Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
Hong-Xia Zhou, Chun-Lai Zhang2*, Yue-Hong Li2, Yu-Xin Zhang, Zi-Feng Wei, Xi Wang, Meng Ling-Li
1Anatomy teaching and research section, Hebei Union University, Tangshan 063009, China
2Worker Hospital Affiliated to Hebei Medical University, Tangshan 063000, China
Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
Hong-Xia Zhou1, Chun-Lai Zhang2*, Yue-Hong Li2, Yu-Xin Zhang1, Zi-Feng Wei1, Xi Wang, Meng Ling-Li1
1Anatomy teaching and research section, Hebei Union University, Tangshan 063009, China
2Worker Hospital Affiliated to Hebei Medical University, Tangshan 063000, China
Objective:To observe the protection effect of rhTPO and granulocyte colony stimulating factor (G-CSF) on brain nerve after hypoxic ischemic brain damage (HIBD) in neonatal rats, exploring new ways for the laboratory basis of treatment for hypoxic ischemic encephalopathy, and provide for possible.Methods:A total of 120 newborn SD rats aging 7 d were randomly divided into control group, model group, TPO group and G-CSF group, using the method of blockingleft carotid artery to establish HIBD model. The left carotid artery was only seperated rather than blocked in the control group; after modeling, saline injection, rhTPO treatment and G-CSF treatment were adopted in the model group, TPO group and G-CSF group respectively. Then 10 rats of 4 groups were executed at Day 3, 7, 14 after modeling, brain tissue was extracted to observe the brain damage; Immunohistochemical method was used to observe the histopathological changes of brain tissue and changes of nest protein (nestin) expression.Results:Injured brain mass of model group, TPO group and G-CSF group were significantly higher than that of control group at corresponding time point (P<0.05). Injured brain mass of TPO group and G-CSF group were significantly lower than that of model group (P<0.05), and with the increase of age, more significant increasing trend. At Day 3 after modeling, the expression of nestin positive cells in cerebral cortex of model group, TPO group and G-CSF group increased significantly than that of control group (P<0.05); nestin positive cells of G-CSF group outnumbered TPO group significantly (P<0.05).Conclusions:The early TPO, G-CSF treatment of HIBD rats can improve brain function after hypoxia ischemia by neural protection. G-CSF can promote the differentiation of neural cells proliferation, and reduce degeneration and necrosis of nerve cells.
ARTICLE INFO
Article history:
Received 10 April 2014
Received in revised form 15 May 2014
Accepted 15 July 2014
Available online 20 September 2014
TPO
1. Introduction
Hypoxic ischemic brain damage (HIBD) is clinical common as a brain injury syndrome caused by brain tissue hypoxia and ischemia due to perinatal suffocating[1-3]. Medium and serious HIBD have very high mortality rate and disability rate. With the in-depth clinical study of HIBD, a lot of new treatment strategies and measures has been explored, such as neural stem cells transplantation, but the treatment effect is still not ideal[4,5]. TPO is a hematopoietic growth factors secreted by the liver, can promote the differentiation and proliferation of colony-forming unit-megakaryocyte, and the maturity of the megakaryocyte and the release of platelet[6]. Studies have shown that[7], a large numbers of TPO receptors located in the central nervous system, can improve the sensorimotor function after injury, granulocyte colony stimulating factor (G-CSF) can strengthen the function of neutrophils. Studies have shown that[8], subcutaneous injection of G-CSF can promote CD34+bone marrow stem cells to migrate and differentiate into neurons and microglia. And its receptor can regulate apoptosis of related gene in the nuclei and neuron apoptosis process as antiapoptotic factor. To observe the protective effect of rhTPO and G-CSF on nerve damage in HIBD rats, 7 d aged SD rats were selected for establishing HIBD model usingleft carotid artery ligation method, rhTPO, G-CSF treatments were used respectively to observe the injured brain mass and positive expression of nestin cells in cerebral cortex, providing experiment basis for clinical treatment, and discuss the possibility the mechanism of treatment.
2. Materials and methods
2.1. Experimental animals and instrument and reagent
A total of 120 clean level SD rats were offered by the experimental animal center gender unlimited. They aged 7 d, weighting (14.1±2.5) g, class Ⅱ, with free access to food and water. Experiments on animals were strictly followed by the regulations on the administration of experimental animals. Hypoxia box (Korean LockLock seal box); BDM130/200 digital biological microscope, OPTPro2008 microscopic image collection and analysis system (OTT Chongqing Optical Instrument Co., LTD.); CY-12-C digital oxygen meter (Jiandeshi Meicheng Electrochemical Analysis Instrument Factory Production); XK06-9 J classification of blood corpuscle counter (Jiangsu Jiangyan City Purchase Medical Equipment Co., LTD.); MEB25200 evoked potentiometer (Photoelectric Japanese Company). Human recombinant platelet hormone injection, recombinant human granulocyte stimulating factor injection (Kirin Kunpeng Biological Pharmaceutical Co., LTD. Preparation Production); 0.01 M sodium citrate buffer solution provided research extension biological technology co., LTD. (Shanghai); White blood cells diluent, platelet diluent (purchased from Nanjing to build biological engineering research institute); Immunohistochemical staining kits, DAB chromogenic reagent box, wood grain-eosin staining kits (Beijing Bo Orson Biological Technology Co., LTD., production).
2.2. Modeling
After anesthesia, the rats were fixed in supine position, neck skin was disinfected conventionally, longitudinal incision on the left 2 mm along the center of neck was separated to expose the left carotid artery, which was ligated using double strands. Then the incision was blocked after carotid artery ligation in the middle of two ligation rings. Postoperative 1 h, the rats in homemade hypoxia box was removed for 2 h. Boxes were filled with nitrogen oxygen mixed with gas at a speed of 2.0 L/min to maintain oxygen volume fraction of 8% in the warehouse. After completion of modeling, the rats were moved to feed with female rats. Based on the neurobehavioral function, the rats were evaluated into 1-4 ranks of successful modeling. Evaluation criteria: 0: normal rats nerve function; 1: mild neurologic injury, contralateral forelimb cannot fully extended when holding the mouse tail after brain injury; 2: moderate injury in nervous function, tend to move toward the opposite of the injured side; 3: difficulty in walk, severely tend to move toward the damaged side; 4: unable walk, consciousness was obvious lost.
2.3. Animal grouping
A total of 120 newborn SD rats aging 7 d were randomly divided into control group, model group, TPO group and G-CSF group, using the method of blockingleft carotid artery to establish HIBD model. The left carotid artery was only seperated rather than ligated in the control group; after modeling, saline injection, rhTPO (15 U/10 g) and G-CSF (0.5 g/10 g) were adopted in the model group, TPO group and G-CSF group for 5 d, respectively.
2.4. Experimental methods
Ten rats from each group were randomly selected on day 3, 7, 14 after modeling, heart was exposed after anesthesia inhalation; left ventricle was connected with infusion devices after exposing the a small hole in vein. Saline was quickly injected till the liver and lung became white. Infusion of 4% paraformaldehyde was performed until clear liquid flowed from the right auricle. When limbs, head and neck showed stiffness, perfusion was stopped. Head of rats was cut in the foramen magnum to expose the brain tissue along the cranial seam and the brain stem , cerebellum and olfactory bulb, evenly split were removed. Brain hemisphere was cut equally for weighing damage degrees of each group, calculation formula was as follows: injured brain mass %=(right brain mass-left brain mass/right brain mass× 100%. After completion of weighing, samples were placed in 4% paraformaldehyde fixing bottle in the 4 ℃ refrigerator for 48 h. Then conventional gradient ethanol dehydration, xylene transparentation and paraffin embedding were conducted, followed by coronary slicing in 4 µm thickness, histological observation was performed after HE staining.
2.4.1. HE Dyeing method
Biopsy was soaked using xylene and roasted for dewaxing, gradient ethanol was used for dehydration, followed by hematoxylin staining; using 70% alcohol to separate colour when the specimens became blue. They were rinsed with distilled water, then stained using 1% eosin for 5-10 min, dehydrated with 70%, 80%, 90% ethanol, immersed in transparent xylene and sealed using neutral resin.
2.4.2. nestin detection
Gradient ethanol was used for biopsy dehydration, thenrinsed three times with distilled water , blocked with H2O2for 10 min, then rinsed for three times; slices were placed in 0.01 M sodium citrate buffer (pH 6.0), then microwaved for two times, with 10 min each, and washed by PBS for three times; blocked at room temperature for 20 min after adding goat serum. They were added with diluted nestin fluid resistance of 50 µL (1:80) for 2 h and washed with PBS for three times; then were incubated for 20 min at 37 ℃ after added with 50 µL of biotin HuaEr, and washed with PBS for three times, incubated for 15 min at 37 ℃ after added with 50 µL of chain mildew avidin working drops and washed with PBS for three times. In accordance with the manual struction, DAB was conducted chromogenically followed by rinsing with distilled water, and hematoxylin redyeing for 1 min, then rinsed by distilled water. Tissue was soaked in Na2HPO4solution for 2 min, conventional dehydration, transparentation and sealled using neutral resin were performed after specimens became blue.
2.5. Results determination
nestin positive cells number was used to determine the number changes of neural stem cells, nestin positive cells dyeing in tan were mainly expressed in the cytoplasm, axons and dendrites of neural cells, five horizons (×40) were randomly selected to observe the positive cell count using analysis software, and the average number was calculated per unit area.
2.6. Statistical analysis
Using SPSS19.0 statistical software to analyze data. Data was expressed as mean±sd, and analyzed byttest,P<0.05 was considered as statistically significant difference.
3. Results
3.1. Comparison of injured brain mass after modeling at different time points
Injured brain mass of model group, TPO group and G-CSF group were significantly higher than that of control group at corresponding time point (P<0.05). Injured brain mass of TPO group and G-CSF group were significantly lower than that of model group (P<0.05), and with the increase of age, more significant increasing trend. The brain damage % of TPO group at 14 d was higher than that of G-CSF group, differences between groups was statistically significant (P<0.05) (Table 1).
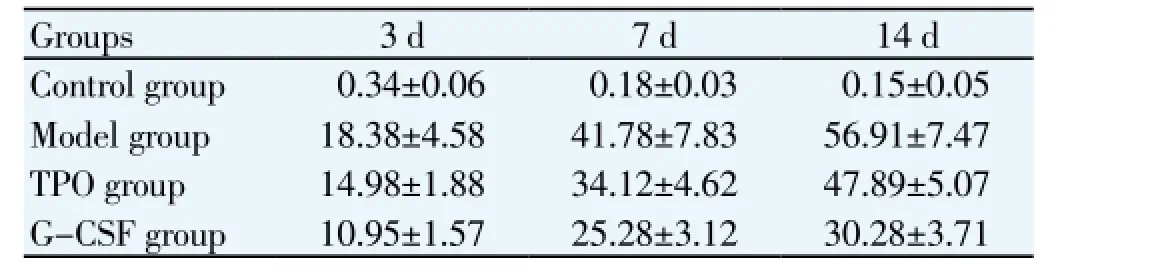
Table 1 Comparison of injured brain mass after modeling at different time points (%).
3.2. Histological observation
Histological slicing on day 14 after modeling showed that in control group, cerebral cortex neurons and glial cells were normal, the number and arrangement of nerve cells were normal, with nucleus in the center position, and no lack of nerve cells. In the model on day 14, intercellular space was increased, nerve cells showed obvious shrinkage, axons and dendrons could not be recognized at high magnification, because part of the nuclears appeared cracking phenomenon. TPO group showed that the damage degree was reduced compared with the model group, with more functional nerve cells in normal size, shape and arrangements. G-CSF group showed microscopically that the extent of cell damage was significantly slighter than model group, with more functional nerve cells and nuclears in normal size, shape and arrangements., and dendritic typical structure was clear (Figure 1).
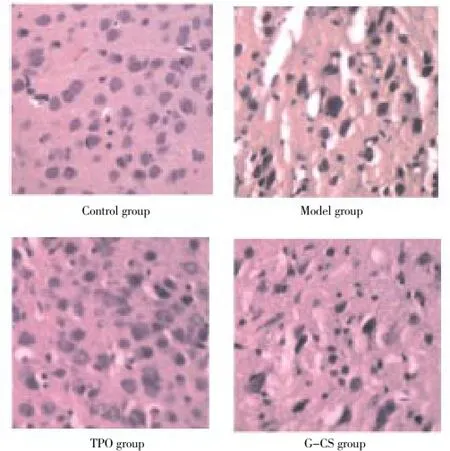
Figure 1. Histological changes of brain tissue on day 14 after modeling (HE, ×400).
3.3. Expression of nestin
There was no positive expression of nestin in observation group; a small amount of nestin positive expression wasvisible within the cytoplasm, axons and dendrites in the model group on day 3 after modeling, increased slightly on day 7, and declined on day 14. In TPO group, the small amount of nestin positive expression was gradually declined with time. In G-CSF group, nestin protein positive expression on day 7 was significantly higher compared with that on day 3, and declined on day 14 (Figure 2).
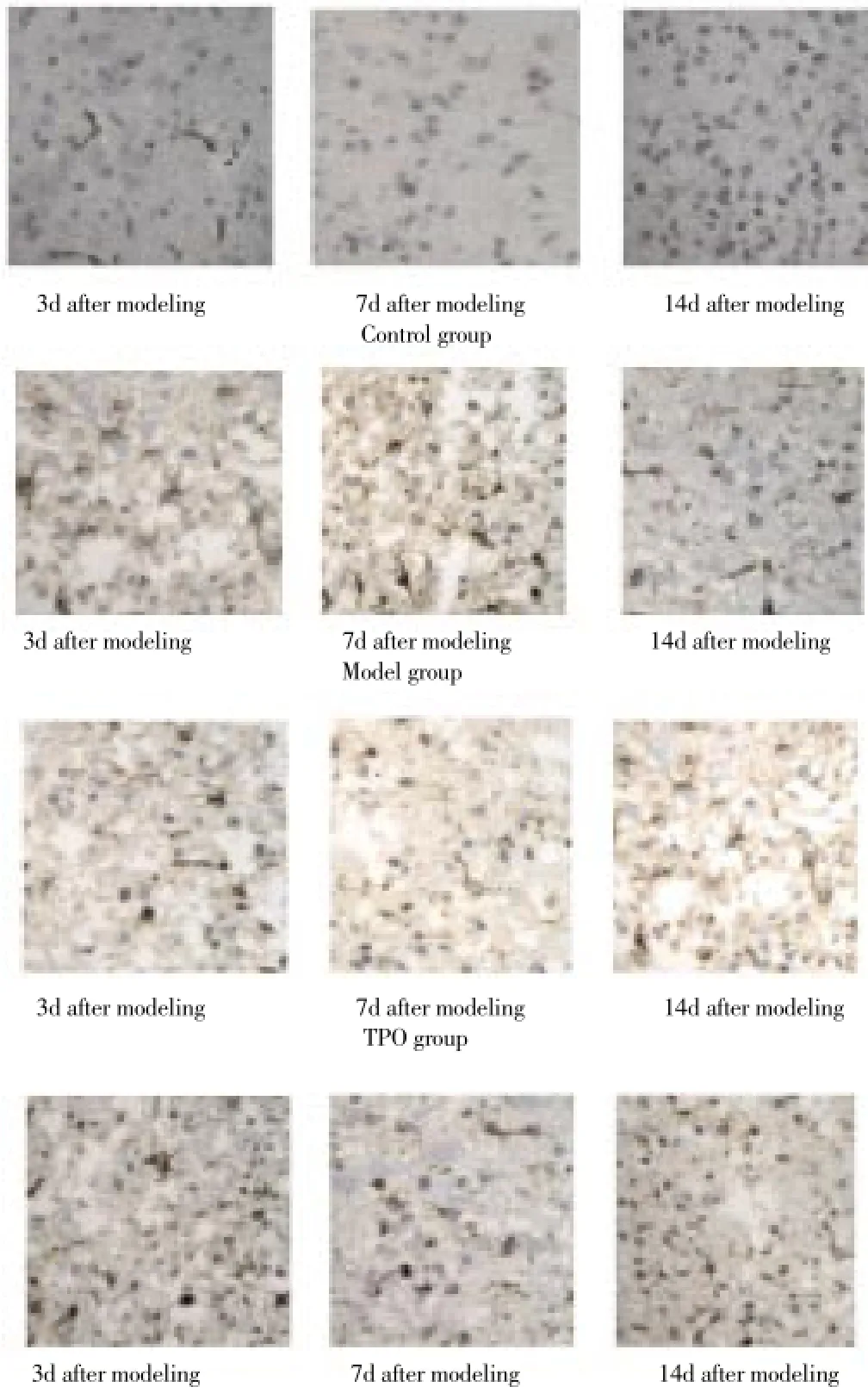
G-CSF groupFigure 2. Expression of nestin cells in brain tissue at different time points.
Expression of nestin cells in brain tissue of model group, TPO group and G-CSF group were significantly higher than that of control group at different time points (P<0.05); nestin cells of G-CSF group were all more than that of TPO group at corresponding time points (P<0.05) (Table 2).

Table 2 Expression of nestin cells in brain tissue at different time points.
4. Discussion
Pathological mechanism of HIBD brain injury is complicated and has not been fully elucidated. Studies have considered[9-12], selective neuronal necrosis caused by hypoxia and ischemia is a prime requisite of brain structure and function damage. It was regarded that[13], necrosis is the only cause of HIBD related nerve cell death. Appropriate treatment given in early HIBD can effectively reduce the brain damage by effecting the process of cell apoptosis. Other studies showed that[14] expression of genes related to apoptosis was observed in the brain tissue after HIBD hypoxia ischemia resistance of neurons increased significantly after being transfected by antiapoptotic gene, showing that apoptosis process involved in the process of HIBD brain damage. Blood supply path in SD rats has high approximation with humans’, formed by internal carotid artery and vertebral artery Willis ring, unilateral carotid artery ligation and hypoxia cavity can set up the HIBD model, ligating side brain rapidly to form hypoxic ischemic brain damage. This study adopted the method of the left carotid artery ligation to set up HIBD model in the mid-point, avoiding the blood vessels from recirculation thoroughly, to induce the corresponding morphologyand function changes of nervous system.
TPO can promote growth of neural stem cells and phosphorylate AKT, activating the PI3-K/AKT signaling pathway, and inhibiting cell apoptosisof of neural progenitor cells C17.2[15-17]. In this study, the expression of nestin cells in brain tissue was analyzed at different time points to observe changes of the rats organization neural precursor cells. After modeling in TPO group of rats expression of nestin positive cells was significantly higher than that of control group, due to hypoxia ischemia of brain tissue. After treatment, histological observationshowed that brain injury and injured brain mass of TPO group was significantly reduced than that of model group, showed that the TPO can reduce brain damage degree after HIBD, and effectively improve the development of the nervous system and restore nerve function, can reduce the influence of hypoxia ischemia on neural development. G-CSF is hematopoietic growth factors, can promote the proliferation and differentiation of hematopoietic stem cells[18-20]. The research found that[21-23], G-CSF can enhance local inflammatory response. This study results showed that injuried brain mass of model group, TPO group and G-CSF group were significantly higher than that of control group at corresponding time point (P<0.05). Injuried brain mass of TPO group and G-CSF group were significantly lower than that of model group (P<0.05), and with the increase of age, more significant increasing trend. At Day 10 after modeling, the expression of nestin positive cells in cerebral cortex of model group, TPO group and G-CSF group increased significantly than that of controlgroup (P<0.05). The histological examination showed that damage degrees in groups TPO and G-CSF were lighter than that of model group; suggesting that TPO and G-CSF treatment in early HIBD can protect neurons.
This study results show that the TPO and G-CSF treatment in early HIBD can improve brain function after hypoxia ischemia in newborn rats, beneficial to the growth of brain function. G-csf can promote the differentiation and proliferation of neural precursor cells, reduce degeneration and necrosis of the nerve cells, so as to achieve protective effect on HIBD rats’ nerve.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Anderson CA, Arciniegas DB. Cognitive sequelae of hypoxicischemic brain injury: areview. NeuroRehabilitation 2010; 26(1): 47-63.
[2] Ceng Z, Zhou ZY, Song YY, Liu Q, Lv JJ, Cai YJ, et al. Protection of erythropoietin on hypoxic ischemic brain damage in newborn rats. J Jinan Univ (Nat Sci & Med Ed) 2011; 32(2): 155-159.
[3] Lu-Emerson C, Khot S. Neurological sequelae of hypoxicischemic brain injury. NeuroRehabilitation 2010; 26(1): 35-45.
[4] Zhou J, Li J, Rosenbaum DM, Barone FC. Thrombopoietin protects the brain and improves sensorimotor functions: reduction of stroke-induced MMP-9 upregulation and blood-brain barrier injury. J Cerebral Blood Flow & Metab 2011; 31(3): 924-33.
[5] Acioly MA, Carvalho CH, Koerbel A, Löwenheim H, Tatagiba M, Gharabaghi A. Intraoperative brainstem auditory evoked potential observations after trigeminocardiac reflex during cerebellopontine angle surgery. J Neurosurg Anesthesiol 2010; 22(4): 347-353.
[6] Liu Q, Ceng Z, Zhou ZY, Cai YJ, Chen MY, Song YY. The influence of EPO on NSE expression for hypoxic ischemic brain damage in newborn rats. J Jinan Univ (Nat Sci & Med Ed) 2011; 32(6): 593-597.
[7] Zhao H, Zhang QR, Lu XM, Zhang HP, Chen HX. The influence of protein - 1, matrix metalloproteinase - 9 expression on after cerebral ischemia reperfusion in rats. Chin J Reh Med 2011; 26(6): 550-552.
[8] Niu ZL, Tang XJ, Li LL, Chen LX, Yao XL. Dynamic expression of TNF alpha and SOCS-3 on focal cerebral ischemia reperfusion. J Pract Cardio-Cerebral Pulmonary Vascular Dis 2011; 19(1): 3-5.
[9] Zhang XY, Chen CL, Li HX. Red protein expression after whole cerebral ischemia reperfusion in rats. J Pract Cardio-Cerebral Pulmonary Vascular Dis 2010; 18(2): 103-105.
[10] Zhang XY, Chen CL, Li HX, Han JQ. Correlation of red protein and brain caspase 3 in hippocampal CA1 area of rats. J Shandong Med 2010; 40(1): 40-41.
[11] Duan CH, Fu QL, Wang CJ, Zhou CY, Xi HX, Hai J, et al. Effect of ginkgo biloba extract on bax and iglial protein expression in schemia-reperfusion injured rats. Chin Exp Surg Mag 2012; 29(1): 97-99.
[12] Mei SL, Gao JX, Yang CJ. Effect of Ginkgo biloba extract on HIF-1 VEGF expression and angiogenesis in focal cerebral ischemia rats. J Emergency Med Chin 2009; 29 (8): 724-726.
[13] Fan YY, Hu WW, Dai HB, Fu QL, Wei EQ, Luo JH, et al. Activation of the central histaminergic system is involved in hypoxia-induced stroke tolerance in adult mice. J Cereb Blood Flow Metab 2011; 31(1): 305-314.
[14] Lin X, Li M,, Hu YZ, Han ZT, Zhang HH, Shang AJ, et al. The relationship of expression changes of craniocerebral red protein and neuron apoptosis after brain trauma. Chin J Appl Physiol 2010; 26(1): 39-44.
[15] Li W, Wu Y, Ren C, Lu Y, Gao Y, Zheng, X, et al. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins 2011; 79(1): 115-125.
[16] Liu C, Jia YJ, Jiang XQ, Zhang Z, Cheng X, Min LQ. Effect of ceftriaxone sodium on expression of Fas and NF-κB in focal cerebral ischemia rats. Chin J Gerontol 2012; 32(19): 4210 -4212.
[17] Chen XL, Jiang L. The influence of exercise rehabilitation on spatial learning and memory after hypoxic ischemic brain damage in newborn rats. Chin Contemporary J Pediatrics 2010; 12(5): 363- 367.
[18] Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 2009; 61(1): 85-100.
[19] Liu CJ, Guo YK, Li YL. Early intervention on synaptic plasticity after hypoxic ischemic brain damage in the newborn rat cerebral cortex. maternal and child health care in China 2011; 26(11): 1702-1705.
[20] Qu YX, He HM, Li KH, Wang CH, Xu LX. Effect of resveratrol glucoside on the learning and memory and hippocampal synaptic in newborn rats after hypoxic ischemic brain damage. J Pediatric Emergency Med Chin 2010; 17(5): 434-436.
[21] Ancora G, Soffritti S, Lodi R, Tonon C, Grandi S, Locatelli C, et al. A combined a- EEG and MR spectroscopy study in term newborns with hypoxic- ischemic encephalopathy. Brain Dev 2010; 32(10): 835-842.
[22] Chan KC, Khong P, Lau HF, Cheung PT, Wu EX. Late measures of microstructural alterations in severe neonatal hypoxicische-mic encephalopathy by MR diffusion tensor imaging. Int DevelNeurosci 2009; 27(6): 607-615.
[23] Rutherford M, Malamateniou C, McGuinness A, Allsop J, Biarge MM, Counsell S. Magnetic resonance imaging in hypoxicischaemic encephalopathy. Early Hum Dev 2010; 86(6): 351-360.
ment heading
10.1016/S1995-7645(14)60124-3
*Corresponding author: Chun-Lai Zhang, M.D., Chief Physician, Worker Hospital Affiliated to Hebei Medical University, Tangshan 063000, China.
E-mail: zhxiatg@163.com
Foundation project: It is supported by Social Science Fund Project of Hebei Province, No: HB13LJ003.
G-CSF
HIBD
Nerve protection
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Sonic Hedgehog signaling pathway in primary liver cancer cells
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
