Philippines[7-10]. In Sarawak, environmental impact assessment (EIA) survey in upper Lupar River and upper Rejang River found eight individuals who were active schistosome eggs excretes. A sero-survey found 16.2% were seropositive for schistosomiasis, however the snail host could be identified and theS. malayensis-like infection among the Penan and other interior tribes (Orang Ulu) in upper Rejang River Basin was reported[11,12].
The research for a new and natural source to be used as a protection for schistosomiasis was increased in the last decade. The medicinal plants provide a new, available andcheap source for developing new drugs nowadays. Natural products account for more than 40% of all pharmaceuticals on the market today, where from 1941 to 2002, over 50% of all the drugs, or new drug entities, available for cancer treatment were derived from natural resources[13].
Aristolochiaceae family is widely distributed in tropical, subtropical and temperate regions of the world. They are known to occur in Asia, Africa, North and South America and Australia. It is represented by three genera: Aristolochia, which consists of approximately 100 species, Holostylis and Euglypha, each of which consists of only one species Holostylis reniformis and Euglypha rojasiana[14]. The latter have been suggested to be included in the Aristolochia genus[15]. With regard to synonyms, more than 300 names have been proposed for these species. The majority of theAristolochialesare tropical, though a number of them range as far north as Canada, Scandinavia, and Northern Japan. Aristolochia species are herbaceous perennials, undershrubs or shrubs, often scandent, scrambling, twining, sometimes lianas, usually with prostrate or tuberous rhizomes or rootstocks, and alternate, pinnate, polymorphic or lobed leaves[16-18]. In traditional medicine, these plants are known as “one thousand men” and have been mainly used as abortifacients, stomachics, antiophidians, antiasthmatics, expectorants, and, recently, in slimming therapies[14].
The selection of Aristolochia gehrtii (A. gehrtii) leaves for the current study was due to its major bioactive alkamides constituents[19], where amides have antioxidant activity[20]. The purpose of the present study was to discover a new natural adjunctive therapeutic agent to treat S. malayensislike infection complications in liver tissue. To accomplish this, we measured several oxidative stress and apoptosis parameters to determine if S. malayensis induces oxidative stress in liver and if this stress could be prevented by the use of A. gehrtii leaves extract.
2. Materials and methods
2.1. Plant material and ethanolic extract
A. gehrtii leaves were provided from Penang Botanical Garden, Penang, Malaysia in September 2012. The plant was botanically identified authenticated at School of Pharmaceutical Science, Universiti Sains Malaysia, Malaysia. Voucher specimen of each plant was deposited at the herbarium of the pharmacy school. The leaves were crushed, pulverized and then weighed and prepared for extraction.
A. gehrtii leaves (5 kg) were air-dried in an oven at 40 ℃ for 4 d and then the dry leaves was cut and pulverized. Then, they were subjected to size reduction to get coarse powder. The powdered material was subjected to successive extraction in a Soxhlet apparatus using solvent petroleum ether (40-60 ℃) and ethyl alcohol. This powder was vacuum dried in a desiccator’s till it was free from moisture[21].
2.2. Mice
Forty male albino mice (20-25 g) were obtained from the animal house of the Advanced Medical and Dental Institute, Universiti Sains Malaysia, Malaysia and were kept in special plastic cages. The animals were maintained on a commercial balanced diet and tap water. The experiments were performed after approval from the ethics committee of the Universiti Sains Malaysia and in accordance with recommendations for the proper care and use of laboratory animals (NIH publication no. 85:23 revised 1985).
2.3. Estimation of lethal dose 50 (LD50) of the extract
Twenty-four mice were orally administered the leaves extract with different four concentrations (1, 3, 5 and 7 mg/kg, bwt). Six rats were kept as control group throughout the entire experimental period. Mortality was assessed and counted in the different groups. LD50was calculated according to the method of Behrens and Karber[22]. Finally, 1/10 of the LD50was found to be safe[23].
2.4. Thin layer chromatography (TLC) for isolation of leaves extract alkamide
TLC was used to resolve and isolate the cinnamoylamide constituent from leaves extract. Precoated silica gel plates (20×20 cm, Brinkman Silplate F-22, with fluorescent indicator, 0.25, 0.50, or 2.00 mm gel thickness) were used in all separations. With these plates, the substituted amide present was easily detected as yellow or golden bands by viewing the developed plates under long-wave ultraviolet light. Amide was isolated by applying the extract as bands to TLC plates, developing in appropriate solvent systems, locating the compounds under ultraviolet light, and then scraping and eluting with chloroform or acetone. Compound was crystallized directly from appropriate solvents (usually ether or ether-hexane), or if necessary, the compound was subjected to further cleanup by TLC. The solvent systems used and the numbers used to designate them are as follows: 1, methylene chloride; 2, chloroform-ethyl acetate (2:1, v/v); 3, methylene chloride-ether (2:1, v/v); 4, benzene-ether (20:1, v/v); 5, hexane-ethyl acetate-methanol (5:5:1, v/v); and 6, methylene chloride-ether (1:1, v/v)[19]. This process was taken 2 months from ethanolic extract until isolation of cinnamoylamide powder was obtained. The isolation ratio=1:5 where 60 g of cinnamoylamide ingredient obtained from 300 g of the extract. The dose of cinnamoylamide used in this study was chosen[19].
2.5. Test for purity, quality and stability of the extract and cinnamoylamide
Purity tests (microbiological, pesticide residues, heavy metals, radioactive residues, chemical, foreign organic matter and sulfated ash) were performed in accordance with Malaysian accepted protocol requirements and accredited to ISO/IEC 17025 consulting WHO guidelines on stability and quality controls methods for medicinal plants[24,25]. The leaves extract and amide were stored in a tightly cooling (-40 ℃) sealed container away from heat and light that prevented a loss of extract solvent and entry of water; plant extract or amide was dissolved in 1 mL distilled water and given orally to individual rat.
2.6. Experimental design
The mice model had been established and divided into four equal (10 mice each) groups as follows: (Ⅰ) Control group including non-infected healthy, parasite free mice as a control group; (Ⅱ) S. malayensis infected group represented by subcutaneously infected with 50 cercariae ofS. malayensis; (Ⅲ)A. gehrtiileaves treated group comprisingS. malayensisinfected group pretreated daily with oral dose of A. gehrtii leaves extract (200 mg/kg, bwt) for 7 d before Schistosoma infection; (Ⅳ) Cinnamoylamide treated group including S. malayensis infected group pretreated daily with oral dose of cinnamoylamide (250 mg/kg, bwt) for 7 d before Schistosoma infection.
All mice were anesthetized 24 h after the last oral administration by inhalation with diethyl ether solution. All mice were massed at the beginning and the end of the study.
2.7. Blood sampling and handling
Blood samples were collected from retro-orbital plexus of mice using capillary tubes[26] into clean centrifuge tubes. Part of blood sample was collected in the presence of using EDTA as an anticoagulant for blood, while the other part of the blood sample was allowed to coagulate and centrifuged at 4 000 r/min for 15 min to separate blood serum which stored at -20 ℃.
2.8. Biochemical analysis
To evaluate liver function, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase (ALP), total protein, albumin, globulin and albumin/globulin ratio were determined colorimetically using kits reagents obtained from Biomeriéux Company, France. To detect lipid fractions, serum total cholesterol, low-density lipoproteins (LDL), triglycerides and high-density lipoprotein (HDL) were determined colorimetically using kits reagents also obtained from Biomeriéux Company, France. Antioxidants enzymes including blood superoxide dismutase (SOD), glutathione peroxidase (GPx), serum lipid peroxidation (MDA) and plasma nitrate and nitrite concentrations as an indicator of nitric oxide (NO) generation were determined colorimetically using kits reagents purchased from Randox Company, British.
2.9. Immunohistochemical detection of p53 and bcl-2 expression in liver tissue
Immunohistochemical staining procedure for p53 and bcl-2 was performed using the methods described by Hsu and Raine[27] and Eliaset al[28].
2.10. Histopathological examinations
Specimens of liver were fixed in 10% (v/v) neutral formalin solution and then processed for routine technique for embedding in paraffin. Blocks were sectioned at a thickness of 5 µm and stained with hematoxylin and eosin for histopathological examination, which were performed under light microscopy and documented by an Olympus microphotocamera.
2.11. Statistical analysis
The results are expressed as mean ± standard error (SE). Statistical significance was determined through oneway analysis of variance (ANOVA), followed by Student’s t test. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Phytochemical composition and LC50of the extract
Phytochemical studies of the plant leaves extract showed the presence of six alkamides in ethanolic extract of the plant leaves as a major ingredient. The concentration of each compound were 90.2, 47.4, 50.0, 121.2, 154.8 and 119.8 mg/g extract for alkamides compounds 1, 2, 3, 4, 5 and 6, respectively; so compound 5 (cinnamoylamide) was chosen for such study. Other chemical ingredients of the plant leaves extract included five lignans, three terpenes, two benzoic acid derivatives, and six phenethyl derivatives.
The maximum non-lethal dose was found to be 2 000 mg/kg; hence 1/10 of the dose was taken as effective dose (200 mg/kg, bwt). In the present study, there were no observable differences of body weight, food or drink intake during experimental period of the study.
3.2. Liver function
The results presented in Table 1 indicated that S. malayensis caused a significant increase (P<0.05) in serum AST, ALT,ALP and bilirubin levels in mice. The prereatment with either A. gehrtii leaves extract or cinnamoylamide for 7 d before S. malayensis infection inhibited the increase in AST, ALT, ALP and bilirubin as compared to the S. malayensisinfected mice.
Table 1 revealed the protective role of A. gehrtii leaves extract or cinnamoylamide on serum total protein, albumin, globulin levels, and albumin/globulin ratio in S. malayensisinfected mice. It is obvious that S. malayensis caused a significant decrease (P<0.05) in serum total protein, albumin, globulin levels, and albumin/globulin ratio as compared with control healthy mice. While eitherA. gehrtiileaves extract or cinnamoylamide pretreated for 7 d before S. malayensis infection induced a significant increase in total protein albumin, albumin, globulin levels, and albumin/globulin ratio in S. malayensis-infected mice as compared with S. malayensis-infected mice. On the other hand, A. gehrtii leaves extract or cinnamoylamide pretreatment to S. malayensis-infected mice revealed an insignificant increase in serum albumin and albumin/ globulin ratio while significant increase in serum total protein (P<0.05) compared to S. malayensis-infected mice group.S. malayensis-infected mice +A. gehrtiileaves extract or cinnamoylamide exhibited a highly significant increase (P<0.01) in serum globulin.
3.3. Lipid fractions
The data presented in Table 2 showed that S. malayensis induced a significant increase (P<0.05) in serum cholesterol on the contrary; A. gehrtii leaves extract or cinnamoylamide treatment to S. malayensis-infected mice exhibited an insignificant increase in serum cholesterol compared to healthy mice.
The level of serum LDL in normal mice after being infected with S. malayensis and then treated with A. gehrtii leaves extract or cinnamoylamide was exhibited in Table 2. Analysis of the changes occurred in LDL after S. malayensisinfection showed a significant increase (P<0.05) in serum LDL, but administration with A. gehrtii leaves extract or cinnamoylamide to mice infected with S. malayensis revealed an insignificant increase in LDL compared to healthy mice.
Analysis of serum triglycerides in normal, S. malayensis infected- or pretreated with eitherA. gehrtiileaves extract or cinnamoylamide toS. malayensisinfected mice was shown in Table 2. Serum triglycerides after S. malayensis infection revealed a significant increase (P<0.05) in serum triglycerides, while A. gehrtii leaves extract or cinnamoylamide pretreatment to S. malayensis infected mice revealed an insignificant increase in serum triglycerides compared to healthy normal mice.
3.4. Antioxidants enzymes
It was found that a significant decrease (P<0.05) in blood SOD and GPx were recorded in association withS. malayensisinfection and the data were tabulated in Table 3. On the contrary, the pretreatment with A. gehrtii leaves extract or cinnamoylamide to S. malayensis infected mice induced insignificant increase in blood SOD and GPx when compared with healthy normal group. On the other hand, A. gehrtii leaves extract or cinnamoylamide to S. malayensis infected group showed a significant increase (P<0.05) in blood SOD but a highly significant increase (P<0.01) in blood GPx compared to the S. malayensis infected group.
S. malayensis caused a significant increase (P<0.05) in serum MDA and plasma NO as compared with the normalhealthy mice. A significant decrease (P<0.05) was observed in serum MDA and plasma NO in either group pretreated with A. gehrtii leaves extract or cinnamoylamide before S. malayensis infection as compared with those infected with S. malayensis (Table 3).
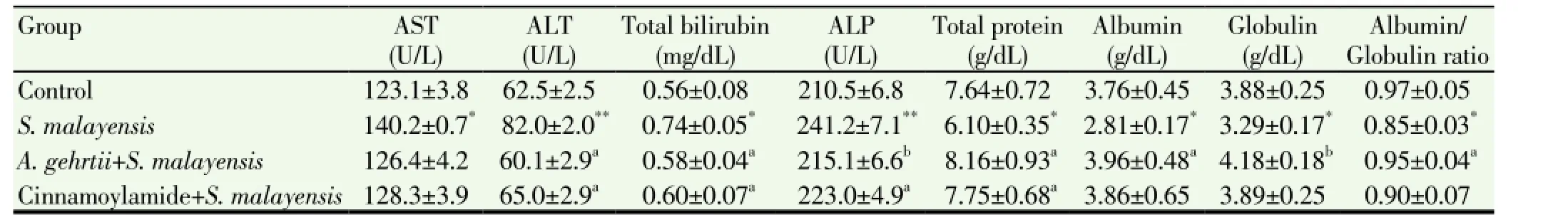
Table 1 Effect of A. gehrtii and cinnamoylamide on liver function of S. malayensis infected mice.

Table 2 Effect of A. gehrtii and cinnamoylamide on lipid fractions of S. malayensis infected mice (mg/dL).
3.5. Anti-apoptotic activity
Figure 1 shows the photomicrographs of immunohistochemically stained liver tissue sections revealing that saline administration induced normal amount of expressed p53 (Figure 1A). On the other hand, the amount of p53 was potentially decreased in S. malayensis infected mice (Figure 1B) as compared to control healthy mice. The pretreatment of S. malayensis infected mice with A. gehrtii leaves extract or cinnamoylamide increased the amount of expressed p53 (Figure 1C & 1D) as compared to S. malayensis infected mice.
Figure 2 exhibits the photomicrographs of immunohistochemically stained liver tissue sections revealing that normal mice showed normal amount of expressed bcl-2 (Figure 2A). On the other side, the amount of bcl-2 was potentially increased in S. malayensis infected mice (Figure 2B). The treatment of S. malayensis infected mice with either dose of A. gehrtii leaves extract or cinnamoylamide potentially decreased the elevated amount of expressed bcl-2 (Figure 2C & 2D) as compared toS. malayensisinfected mice.
3.6. Histology studyFigure 3 reveals the histopathology results in S. malayensis infected mice as well as A. gehrtii leaves extract or cinnamoylamide potentially pretreated groups. The structureof the control liver showed normal hepatocytes, vascular sinusoids, and centrolobular vein (Figure 3A). S. malayensis infection caused liver granuloma with ova embedded in dense fibrous tissue. The thin-walled, non-striated helminth ova are not operculated and contain nonvital miracidial cells (Figure 3B). Examination of liver sections of rats pretreated withA. gehrtiileaves extract or cinnamoylamide prior to S. malayensis infection showed preserved hepatic lobular architecture. The hepatocytes were within normal limit and preserved its plate pattern. Liver almost returned to the normal pattern (Figure 3C & 3D).

Table 3 Effect of A. gehrtii and cinnamoylamide on antioxidant idexes of S. malayensis infected mice.
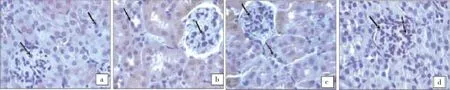
Figure 1. p53 expression in control, S. malayensis-infected, cinnamoylamide and A. gehrtii extract pretreated mice.(a) Immunohistochemically-stained liver tissue sections showing apoptotic marker, p53 expression in control mice. There is almost negligible expression of p53 in the liver sections of control group. (b) The tissue sections showing the effect of S. malayensis on apoptotic marker p53 expression. S. malayensis decreased strongly p53 expression in liver sections. (c) There was partial expression of p53 as evidenced by weak immuno-staining in the mice liver treated with cinnamoylamide. (d) The expression of p53 was increased markedly on administration of A. gehrtii leaves extract which was well evident by the positive staining.
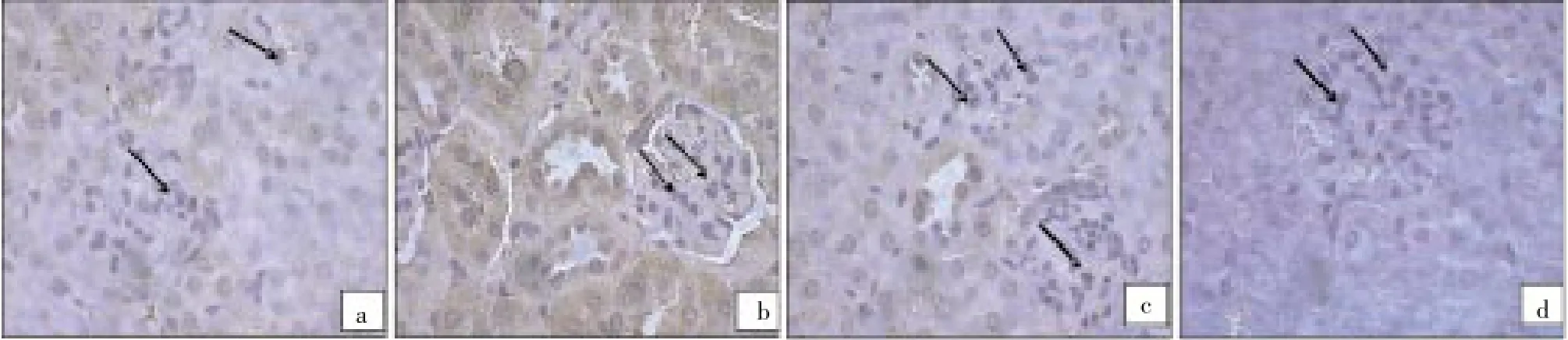
Figure 2. bcl-2 expression in control, S. malayensis-infected, cinnamoylamide and A. gehrtii extract pretreated mice.(a) Immunohistochemically-stained kidney tissue sections showing bcl-2 expression in control healthy group which almost shows normal amount of expressed bcl-2. (b) S. malayensis strongly induced bcl-2 expression in liver sections. (c) There is partial inhibition of bcl-2 expression as evidenced by weak immune-staining in the mice liver treated with cinnamoylamide. (d) A. gehrtii leaves extract treatment showed lesser expression which implies that bcl-2 has been inhibited.
Figure 3. Liver histology in control, S. malayensis-infected, cinnamoylamide and A. gehrtii extract pretreated mice.(a) Shows the control group with preserved hepatic architecture (H&E, ×200). (b) Shows S. malayensis liver granuloma with ova embedded in dense fibrous tissue (arrows). The thin-walled, non-striated helminth ova are not operculated and contain nonvital miracidial cells was found (H&E, ×200). (c) Shows pretreatment of cinnamoylamide to S. malayensis infected mice with preserved hepatic lobular architecture. The hepatocytes are within normal limit and preserve its plate pattern. Liver almost returns to the normal pattern (H&E, ×200). (d) Shows pretreatment of A. gehrtii leaves extract to S. malayensis infected mice with large reserved hepatic lobular architecture and the liver almost returns to the normal pattern (H&E, ×200).
4. Discussion
In Malaysia, S. malayensis, in addition to Schnistosoma spindale, Schnistosoma nasale, Schnistosoma incognitum,Trichobilhazia brevis, andPseudobilharziella lonchurae, is known to occur in wildlife[29]. TheRobertsiellasnails are the intermediate host of S. malayensis and have been found almost exclusively in small rivers[30]. Several attempts to recover eggs from feces from the Orang Asli population in peninsular Malaysia[31] were unsuccessful, however, which was attributed to the zoonotic nature of S. malayensis and thus missing adaptation to the human host. The rodent (jungle rat) feces could have contaminated the water with the eggs of S. malayensis.
The mechanism of A. gehrtii protection in S. malayensis infection was dependant on the antioxidant activity of the plant amide to reduce the oxidative stress produced byS. malayensisinfection, where amides at lower concentrations have the ability to reduce schistosome pairing and motility. This effect was due to the functional group of the amine compounds which influenced the schistosomicidal activity[32].
The aim of the present study was to evaluate the protective role of of A. gehrtii leaves extract in liver toxicity and apoptosis occurred in S. malayensis infection in male albino mice.
The clinical and diagnostic values associated with the changes in blood enzyme concentrations such as AST, ALT, ALP, and serum bilirubin have long been recognized[33,34]. Increased levels of these diagnostic markers of hepatic function in S. malayensis infection in male mice are implicative of the degree of hepatocellular dysfunction caused byS. malayensisinfection. Comparatively lower levels of these parameters in the pretreatment with A. gehrtii leaves extract or cinnamoylamide groups to S. malayensis mice show the ability of the phyto-constituents to protect the liver against harmful effects of S. malayensis.
Albumin is the most abundant circulatory protein and its synthesis is a typical function of normal liver cells. Low levels of albumin have been reported in the serum of patients and animals with hepatocellular cancer[35]. The fall in the serum albumin levels could probably contribute to the low total protein levels observed inS. malayensisinfected mice. Considerably higher albumin and total protein levels were seen inA. gehrtiileaves extract or cinnamoylamide treated groups as compared to S. malayensis group, indicating that one of the mechanisms by which the phytoconstituents exhibit their protective effects during cancer is by enhancing the levels of albumin and thereby total protein levels. Thus, based on our preliminary biochemical findings, we suggest possible preventive effects of A. gehrtii leaves extract and 0S. malayensis infection. S. malayensis increased significantly serum cholesterol, LDL, and triglycerides, while A. gehrtii leaves extract or cinnamoylamide pretreatment directed serum cholesterol, LDL, and triglycerides to the normal levels. This effect may be related to amides-containing plant which protects LDLs from oxidation. Lipids targeted for cellular metabolism are mobilized from the intestine as: (Ⅰ) triglyceridesrich chylomicrons; (Ⅱ) triglycerides-rich very-lowdensity lipoproteins, which are subsequently converted to cholesterol-rich LDL; and (Ⅲ) as cholesterol-phospholipidrich HDL, which removes cholesterol from peripheral cells and transports it to the liver[36]. Such findings are in agreement with that of Rashed et al[37], Xu et al[38], and Parket al[39] who found that the amides potently inhibited rabbit liver acyl-CoA: cholesterol O-acyltransferase, and also decreased significantly the plasma cholesterol (42%-68%) in an acute cholesterol-fed rat model.
S. malayensis decreased significantly blood GPx and SOD activities but increased serum MDA and plasma NO. On the contrary, A. gehrtii leaves extract or cinnamoylamide pretreatment increased blood GPx and SOD activities but decreased serum MDA and plasma NO. This observation could be related to the antioxidant effect of amidescontaining plant. Such findings are accepted with that of Marinovaet al[40] and Storozhoket al[41] who found thatcinnamoyl- and hydroxycinnamoyl amides of biogenic amines exhibited an excellent antioxidant activity, higher than or comparable with that of caffeic acid.
In the current study, anti-apoptotic activity of A. gehrtii leaves extract or cinnamoylamide was also recorded. P53 and bcl-2 are closely related to the majority of human toxicity and cancer[42]. Apoptotic marker p53 is a critical regulator of apoptosis in many cells. It stimulates a wide network of signals that act through either extrinsic or intrinsic pathways of apoptosis[43] by activating the transcription of downstream genes such as p21 and Bax to induce apoptotic process which inhibit the growth of cells with damaged DNA. On the other hand, bcl-2 has been reported to function primarily by blocking the apoptosis pathway[44]. Bcl-2 gene product is a negative regulator of apoptosis, which forms a heterodimer complex with Bax and neutralizes the effect of pro-apoptosis[45]. Our results indicated that S. malayensis decreased p53 expression but increased bcl-2 expression. On the other side, the treatment with A. gehrtii leaves extract or cinnamoylamide to S. malayensis infected mice increased p53 expression but decreased bcl-2 expression. These results are in agreement with that of Mulware[46] who reported that parasite or environmental toxicant cause induction of oxidative-induced DNA damage by ROS may lead to isolated base lesions or single-strand breaks, complex lesions like double-strand breaks, and some oxidative generated clustered DNA lesions which are linked to cell apoptosis and mutagenesis. On the other side, the A. gehrtii leaves extract or cinnamoylamide has antiapoptotic activity, where the plant leaves extract, an inhibitor of p53, abrogated glucolipotoxicity-induced ROS generation and p53 expression[47].
Histopathological studies of liver tissues of normal, S. malayensis infected group and A. gehrtii leaves extract or cinnamoylamide +S. malayensis-infected groups indicate that either plant extract or alkamide has cytoprotective properties.
Data from the present study highlights S. malayensis fatal effects on oxidative stress and apoptosis in the liver of male albino mice. Pretreatment of A. gehrtii showed significant reduction in liver oxidative stress and apoptosis in S. malayensis evidenced in histological examinations, and this effect was associated with cinnamoylamide ingredient of the plant leaves. However, further clinical studies are warranted to establish its effectiveness and it will be interesting to see whether A. gehrtii reverses existing S. malayensis liver toxicity and apoptosis, like in real case scenarios.
Conflict of interest statement
The authors declare that there are no conflicts of interests.
[1] Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: Systematic review meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006; 6: 411-425.
[2] Bichler KH, Feil G, Zumbragel A, Eipper E, Dyballa S. Schistosomiasis: A critical review. Curr Opin Urol 2001;11: 97-101.
[3] McManus DP, Gray DJ, Li Y, Feng Z, Williams GM, Stewart D, et al. Schistosomiasis in the People’s Republic of China: the Era of the Three Gorges Dam. Clin Microbiol Rev 2010; 23: 442-466.
[4] Wang W, Liang YS, Hong QB, Dai JR. African schistosomiasis in mainland China: Risk of transmission and countermeasures to tackle the risk. Parasit Vectors 2013; 6: 249.
[5] Bergquist R, Tanner M. Controlling schistosomiasis in Southeast Asia: A tale of two countries. Adv Parasitol 2010; 72:109-144.
[6] Conlan JV, Sripa B, Attwood S, Newton PN. A review of parasitic zoonoses in a changing Southeast Asia. Vet Parasitol 2011; 182: 22-40.
[7] Oliveira G, Rodrigues NB, Romanha AJ, Bahia D. Genome and genomics of schistosomes. Can J Zool 2004; 82: 375-390.
[8] Leshem E, Meltzer E, Marva E, Schwartz E. Travel-related schistosomiasis acquired in Laos. Emerg Infect Dis 2009; 15: 1823-1826.
[9] Krishnasamy M, Chong NL, Ambu S, Jeffery J, Oothuman P, Edariah AB. Schistosomiasis in Malaysia, with special reference to Schistosoma spindale, the causative agent of cercarial dermatitis. Trop Biomed 2001; 18: 51-64.
[10] Akinwale PO, Tang TH, Doblin AS, Cheah, HL. Molecular survey of fresh water snail intermediate host of Schistosoma malayensis in northern peninsular Malaysia. Continent J Biol Sci 2013; 6: 33-41.
[11] Agrawal MC. Schistosomes and schistosomiasis in South Asia. New Delhi: Springer 2012; p. 101-112.
[12] Chandra SK, Pathmanathan R. Schistosomiasis in Malaysia. Rev Infect Dis 1987; 5: 1026-1037.
[13] Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 2003; 66: 1022-1037.
[14] Lopes LMX, Nascimento IR, Da Silva T. Phytochemistry of the Aristolochiaceae family. In: Mohan RMM, editor. Research advances in phytochemistry. Vol 2. Kerala: Global ResearchNetwork; 2001, p. 19-108.
[15] Zhang J, Xiao Y, Feng J, Wu SL, Xue X, Zhang X, et al. Selectively preparative purification of aristolochic acids and aristololactams from Aristolochia plants. J Pharm Biomed Anal 2010; 52: 446-451.
[16] Kumar V, Prasad AK, Parmar VS. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat Prod Rep 2003; 20: 565-583.
[17] Francisco CS, Messiano GB, Lopes LMX, Tininis AG, de Oliveira JE, Capellari LJr. Classification of Aristolochia species based on GC-MS and chemometric analyses of essential oils. Phytochemistry 2008; 69: 168-175.
[18] Li J, Zhang L, Jiang Z, Shu B, Li F, Bao Q, et al. Toxicities of aristolochic acid i and aristololactam I in cultured renal epithelial cells. Toxicol in vitro 2010; 24: 1092-1097.
[19] Navickiene HMD, Lopes LMX. Alkamides and phenethyl derivatives from Aristolochia gehrtii. J Braz Chem Soc 2001; 12: 467-472.
[20] Zhang X, Tobwala Sh, Ercal N. N-acetylcysteine amide protects against methamphetamine-induced tissue damage in CD-1 mice. Hum Exp Toxicol 2012; 31: 931-944.
[21] Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants (including the supplement). New Delhi: Council of Scientific and Industrial Research; 1986, p. 402-405.
[22] Behrens H, Karber S. Determination of LD50. Arch Fur Exp Path Pharm 1953: 3: 177-372.
[23] Koriem KM, Arbid MS, El-Gendy NF. The protective role of Tropaeolum majus on blood and liver toxicity induced by diethyl maleate in rats. Toxicol Mech Methods 2010; 20: 579-586.
[24] Farnsworth NR, ed. NAPRALERT database. Chicago: University of Illinois at Chicago; 2001, p. 47-51.
[25] Leung AY, Foster S. Encyclopedia of common natural ingredients used in food, drugs and cosmetics. New York: John Wiley and Sons, Inc.; 1996, p. 84-92.
[26] Schermer S. The blood morphology of laboratory animals. 3rd ed. Philadelphia: F. A. Davi. Co.; 1967, p. 42-45.
[27] Hsu SM, Raine L. Advances in immunochemistry. In: DeLellis RA, editor. New York: Mason Publishing USA, Inc.; 1984, p. 31-42.
[28] Elias JM, Margiotta M, Gaborc D. Sensitivity and detection efficiency of the peroxidase antiperoxidase (PAP), avidin-biotin peroxidase complex (ABC), and peroxidase-labeled avidin-biotin (LAB) methods. Am J Clin Pathol 1989; 92: 62-67.
[29] Latif B, Heo CC, Razuin R, Shamalaa DV, Tappe D. Autochthonous human schistosomiasis, Malaysia. Emerge infect disease 2013; 19: DOI: 10.3201/eid1908.121710.
[30] Attwoodi SW, Lokman HS, Ong KY. Robertsiella silvicola, a new species of triculine snail (Caenogastropoda: Pomatiopsidae) from Peninsular Malaysia, intermediate host of Schistosoma malayensis (Trematoda: Digenea). J Mollus Stud 2005; 71: 379-391.
[31] Chuah C, Jones MK, Burke ML, Owen HC, Anthony BJ, McManus DP, et al. Spatial and temporal transcriptomics of Schistosoma japonicum-induced hepatic granuloma formation reveals novel roles for neutrophils. J Leukocyte Biol 2013; 94: 353-365.
[32] Foster JM, Marshall I, Svoboda JA, Thompson MJ, Rees HH. Effect of insect-growth-disrupting amines and amides on Schistosoma mansoni in vitro. J Parasitol 1996; 82: 340-343.
[33] Friedman LS, Keeffe EB. Handbook of liver disease. 3rd ed. Saunders: Elseiver; 2013, p. 249-268.
[34] Hayes AW, Kruger CL. Hayes’ principles and methods of toxicology. 6th ed. Boca Raton: CRC Press 2014, p. 1031-1047.
[35] Doungngern T, Huckleberry Y, Bloom JW, Erstad B. Effect of albumin on diuretic response to urosemide in patients with hypoalbuminemia. Am J Crit Care 2012; 21: 280-285.
[36] Palchykovska LG, Vasylchenko OV, Platonov MO, Starosyla DB, Porva JI, Rymar SJ, et al. Antiviral properties of herbal flavonoids-inhibitors of the DNA and RNA synthesis. Biopolym Cell 2013; 29: 150-156.
[37] Rashed MM, Shallan M, Mohamed DA, Fouda K, Hanna LM. Hypolipidemic effect of vegetable and cereal dietary mixtures from Egyptian sources. Grasis Y Aceities 2010; 61: 261-270.
[38] Xu MZ, Lee WS, Kim MJ, Park DS, Yu H, Tian GR, et al. Acyl-CoA: cholesterol acyltransferase inhibitory activities of fatty acid amides isolated from Mylabris phalerate Pallas. Bioorg Med Chem Lett 2004; 14(16): 4277-4280.
[39] Park YD, Lee WS, An S, Jeong TS. Human acyl-CoA: cholesterol acyltransferase inhibitory activities of aliphatic acid amides from Zanthoxylum piperitum DC. Biol Pharm Bull 2007; 30: 205-207.
[40] Marinova E, Georgiev L, Totseva I, Seizova K, Milkova T. Antioxidant activity and mechanism of action of some synthesized phenolic acid amides of aromatic amines. Czech J Food Sci 2013; 31: 5-13.
[41] Storozhok NM, Medyanik NP, Krysin AP, Krekov SA, Borisenko VE. Synthesis, structure, and antioxidant activity of hybrid N-substituted salicylic acid amides. Russian J Org Chem 2013; 49: 1031-1034.
[42] Gu Q, Hu C, Chen Q, Xia Y, Feng J. Development of a rat model by 3,4-benzopyrene intra-pulmonary injection and evaluation of the effect of green tea drinking on p53 and bcl-2 expression in lung carcinoma. Cancer Detect Prev 2009; 32: 444-451.
[43] Yerlikaya A, Okur E, Ulukaya E. The p53-independent induction of apoptosis in breast cancer cells in response to proteasome inhibitor bortezomib. Tumour Biol 2012; 33: 1385-1392.
[44] Hussein AM, Ahmed OM. Regioselective one-pot synthesis and anti-proliferative and apoptotic effects of some novel tetrazolo[1,5-a]pyrimidine derivatives. Bioorg Med Chem 2010; 18: 2639-2644.
[45] Chaudhary SC, Siddiqui MS, Athar M, Alam MS. D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum Exp Toxicol 2012; 31: 798-811.
[46] Mulware SJ. Comparative trace elemental analysis in cancerous and noncancerous human tissues using PIXE. J Biophys 2013; 2013: 192026.
[47] Wang HJ, Lee EY, Han SJ, Kim SH, Lee BW. Dual pathways of p53 mediated glucolipotoxicity-induced apoptosis of rat cardiomyoblast cell: Activation of p53 proapoptosis and inhibition of Nrf2-NQO1 antiapoptosis. Metabolism 2012; 61: 496-503.
ment heading
10.1016/S1995-7645(14)60117-6
*Corresponding author: Dr Khaled M. M. Koriem, Integrative Medicine Cluster, Advanced Medical and Dental Institute (AMDI), Universiti Sains Malaysia (USM), No. 6 Tingkat 1, Persiaran Seksyen 4/9 Bandar Putra Bertam, 13200 Kepala Batas, Pulau Pinang, Malaysia.
Tel: 604-5622413
Fax: 604-5622349
E-mail: kkoriem@yahoo.com
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Sonic Hedgehog signaling pathway in primary liver cancer cells
