Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
First Hospital Affiliated to Hebei Northern Institute, Zhangjiakou, Hebei 075000, China
Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
Fang-Jiang Li*, Tao Hsu, Hui-Xian Li, Jin-Zheng Shi, Mei-Ling Du, Xiao-Yuan Wang, Wen-Ting Zhang
First Hospital Affiliated to Hebei Northern Institute, Zhangjiakou, Hebei 075000, China
Objective:To investigate the protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury (I-RI) in rats.Methods:A total of 60 SD rats were randomly divided into control group, model group, lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group with 15 in each. The I-RI model was established in model group, the lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group by method of seaming along left anterior descending coronary artery myocardial, control group was only opened the chest without seaming, ST-elevation within 2 min was regarded as modeling success. Model group did not adopted any intervention, lithium chloride intervention group was treated with lithium chloride injection 15 mg/kg by jugular venipuncture preoperatively, L-arginine methyl ester + lithium chloride intervention group was treated with intraperitoneal injection of 30 mg•kg-1•d-1L-arginine methyl ester 7 d before the test, and intravenous catheter of 15 mg/kg lithium chloride preoperatively. The hydroxybutyric acid dehydrogenase (HBDH), creatine kinase isoenzyme (CK-MB), superoxide dismutase (SOD), malondialdehyde (MDA) level and nitric oxide synthase (NOS) activites were tested. Each large area of myocardial ischemia tissue was extracted for determination of the MDA content, SOD activity in tissue and serum, and morphological changes of myocardial tissue.Results:SOD activity was highest in lithium chloride intervention group, followed by L-arginine methyl ester + lithium chloride intervention group, control group and model group (P<0.05); SOD activity was highest in L-arginine methyl ester + lithium chloride intervention group intervention group, followed by lithium chloride intervention group, control group and model group (P<0.05). MDA content of myocardial tissue was highest in model group, followed by L-arginine methyl ester + lithium chloride intervention group, the lithium chloride intervention group and control group (P<0.05); serum MDA content was highest in L-arginine methyl ester +lithium chloride intervention group, followed by model group, lithium chloride intervention group and control group (P<0.05). Compared with the control group, serum NOS was significantly higher in model group, lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group (P<0.05), there was no statistical difference of serum NOS activity between the three groups (P>0.05); HBDH and CK-MB of plasma were highest in model group, followed by L-arginine methyl ester + lithium chloride intervention group, lithium chloride intervention group and control group (P<0.05). A significantly lighter myocardial damage was observed microscopically in lithium chloride intervention group than that in L-arginine methyl ester + lithium chloride intervention group and model group. Conclusions: lithium chloride pretreatment can significantly reduce the myocardial I-RI, maintain structure and function of myocardial cells, improve the antioxidant ability of myocardial tissue, play an effective role on protecting myocardial I-RI.
ARTICLE INFO
Article history:
Received 10 April 2014
Received in revised form 15 May 2014
Accepted 15 July 2014
Available online 20 September 2014
Myocardium
1. Introduction
Ischemia-reperfusion injury (I-RI) refers to the pathological process of cell structure and metabolism impairment aggravating induced by reentry of the blood supply to the tissues after a period time of ischemia[1]. As the development of heart transplant surgery, the prevention and cure of myocardial I-RI has become the hot topic in clinical study[2]. According to WHO statistics[3], acute obstructive coronary artery disease is becoming the major cause of deaths worldwide, in the process of treatment, the existing operation and drug treatment of coronary arteryrecanalization can all cause I-RI[3]. With the deepening of clinical research on I-RI and the development of myocardial protection drugs, drug pretreatment has become the main method of myocardial protection. Lithium is widely existed in the body’s bones, muscles and major organs, is one of the trace elements in the human body, is also the potential of the myocardial protective drugs. Studies have confirmed that[4], after a period of time, lithium chloride pretreatment can reduce nerve - mental symptoms of I-RI recovery phase in rats. Another study showed[5-7], lithium chloride has antiapoptotic effect on myocardial reperfusion, which can effectively reduce myocardial I-RI. To observe the protective effects and mechanism of lithium chloride on rat myocardial I-RI, the author selected SD rats for myocardial I-RI modeling after using lithium chloride pretreatment.
2. Materials and methods
2.1. Experimental animals
A total of 60 healthy adult SD rats were selected, weighting 250-300 g, male and female unlimited. They were purchased from Nanjing University Laboratory Animal Center, and provided with free food and water.
2.2. Instrument and reagent
Small animal breathing machine, electrocardiograph (Nanjing Yu’s Biotechnology Company); MICROM HM325 tissue slicing machine (Zeiz Company, Germany); Spectrophotometric instrument (Olympus Company, Japan); BH-2 optical microscope (Olympus Company, Japan); MDA determination kit (MDA2003), SOD determination kit (GMS50002.1), anhydrous lithium chloride, L-arginine methyl ester, heparin injection, 2% pentobarbital sodium, bought from Nanjing Institute of Biological Engineering.
2.3. model establishMENT
All rats had intraperitoneal injection of 2% sodium pentobarbital for anesthesia, followed by endotracheal intubation assisted mechanical ventilation. Indwelling tube was placed in the jugular vein. Skin incision was performed along the left collarbone midline between 4th rib, and muscle blunt separation was carried out. Pericardium was cut after thoracotomy; the left anterior descending coronary artery was along the joint of left atrium and pulmonary arterial cone using 6-0 prolene string. String was cut down after 30 min of artery blocking, blood supply was restored, closed chest, and appearance of ST-elevation within 2 min was regarded as modeling success.
2.4. Animal grouping and pretreatment
A total of 60 SD rats were randomly divided into control group, model group, lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group with 15 in each. The I-RI model was set up in model group, the lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group by method of seaming along left anterior descending coronary artery myocardial. Rats in control group was only opened the chest without seaming, ST-elevation within 2 min was regarded as modeling success. Model group did not adopted any intervention, lithium chloride intervention group was treated with lithium chloride injection 15 mg/kg by jugular venipuncture preoperatively, L-arginine methyl ester + lithium chloride intervention group was treated with intraperitoneal injection of 30 mg•kg-1•d-1L-arginine methyl ester 7 d before the test, and intravenous catheter of 15 mg/kg lithium chloride preoperatively. After 240 min of reperfusion, venous blood was extracted and centrifuged, the serum was saved in freeze. After sacrificing the rats, ischemic ventricular wall was sheared,for REDOX index and morphological observation.
2.5. Biochemical index determination
The hydroxybutyric acid dehydrogenase (HBDH), creatine kinase isoenzyme (CK-MB), superoxide dismutase (SOD), malondialdehyde (MDA) level and nitric oxide synthase (NOS) activites were detected.
2.6. Tissue morphology observation
0.3 cm×0.3 cm×0.3 cm size of ischemic myocardial tissue was processed under conventional fixation, embedding, sectioning and HE staining, to observe the histomorphology changes of the damaged myocardium.
2.7. Statistical analysis
Data were analyzed using SPSS19.0 statistical software, and expressed as mean±sd value. Comparison between groups was analyzed using single factor analysis of variance withqinspection, qualitative data was analyzed with Χ2test, andP<0.05 was regarded as significant difference.
3. Results
3.1. Changes of SOD activity and MDA level of myocardium and serum
SOD activity of myocardium was highest in lithium chloride intervention group, followed by L-arginine methyl ester + lithium chloride intervention group, control group and model group (P<0.05); serum SOD activity was highest in L-arginine methyl ester + lithium chloride intervention group, followed by lithium chloride intervention group, control group and model group (P<0.05). MDA content of myocardial tissue was highest in model group, followed by L-arginine methyl ester + lithium chloride intervention group, the lithium chloride intervention group and control group (P<0.05); serum MDA content was highest in L-arginine methyl ester +lithium chloride intervention group, followed by model group, lithium chloride intervention group and control group (P<0.05), as shown in Table 1.
3.2. NOS, CK-MB and HBDH level
Compared with the control group, serum NOS was significantly higher in model group, lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group (P<0.05), there was no statistical difference of NOS activity between the three groups (P>0.05); HBDH and CK-MB of plasma were highest in model group, followed by L-arginine methyl ester + lithium chloride intervention group, lithium chloride intervention group and control group (P<0.05), as shown in Table 2.
3.3. Tissue morphology observation
Control group showed no pathological changes of myocardial tissue; myocardial tissue of model group, lithium chloride intervention group and L-arginine methyl ester + lithium chloride intervention group was microscopically showed with different degrees of pathological damage; Irregular cell nuclei, hyperchromatic cytoplasm, inflammatory cells infiltration and myocardial fibrosis were observed in model group. Some irregular cell nucleus and fibrous degeneration were observed in Lithium chloride intervention group, the hyperchromatic cytoplasm was not obvious, inflammatory cells infiltration degree was slighter than that of model group. The myocardial damage degree of L-arginine methyl ester + lithium chloride intervention group were between model group and lithium chloride intervention group, the results are shown in Figure 1.
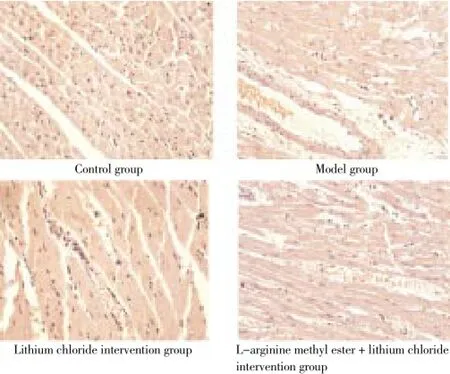
Figure 1. Tissue morphology observation (HE×200).
4. Discussion
Myocardial I-RI refers to the pathological phenomena of aggravating myocardial tissue damage degree induced by blood supply restore after blood supply of myocardial tissue ischemia for a period of time. Damage of schemic myocardium after restore blood flow can be aggravated on the ultrastructure, function and the electrophysiologicalchange, which resulted in the pathological basis of ischemia-reperfusion injury[8]. Clinical symptoms of myocardial I-RI are often characterized by abnormal changes of reperfusion myocardial enzyme, arrhythmia and myocardial ultrastructure[9-12]. Studies have shown that[13], ischemic heart disease perioperative cardiac events is the important cause of perioperative death. Therefore, to prevent or alleviate perioperative I-RI is of great significance in the process of treatment.
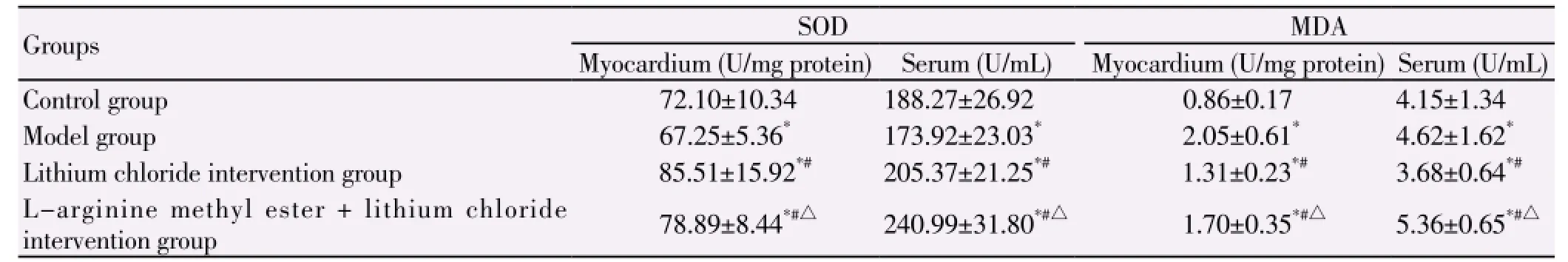
Table 1 Changes of SOD activity and MDA level in serum and myocardium.
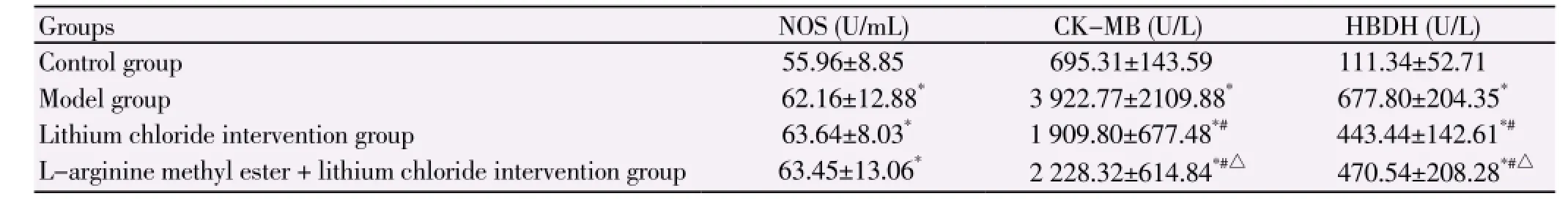
Table 2 NOS, CK-MB and HBDH level.
Mechanism of myocardial I - RI is not fully clear. Studies have shown that[14-16], myocardial energy metabolism disorder, calcium overload and free radical, neutrophils and apoptosis may participate in myocardial I-RI. Preconditioning refers to adding damage to tissues and organs in advance to stimulate adaptation and tolerance of the body tissues against harmful events[17]. Studies have shown that[18-20], repeatedly, transient myocardial ischemiareperfusion can reduce myocardial cell damage due to long ischemia-reperfusion. According to the theory of the ischemic preconditioning protection mechanism, clinical used drugs as alternative ischemic stimulus in clinical application, in order to make the body produce medicine rational preadaptation, which is easy to control. Clinical use of inorganic lithium ion is more focused on the treatment of mental symptoms, recent studies have shown that[21], lithium salts can serve as an antioxidant to against ischemic anoxia. Other scholars found[22-24], lithium chloride pretreatment can reduce ischemia-reperfusion damage of neurons in rats, and can reduce the cerebral infarction volume. Therefore, the author carried on the study of lithium chloride pretreatment on myocardial I-RI.
In cardiac surgery, filling and began, with the oxygen molecules after myocardial ischemia can produce a large number of oxygen free radical, affecting lipid peroxidation of myocardial cell membrane, cell membrane and mitochondrial membrane, and increase membrane permeability, causing membrane dysfunction such as membrane receptors, ion channels dysfunction[16]. Studies have confirmed that[22,23], inhibition of oxygen free radicals, can reduce the I-RI. SOD is important metal antioxidant enzymes in the body by damaging free radicals and preventing the toxic effects of oxygen, so SOD activity can indirectly reflect the clearance ability of oxygen free radicals. MDA is the final production of lipid peroxides, its content changes can indirectly reflect the damage severity of tissue cells by free radicals, and can reflect the lipid peroxidation degree. Therefore, these two indicators can reflect oxygen free radical scavenging ability and damage severity of tissue cells attack by free radicals.
This study showed that compared with model group, lithium chloride intervention group had higher level of serum SOD and NOS activity (P<0.05), lower plasma HBDH, MDA and CK-MB content (P<0.05), suggesting the lithium chloride pretreatment can reduce the attacks of free radicals on cell membrane lipid peroxidation, directly decrease the mitochondrial membrane damage, protect myocardial I-RI. In this study, the degree of myocardial damage in the lithium chloride intervention group rat model group and L-arginine methyl ester + lithium chloride intervention group were significantly reduced, also confirmed that the lithium chloride pretreatment can reduce myocardial I-RI effectively.
This study showed that the drug preconditioning of lithium chloride can strengthen the antioxidant capacity of myocardial tissue, reduce myocardial I-RI injury, and play an effective role in protecting myocardial I-RI.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innateadaptive immune-mediated tissue inflammation. Am J Transplant2011; 11: 1563-1569. [PMID:21668640DOI: 10.1111/j.1600-6143.2011.03579.x]
[2] Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013; 10: 79-89. [PMID:23229329 DOI: 10.1038/nrgastro.2012.225]
[3] Chen W, Guo LL, Wang J. research progress of traditional Chinese medicine on prevention and control of myocardial ischemiareperfusion injury. Liaoning Trad Chin Med 2012; 39(6): 1-5.
[4] Su HJ, Xiang XY, Zhao XJ, Kong LW, Du DY, Zhang WM, et al. Protective experimental study of Shenfu Zhusheye on myocardial ischemia-reperfusion injury in rats. J Chongqing Med Univ 2010; 1(35): 60-64.
[5] Piguet AC, Dufour JF. PI(3)K/PTEN/AKT path-way. J Hepatol 2011; 54: 1317-1319. [PMID: 21168457 DOI: 10.1016/ j.jhep.2010.12.013]
[6] Keyes KT, Xu J, Long B, Zhang C, Hu Z, Ye Y. Phar-macological inhibition of PTEN limits myocardial infarct size and improves left ventricular function postinfarction. Am J Physiol Heart Circ Physiol 2010; 298: H1198-H1208. [PMID: 20097771 DOI: 10.1152/ajpheart.00915.2009]
[7] Ruan H, Li J, Ren S, Gao J, Li G, Kim R, et al. Inducible and cardiac specifc PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol 2009; 46: 193-200. [PMID: 19038262 DOI: 10.1016/j.yjmcc.2008.10.021]
[8] Shi GD, Ouyang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun 2011; 404: 941-945. [PMID: 21185267 DOI: 10.1016/j.bbrc.2010.12.085]
[9] Lai JP, Bao S, Davis IC, Knoell DL. Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury. Br J Pharmacol 2009; 156: 189-200. [PMID: 19134000 DOI: 10.1111/j.1476-5381.2008.00020.x]
[10] Tu Y, Wan L, Fan Y, Wang K, Bu L, Huang T, et al. Ischemic postconditioning-mediated miRNA-21 protects against cardiac ischemia/reperfusion injury via PTEN/Akt pathway. PLoS One 2013; 8: e75872. [PMID: 24098402 DOI: 10.1371/journal. pone.0075872]
[11] Ren F, Duan Z, Cheng Q, Shen X, Gao F, Bai L, et al. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an in-terleukin-10-mediated immune regulatory mechanism. Hepatology 2011; 54: 687-696. [PMID: 21567437 DOI: 10.1002/hep.24419]
[12] Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. PTEN-mediated Akt/β-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology 2013; 57: 289-298. [PMID: 22807038 DOI: 10.1002/ hep.25958]
[13] Ren F, Zhang HY, Piao ZF, Wu ZM. Glycogen synthase kinase -3β in the hot liver ischemia-reperfusion injury and its intervention. Chin J Liver Dis 2011; 19: 547-551.
[14] O’Neill S, Ross JA, Wigmore SJ, Harrison EM. The role of heat shock protein 90 in modulating ischemia-reperfusion injury in the kidney. Expert Opin Investig Drugs 2012; 21(10): 1535-1548.
[15] Ren F, Zhang HY, Piao ZF, Zheng SJ, Chen Y, Chen DX, et al. Regulation of activity of glycogen synthase kinase 3 receptor 4 on Tol l mediated inflammatory response in the liver. Chin J Liver Dis 2012; 20: 693-697.
[16] Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol 2012; 56: 359-366. [PMID: 21756853 DOI: 10.1016/ j.jhep.2011.05.023]
[17] Tan L, Wang LY, Xiang HY, Li M. Protective effects and mechanism study of Emodin on cerebral ischemia-reperfusion in rats. J Trad Chin Western Med Cardio-Cerebrovasc Dis 2010; 8(9): 1100-1101.
[18] D’Annunzio V, Donato M, Buchholz B, Pérez V, Miksztowicz V, Berg G, et al. High cholesterol diet effects on ischemiareperfusion injury of the heart. Can J Physiol Pharmacol 2012; 90(9): 1185-1196.
[19] Minguet G, Brichant JF, Joris J. Opioids and protection against ischemia-reperfusion injury: from experimental data to potential clinical applications. Acta Anaesthesiol Belg 2012; 63(1): 23-34.
[20] Minamino T. Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J 2012; 76(5): 1074-1082.
[21] Vatazin AV, Astakhov PV, Zul’karnaev AB, Kantariia RO, Artemov DV. Cellular factors of ischemia/reperfusion injury pathogenesis in the renal transplantation. Ross Fiziol Zh Im I M Sechenova 2012; 98(7): 906-914.
[22] Qiao ZK, Zhang ZR, Tian LZ, Wang KL, Zhang R. protection study of Emodin in canine autologous kidney related ischemiareperfusion injury. J Modern Clin Med 2014; 29(2): 131-134.
[23] Bai L, Ren F, Zheng SJ, Zhang J, Chen Y, Duan ZP. regulation role and mechanism of PTEN/PI3K in hepatic ischemiareperfusion injury in mice. J World Chin Digestion 2014; 22(2): 203-209.
[24] Tang LM, He YG, Zhang YD, Zheng H, Zhang GB, Xi JK. research progress of mechanism and protection of myocardial ischemia/reperfusion injury. Med J China Coal Industry 2013; 16(1): 159-162.
ment heading
10.1016/S1995-7645(14)60128-0
*Corresponding author: Fang-Jiang Li, M. B., Supervisor of Postgraduate Students, Chief Physician, First Hospital Affiliated to Hebei Northern Institute, Zhangjiakou, Hebei 075000, China.
E-mail: lfj6789tg@163.com
Tel: 13393246681
Foundation project: It is supported by Target Scientific Research of Zhangjiakou Technology Bureau No. 12110044D-2.
I-RI
Lithium oxide
Myocardial protection
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Sonic Hedgehog signaling pathway in primary liver cancer cells
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
- Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
