Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
ICU, First Hospital of Jilin University, Changchun130041, China
Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
Shu-Jie Zhao*, Shi-Ji Wang, Hong-Xiang Li, Li-Hua Dong, Huai-Jiang He, Zhong-Min Liu, Yu-Shan Wang
ICU, First Hospital of Jilin University, Changchun130041, China
Objective:To tinvestigate the protective effect and mechanism of emodin pretreatment on intestinal mucosa of rats with intestinal ischemia-reperfusion injury.Methods:A total of 50 SD rats were randomly divided into control group, model group, emodin groups of low, medium and high dose, with 10 in each group. Ischemia-reperfusion injury (I-RI) mode was established by using noninvasive clamp on superior mesentericartery (SMA). Control group and model group were pretreated with 0.5% sodium carboxymethyl cellulose solution lavage 2 h before operation, emodin groups of low, medium and high dose were given emodin lavage with 20, 40, 60 mg/kg pretreatment, femoral venous blood before the lavage pretreatment (T0) and 1 h ischemia (T1) , and inferior vena venous blood after 1 h of reperfusion (T2) were extracted from each group of rats for detection of serum level of intestinal fatty acid binding protein (I-FABP), tumor necrosis factor (TNF-α), endotoxin, interleukin 6 (IL-6), and the content of diamine oxidase (DAO); After model establishment, the rats were sacrificed, intestine homogenate was prepared by using blind intestinal tissue to detect intestinal tissue myeloperoxidase (MPO), malondialdehyde (MDA) and superoxide dismutase (SOD) levels. And upper small intestine tissue was retrieved, followed by fixation and conventional HE staining to observe intestinal tissue morphology under light microscopy.Results:In emodin groups of low, medium and high dose at T1 and T2, I - FABP, TNF-α, endotoxin, IL-6 and DAO level were significantly lower than that of model group (P<0.05); in emodin group of low, medium and high dose, MPO and MDA content in intestinal tissue homogenate was significantly lower than that in model group (P<0.05), SOD level was significantly higher than that of model group (P<0.05). Intestinal damage of emodin low, medium and high dose groups were significantly lighter than model group. Conclusions:Emodin pretreatment has certain protective effect on intestinal mucosa in ischemia reperfusion injury.
ARTICLE INFO
Article history:
Received 10 April 2014
Received in revised form 15 May 2014
Accepted 15 July 2014
Available online 20 September 2014
Emodin
1. Introduction
There are 1.2 million heart surgery around the world every year, as the statistics showed[1-3]. The prevention and control of postoperative complications of cardiac surgery has gained extensive clinical attention. Studies have shown that[4,5], postoperative gastrointestinal complications of heart surgery are 0.5% to 4.0%, with main symptoms of perforation, ischemic enteritis and gastrointestinal bleedingetc, which can be as high as 26%-75% mortality rate. How to reduce the incidence of gastrointestinal complications after heart surgery has become urgent. CPB perfusion should first guarantee important organs, thus it can easily lead to decreased gastrointestinal mucosal blood flow, induces intestinal ischemia-reperfusion injury[6-8]. At present, clinical protection against intestinal ischemia-reperfusion injury during CPB after heart surgery is mainly by way of inhibiting gastric acid, but excessive inhibition of gastric acid can lead to intestinal micro environmental damage, and bacterial translocation, with a poor effect[9]. Therefore, seeking a better way to protect intestinal mucosa during CPB is particularly important. Studies have confirmedthat[10], emodin can reduce the inflammatory reaction, and have significant protective effect on the intestinal ischemiareperfusion injury (I-RI). To observe protective effects and mechanism of emodin on intestinal I-RI, we selected SD rat intestinal I-RI model, the results are reported as follows.
2. Materials and methods
2.1.Experimental animals
A total of 50 healthy adult SD rats, male and female unlimited, weight 250-300 g, clean level, were provided by our animal experiment center. They were provided with free food and water, adaptive feed for 5 d, before modeling, limited food for 12 h and water for 4 h, the experiment process on animals were strictly followed by the regulations on the administration of experimental animals.
2.2. Instrument and reagent
Emodin chemical reference substance and sodium carboxymethyl cellulose were bought from Beijing Solarbio Science and Technology Vo., LTD. Anhydrous calcium chloride and 10% chloral hydrate were provided by the national medicine group chemical reagent Beijing Vo., LTD.; TNF-α Elisa kit, endotoxin Elisa kit, IFABP Elisa kit and IL-6 Elisa kit were purchased from RD Company, USA; DAO, SOD, MDA and MPO reagent plates were purchased from Nanjing Institute of Biological Engineering Institute. RH-1650 low temperature centrifuge were provided from Shanghai Lingcheng Biological Technology Co., LTD.; MICROM HM325 tissue slicing machine were provided from Zeiz Company, Germany; BH-2 optical microscope were provided from Olympus Company, Japan.
2.3. Model establishment
Intraperitoneal injection of 10% chloral hydrate was used for anesthesia by dose of 3 mL/kg. They were fixed in supine position. After disinfection, incision laparotomy was performed along epigastric midline, and the root of superior mesenteric artery was separated by blunt separation. Blood flow was blocked by using noninvasive clip at mesenteric artery root, the wounds were sutured. Under light, the artery clip was loosen from the original incision on abdomen to restore the blood perfusion 1 h after ischemia, and was reperfused for 1 h.
2.4. Animal grouping
A total of 50 SD rats were randomly divided into control group, model group, emodin groups of low, medium and high dose, with 10 in each group. Ischemia-reperfusion injury model was established by using noninvasive clamp on superior mesentericartery (SMA), the separation of the superior mesenteric artery root no arterial clip operation was performed in control group after laparotomy. In Control group and model group they were pretreated with 0.5% sodium carboxymethyl cellulose solution 1 mL/100 g lavage 2 h before operation, while in emodin groups of low, medium and high dose they were given emodin lavage with 20, 40, 60 mg/kg pretreatment. In emodin lavage preparation, the corresponding dose of emodin was added to 0.5% sodium methyl cellulose solution for 1 mL/ 100 g .
2.5. Observation indicators
2 mL of femoral venous blood before the lavage pretreatment (T0) and 1 h ischemia (T1) , and inferior vena venous blood after 1 h of reperfusion (T2) were extracted from each group of rats, and the centrifugal supernatant was saved at - 70 ℃. With double sandwich ELISA method serum level of intestinal fatty acid binding protein (I-FABP), tumor necrosis factor (TNF-α), endotoxin, interleukin 6 (IL-6), and the content of diamine oxidase (DAO) were detected. After modeling, the rats were sacrificed, and 2 cm blind intestinal tissue was prepared for saline rinse. They underwent routine dehydration, transparent procession, paraffin embedding, sectioning and HE staining. Pathological changes were observed under optical microscope. Meanwhile, intestine homogenate was prepared using blind intestinal tissue for detection of intestinal tissue myeloperoxidase (MPO), malondialdehyde (MDA) and superoxide dismutase (SOD) levels.
2.6. Statistical analysis
Data were expressed as mean±sd, and analyzed byttest.P<0.05 was regarded as significant difference.
3. Results
3.1. I-FABP, TNF, endotoxin, IL-6 and DAO serum levelsIn emodin groups of low, medium and high dose at T1 and T2, I-FABP, TNF-α, endotoxin, IL-6 and DAO level were significantly lower than that of sham group (P<0.05); IFABP, TNF-α, endotoxin and DAO level were significantly lower than that of control group (P<0.05). There was no statistical difference in serum I-FABP, TNF-α, endotoxin, IL-6 and DAO levels among emodin group of low, medium and high dose at different time points (P>0.05) (Table 1).
3.2. MDA, SOD and MPO levels in intestinal homogenate
MPO and MDA in model group and emodin low, medium and high dose group were significantly higher than that of control group (P<0.05), SOD levels in model group and emodin low, medium and high dose groups were significantly lower than that in control group (P<0.05); SOD level in emodin low, medium and high dose group was significantly higher than that of model group (P<0.05) (Table 2).
3.3. Histological observation
Microscopically, the control group with normal structure of intestinal wall, complete mucous membrane layer and epithelial cells, and smooth surface; model group with part of intestines mucous membrane layer fell off, disordered arranged villus cells in degeneration necrosis, central expanded lacteals and lamina propria capillary, a number of inflammatory cell in infiltration; emodin low, medium and high dose group with similar degree of intestinal tissue lesions, significantly lighter than that in model group, the intestines mucous membrane layer of fluff were in complete structure and order, small amount of epithelial shedding on the top was observable, on the villous surface, a small amount of inflammatory cells were visible, mild expanded central lacteals, with significant degree of pathological changes (Figure 1).
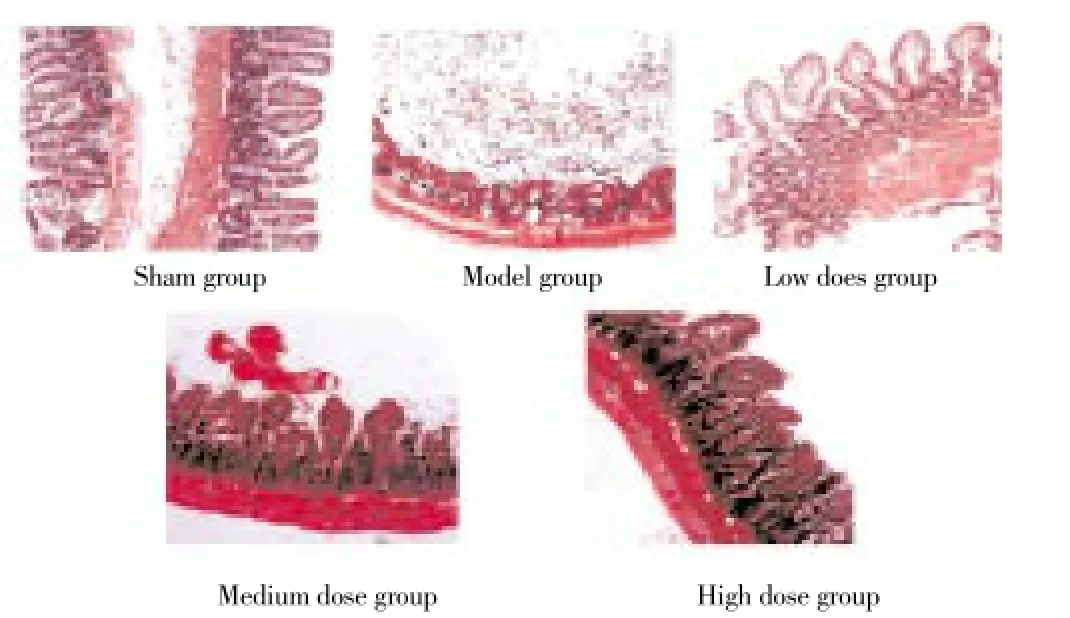
Figure 1. histological changes of rat intestinal mucosa epithelium (HE,×200).
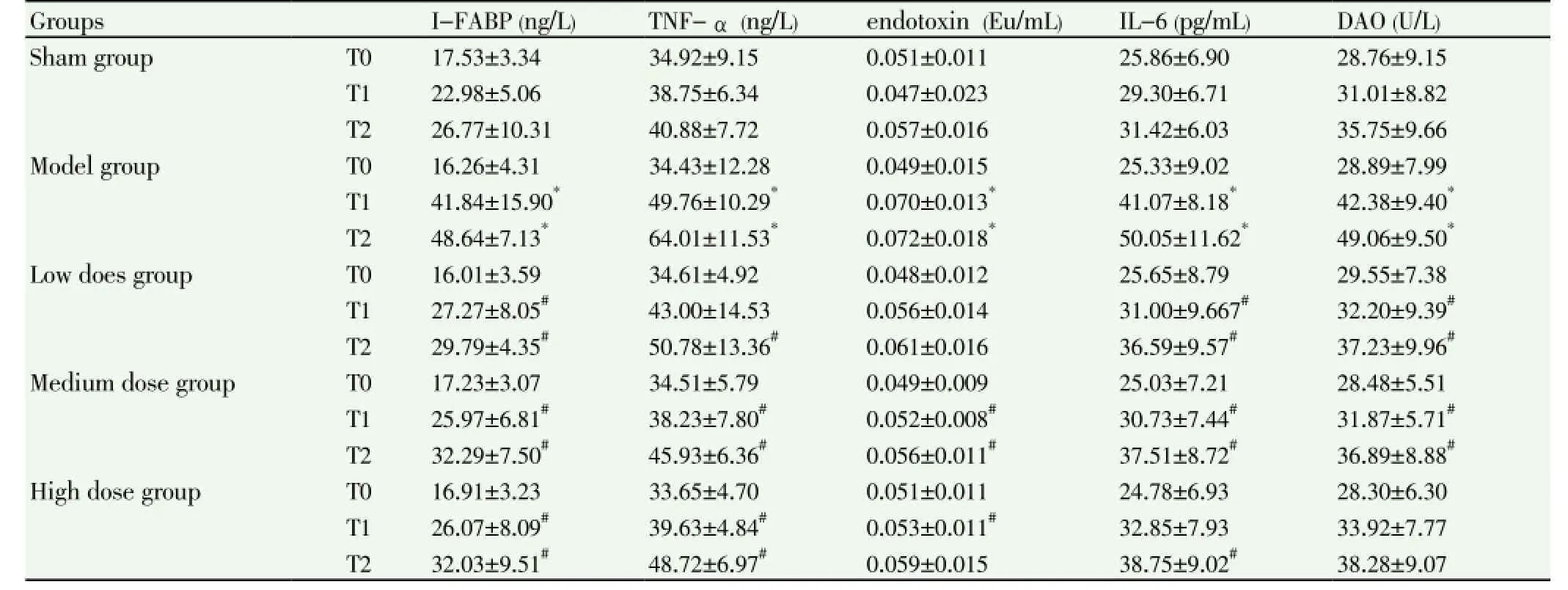
Table 1 Serum I -FABP, TNF-α, endotoxin, IL-6 and DAO level (n=10).
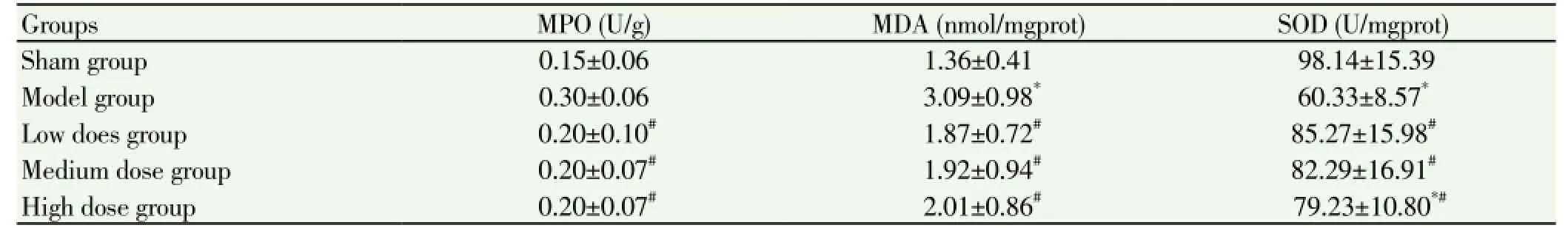
Table 2 MDA, SOD and MPO level comparison among groups (n=10).
4. Discussion
I-RI refers to pathological process of cell structure, metabolism impairment aggravating when restoring blood supply after a period of time ischemia to organs and tissues[11]. I-RI pathogenesis is not fully clear, it is thought to be related with energy metabolic disorders, oxygen free radicals, inflammatory mediators and enzymes activity and content abnormalitie[12-16]. Intestinal organs are the stress center for the body, research has confirmed that in the stress reaction such as major surgery, trauma, it is easy tocause damage of intestinal mucosa[17]. I-RI is a pathological process induced by many diseases, causing damage of intestinal mucosa, and translocation of intestinal endotoxin and bacterial, which induces severe complications like systemic inflammatory response syndrome and multiple organ dysfunction syndrome[18]. At present, the western medicine in clinical treatment of prevention and treatment for intestinal mucosa barrier damage has not gained much breakthrough, so many scholars are studying on traditional Chinese medicine, and have obtained remarkable achievements.
Emodin is the effective component extracted from many traditional Chinese medicines, can reduce the TNF alpha, the release of NO and the production of oxygen free radicals, and can reduce the activation of neutrophils, thus inhibiting excessive inflammation, and protecting against ischemia-reperfusion injury[19]. Modern pharmacology study showed that[20], emodin can improve the blood circulation, promote intestinal peristalsis, reduce the permeability of intestinal mucosa, regulate immune function, antiinflammation, killing microbes etc, play a significant role on protecting intestinal mucosa[21-23]. I-FABP protein in cells has the specific antigen host, expression is confined to small intestine membrane cells and gastric membrane. Studies have confirmed that[24], in the early stage of small intestine ischemia, I-FABP levels increased, after resection the necrosis intestinal part, I-FABP level returned to normal, the level change were positively correlated with gastrointestinal injury severity. In this study, I-FABP concentration of model group was significantly increased after T1, and even higher after T2, while serum I-FABP concentration in emodin low, medium and high dose group at T1 and T2 was significantly lower than that of model group (P>0.05), suggesting that emodin has certain protective effect on intestinal wall after intestinal ischemia-reperfusion injury. TNF-α is activated by soluble polypeptide secreted by neutrophils and endothelial cells, is also an important inflammatory mediators, widely participating in the body inflammatory response. When I-RI happens, mononuclear and macrophages are excessively activated and lead to higher expression of TNF-α mRNA, causing increased inflammatory mediator release and intestinal tissue damage[25]. Emodin low, medium and high dose group, according to the results of this study, had a significantly lower serum TNF level after T1, T2 than that of model group (P<0.05), suggesting emodin can restrain bowel I-RI after the release of TNF-α, so as to reduce the damage of intestinal mucosa. Endotoxin, its chemical nature is lipopolysaccharide, in the case of intestinal barrier function complete, endotoxin seldomly enter blood circulation, when the intestinal barrier function is impaired, endotoxin can pass through the intestinal mucosa into the blood, and its content can reflect the intestinal mucosal barrier function and intestinal I-RI prognosis[26]. In this study, the endotoxin levels in model group after T1, T2 increased significantly than that in control group (P<0.05); endotoxin levels in emodin low, medium and high dose groups at T1 and T2 were significantly lower than that of model group (P<0.05), suggesting emodin is good for maintaining integrity of intestinal mucosal barrier function. IL-6 can promote the production of inflammatory cytokines, increase immunity and inflammation strength, is an early sensitive indicators reflect the tissue damage[27]. Emodin low, medium and high dose group in this study, serum level of IL-6 after T1, T2 was significantly lower than that of model group (P<0.05), indicating that emodin has certain inhibitory effect, can reduce intestinal I-RI inflammatory reaction, thus protect the intestinal mucosal barrier function. DAO mainly distributes on the top of the villus, its active is closely related to the mucosal cells of nucleic acid and protein synthesis, abnormal activity can reflect the damage degree of intestinal barrier immediately[19]. In this study, DAO level in model group after T1, T2 was significantly higher (P<0.05), indicating that after I-RI, intestinal mucosa barrier damage was serious; DAO level in emodin low, medium and high dose groups after T1, T2 was significantly lower than that of model group (P<0.05), also confirmed that emodin pretreatment, can obviously reduce the intestinal mucosa barrier damage after I-RI.
MPO is reliable index of evaluatinge the degree of neutrophil infiltration, its excessive activation cause the intestinal tissue I-RI by mediating a variety of mechanisms[12]. After intestinal I-RI, MDA content made of lipid peroxide decomposition can reflect the degree of oxygen free radical on tissue and cell damage, and SOD is recognized as oxygen free radical scavenger, its vitality can indirectly reflect the oxygen free radical scavenging ability of the body[13]. Studies have confirmed that[21], emodin can improve the cell membrane fluidity, calcium pump activity, reduce oxygen free radicals and reduce damage of important organs. MPO and MDA content in the emodin low, medium and high dose groups was significantly lower than that in model group (P<0.05), while SOD activity was significantly higher than that of model group (P<0.05), indicating that emodin in intestinal I-RI has certain inhibitory effect on inflammatory reaction and can maintain the SOD activity, thus plays a role of protection of intestinal mucosa barrier in I-RI. Histological observation showed that intestinal mucosa damage degree of emodin intervention groups were significantly lighter than that of model group, also shows that emodin has significant protective effect on I-RI damage of intestinal mucosain rats.
Emodin pretreatment has certain protective effect on intestinal mucosa in ischemia reperfusion injury.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Xu JD, Liu S, Wang W, Li LS, Li XF, Li Y, et al. Emodin induces chloride secretion in rat distal colon through activation of mast cells and enteric neurons. Br J Pharmacol 2012; 165(1): 197-207.
[2] Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innateadaptive immune-mediated tissue inflammation. Am J Transplant 2011; 11: 1563-1569 [PMID: 21668640DOI: 10.1111/j.1600-6143.2011.03579.x].
[3] Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013; 10: 79-89. [PMID: 23229329 DOI: 10.1038/nrgastro.2012.225].
[4] RyanTJ, Grant SG. The origin and evolution of synapses. Nat Rev Neurosci 2009; 10(10): 701-712.
[5] Xia XM, Wang FY, Wang ZK, Wan HJ, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol 2010; 16(24): 2994-3001.
[6] Gao L, Hu P, Han X, Jaing LL, Yang J, Ma HJ, et al. In vitro effect of emodin on ileum smooth muscle contraction of rats. J Beijing Univ Chinese Med 2014; 37(1): 43-47.
[7] Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg 2011; 396(1): 13-29.
[8] Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol 2011; 32(6): 256-264.
[9] Cario E. Heads up! How the intestinal epithelium safeguards mucosal barrier immunity through the inflammasome and beyond. Curr Opin Gastroenterol 2010; 26(6): 583-590.
[10] Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Effects of nitric oxide on mucosal barrier dysfunction during early phase of intestinal ischemia/reperflision. Eur J Pharm Sci 2011; 42(3): 246-252.
[11] Kannan KB, Colorado I, Reino D. Hypoxia-inducible factor plays agut-injurious role in intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2011; 300(5): G853-G8561.
[12] Yi ZZ, Ye LJ, Xu C, Shi DG, Wang GF. Protection of Ligustrazine on intestinal ischemia-reperfusion injury. J Trad Chin Med Hunan 2011; (1): 101-102.
[13] Zou JJ, Zhang JH, Xu Y, Yan W. protection function of Zhizhu Decoction for intestinal mucosa in rats. J Trad Chin Med Zhejiang 2009; (8): 568-569.
[14] Chen YP, Zhang JH, Ke YL, Yang Y, Yang Y, Luo D. The influence of Flavored Zhizhu Decoction on early cells apoptosis of small intestinal mucosa in rat with intestinal ischemiareperfusion. Abdominal Surg 2010; 23(1): 49-50.
[15] Gao Y, Luo Y, Chen YP, Zhu L. Protection of Dachengqi Decoction on intestinal ischemia-reperfusion injury of rats. J Chin Pharmacol Clin Med 2013; 29(2): 10-12.
[16] Zhang XS, Chen YY, Li TX. Experimental study on possible effect of ulinastatin and verapamilin the ischemia-reperfusion injury of rat small intestine. Chin Foreign Med Res 2012; 10(30): 4-6.
[17] Shen ZX, Gao HL, Sun W. Research on dynamic alteration of proliferating cell noclear antigen on the model of intestinal ischemia reperfusion in jury in rats. China Sci Technol Information 2010; 18: 214-216.
[18] Zhang FQ, Chen GZ, Yang MH. Influence of ulinastatin combined with ofloxacin on superoxide dismutase and malonaldehyde in septic rat’s intestinal mucosa. Clin J Med Officers 2010; 38(6): 893-895.
[19] Fan LL, Hou MX, Du YR, Sun JZ. The influence of Ulinastatin on serum TNF alpha and IL-6 in rats with intestinal ischemiareperfusion. Modern Biomed Progr 2013; 13(31): 6011-6013.
[20] Wang Y, Zhou DZ, Xia XX, Han PP, Cao LJ. Protection of emodin on intestinal mucosa barrier in acute radioactive enteritis. J Xi ‘an Jiaotong Univ (Med Ed) 2013; 34(2): 248-252.
[21] Liu JX, Zhang JH, Li HH, Lai FJ, Chen KJ, Chen H, et al. Emodin induces Panc-1 cell apoptosis via declining the mitochondrial membrane potential. Oncol Rep 2012; 28(6): 1991-1996.
[22] Wang G, Song L, Wang H, Xing N. Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth and inducing apoptosis in human prostate cancer cells. Oncol Rep 2013: 357-363.
[23] Jelínek M, Balu Íková K, Kopperová D, N McOvá-Fürstová V, Rámek J, Fidlerova J, et al. Caspase-2 is involved in cell death induction by taxanes in breast cancer cells. Cancer Cell Int 2013; 13(1): 42.
[24] Špes A, Sobotic B, Turk V, Turk B. Cysteine cathepsins are not critical for TRAIL- and CD95-induced apoptosis in several human cancer cell lines. Biol Chem 2012; 393(12): 1417-1431.
[25] Leprêtre C, Tchakarska G, Blibech H, Lebon C, Torriglia A. Apoptosis-inducing factor (AIF) and leukocyte elastase inhibitor/ L-DNase II (LEI/LDNaseII), can interact to conduct caspaseindependent cell death. Apoptosis 2013; 18(9): 1048-1059.
[26] Gao W, Xiao F, Wang X, Chen T. Artemisinin induces A549 cell apoptosis dominantly via a reactive oxygen speciesmediated amplification activation loop among caspase-9,-8 and -3.Apoptosis. 2013. DOI:10.1007/S10495-013-0857-Z.
[27] Narender T, Sukanya P, Sharma K, Bathula SR. Preparation of novel antiproliferative emodin derivatives and studies on their cell cycle arrest, caspase dependent apoptosis and DNA binding interaction. Phytomedicine 2013; 20(10): 890-896.
ment heading
10.1016/S1995-7645(14)60129-2
*Corresponding author: Shu-Jie Zhao, M.M., Associate Chief Physician, Associate Professor, ICU, First Hospital of Jilin University, Changchun130041, China.
Tel:15943057605
E-mail: zhaoxjt1965@126.com
Foundation project: It is supported by Special Project of Public Welfare Industry Affiliated to Ministry of Health, Grant No: 201202011.
Ischemia-reperfusion injury
Intestinal mucosa barrier
Protection
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Sonic Hedgehog signaling pathway in primary liver cancer cells
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
- Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
