Sonic Hedgehog signaling pathway in primary liver cancer cells
First Hospital Affiliated to Chinese Medical University, Shenyang 110001, China
Sonic Hedgehog signaling pathway in primary liver cancer cells
Lian-Yi Guo, Pei Liu*, Ying Wen, Wei Cui, Ying Zhou
First Hospital Affiliated to Chinese Medical University, Shenyang 110001, China
Objective:To investigate clinical significance of Sonic Hedgehog (SHH) signaling pathway molecular Shh, Smo and Gli2 in primary hepatocellular carcinoma (HCC) tissue.Methods:A total of 30 HCC tissue samples were collected. Protein expression of SHH signaling pathway molecules Shh, Smo and Gli2 in HCC tissues and para - carcinoma tissue were detected by using immunohistochemical method. Cirrhosis and normal liver tissue specimens were observed as control to analyze the expression of SHH signaling pathway molecular Shh, Smo and Gli2 mRNA in HCC tissues and corresponding para-carcinoma tissues and its relationship with the onset of HCC. Results:There was no expression of Shh, Smo and Gli2 protein in normal liver tissue, while their positive rates were 63.3%, 76.7% and 66.7% in HCC tissues, respectively, with a significantly higher expression level than that in the para - carcinoma tissue (P<0.05). The protein expressions in HepG2 cells were slightly lower than that in Huh7 cells, with no statistical difference (P>0.05); Shh and Smo protein was detected in part of cirrhosis with positive expression, but Gli2 protein was not observable in cirrhosis tissues.Conclusions:In HCC tissues, the high expression level of SHH signaling pathway molecules signal peptide (Shh), membrane protein receiptor (Smo) and nuclear transcription molecular (Gli2) can be indicators of the onset of liver cancer.
ARTICLE INFO
Article history:
Received 10 April 2014
Received in revised form 15 May 2014
Accepted 15 July 2014
Available online 20 September 2014
HCC
1. Introduction
The primary hepatocellular carcinoma (HCC) is a common malignant tumor of the digestive system, its incidence increases year by year in our country, seriously harm human health due to its complicated treatment and poor prognosis[1-3]. The pathogenesis of HCC is unclear[4]. Studies have reported[5-7], activation of Sonic Hedgehog (SHH) pathway exists in the HCC tissue and may promote tumor growth and angiogenesis, indicating that SHH signaling pathway molecules are involved in the onset of HCC process. SHH signaling pathway is a key regulator pathway for cell proliferation in the process of embryonic development. SHH pathway inactivation are observed in both embryonic development and tumorigenesis process, which may have been caused by the disorder expression of cancer gene[8]. Currently, there are many researches about the relationship of SHH signaling pathway and malignant tumor, but the correlation between its expression and HCC is rare reported. To observe the mechanism of SHH signaling pathway in the development of liver cancer, we detected the protein expression of molecules Shh, Smo and Gli2 in 30 HCC tissue samples using immunohistochemical method, and analyzed the relationship between the liver cancer and the expression disorder of SHH signaling pathway, as to provide experimental basis for effective prevention and treatment of liver cancer.
2. Materials and methods
2.1. Specimens collection
A total of 30 (male 22, female 8) HCC patients aged between 37-72 yr [mean (51.7±2.3) yr] who were admitted from 2011.1 to 2012.1 were recruited, the tissue specimens werecollected after surgery. Edmondson level:Ⅰ-Ⅱ 17 cases, Ⅲ-Ⅳ 13 cases; tumor diameter: 24 cases ≥3 cm, 6 cases ≤3 cm, 17 cases of cirrhosis, 12 cases with intrahepatic portal vein invasion; 22 cases with serum AFP > 25 ng/mL. Tissue specimens (5 cm plus) of liver tumor and para-tumor were intraoperative extracted immediately from the 30 samples, then were frozen in liquid nitrogen, followed by SHH pathway molecule detection using immunohistochemical method; respective 10 specimens of cirrhosis and normal liver tissue were set for control observation.
2.2. Instruments and reagents
Immunohistochemical kit, DAB chromogenic agent, PBS (Golden Bridge Biotechnology co., LTD., Beijing Chinese); 1640 (powder), trypsin (GIBCO Co., USA); Rabbit polyclonal antibody against human Shh, rabbit polyclonal antibody against human Smo, rabbit polyclonal antibody against human Gli2, biotin labeled goat rabbit IgG, blocked with goat serum of 5% BSA, HRP conjagated Streptavidin were bought from Wuhan Science and Technology Co., LTD, China&United States. CO2incubator Heareus Company (Germany); BH-2 optical microscope (Olympus, Germany); Inverted microscope (OLYMPUS, Japan); Gel scan imaging system (BioRad Co., US); Image analysis system Imageproplus morphometric analysis software.
2.3. Experimental methods
Immunohistochemical SP method was used to detect SHH signaling pathway molecules Shh, Smo and Gli2 protein expression. After fixation and paraffin embedding, samples were sliced and moved in baker at constant 60 ℃ for 30 min. After conventional dewaxing, 0.3% of Triton X-100 was added, then incubated at room temperature for 10 min, in order to dissolve the cell membrane, nuclear membrane, so that the antibodies could get into cells. 3% hydrogen peroxide was added to inactivate endogenous enzymes, washed three times with PBS. 50 µL of primary antibodies (Shh, Smo: 1:150 dilution, Gli2: 1:100 dilution) was added at room temperature for 30 min. After PBS wash, second antibody was added to rabbit IgG, then was incubated for 30 min at room temperature. HRP conjagated Streptavidin was added then incubated for 30 min at room temperature. They were washed with PBS and DAB for coloration,redyed with hematoxylin followed by dehydration, transparentation and cementing. RT-PCR reaction was conducted strictly according to the kit instructions
2.4. Results determine
Five vision fields were randomly taken under the light microscopy (SP×400), positive cells appeared blond, brown granules microscopically in cytoplasm, result determination was according to the proportion of positive cells to staining intensity[9]. Positive cell percentage of scoring criteria: 0: positive cells ≤5%; 1 point: positive cells > 5%-25%; 2 points: positive cells > 25%-50%; 3 points: positive cells > 50%-75%; 4 points: positive cells > 75%. Dyeing strength criteria: 0: without coloring; 1 point: light yellow; 2 points: tan; three points: brown. Product of the integral above was marked as the dyeing criteria: the negative (-): 0; weakly positive (+) : 1-4 points, positive (++ - +++) : > 4 points.
2.5. Statistics analysis
SPSS19.0 statistical software was used, all data were expressed as mean±sd. Variance was analyzed with ANOVA and F test.P<0.05 was regarded as statistically significant difference.
3. Results
3.1. mRNA expression of SHH pathway in HCC tissues and HCC cell lines
mRNA positive expression rate of Shh, Smo and Gli2 in HCC tissue was 60%, 80%, and 70%, similar to the immunohistochemical detection results; and was 20%, 30% and 30% in para-carcinoma tissue respectively, mRNA expression significantly increased in HCC tissue compared with para-carcinoma tissue(P<0.05). mRNA expression of Shh, Smo and Gli2 in liver cancer cell line HepG2 was slightly lower than that in Huh7 (P>0.05), as shown in Table 1.
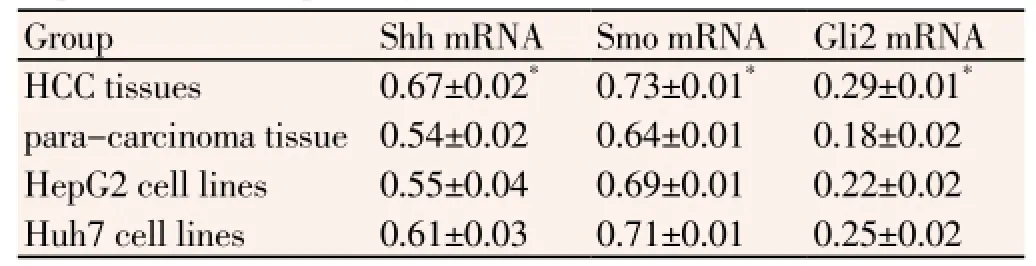
Table 1 Expression of SHH pathway in HCC tissues and HCC cell lines.
3.2. Protein expression of SHH signaling pathway molecules in different tissues
Shh, Smo and Gli2 protein had no expressions in normal liver tissue; Shh and Smo could be detected in part of cirrhosis tissue; Protein expression of Shh, Smo and Gli2 in HCC tissue was 63.3% (19/30), 76.7% (23/30), and 66.7% (20/30), Shh and Smo protein mainly expressed in cytoplasm, with granules in tan or brown, Smo and Gli2 protein positive rate was SHH, Gli2 protein was expressed both in nucleus and cytoplasm, with brown granules or tan granules (Figure 1).
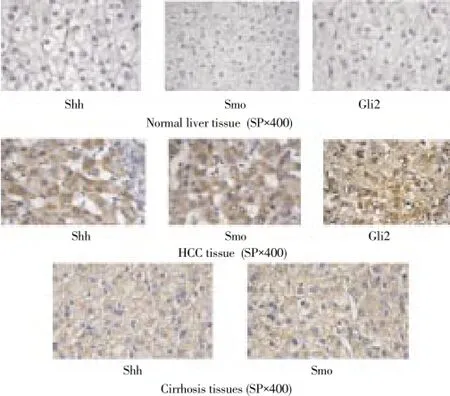
Figure 1. Protein expression of SHH signaling pathway molecules in different tissues.
4. Discussion
HCC is a common digestive system malignant tumor, clinical diagnosis and treatment is difficult. Its pathogenesis has not been entirely clear, the onset process include multiple genes and signaling pathway[9-13]. SHH signaling pathway is involved in the process of embryonic development and tissue polarity regulation, studies have shown that[14-18], activation of SHH signaling pathway may participate in a variety of tumor formations, closely associated with the occurrence and development of malignant tumors. This study is to observe the protein expression of Shh, Smo and Gli2 related to SHH signaling pathway in HCC tissues and HCC cell lines, analyze the function of SHH signaling pathway on the pathogenesis of HCC process, and lay a foundation for the further study of HCC pathogenesis. Hedgehog signaling pathway was firstly found in fruit flies, adjusting the directional differentiation and embryonic development[19]. Studies have reported[20], SHH signaling pathway is not activated in normal liver cells in mature mice. In this study, Shh, Smo and Gli2 showed no protein positive expression in normal liver tissues, confirmed that the SHH signaling pathway is not activated in normal liver tissue, consistent with literature reports. SHH signaling pathway gene present high expression in HCC tissues[21-24], suggesting SHH signaling pathway are highly activated. Positive expression rate of Shh, Smo and Gli2 protein in HCC tissue was 63.3%, 67.7% and 66.7%, respectively according to the results of this study, indicating that positive expression of SHH signaling pathway may participate in the occurrence and development of liver cancer[25]. In this study, HCC cell lines HepG2 showed a lower expression than Huh7, comparison between the two groups has no statistical difference (P>0.05), which may be due to different level of activation of SHH signaling pathway in various liver cancer cell lines. SHH Mrna expression was observed in cancer cell lines of digestive tract’s different parts, showing that the activation of SHH pathway in the digestive tract cell lines is closely related to the occurrence and development of tumors[26].
This study also detected SHH signaling pathway factors in part of cirrhosis tissue, confirmed SHH signaling pathway activated in liver cirrhosis. Other studies on primary biliary cirrhosis tissue, found a high expression level of SHH signaling pathway molecules, suggesting abnormal activation of SHH are closely related to the formation and development of liver cirrhosis, blocking SHH pathway may become a new target for prevention and control of liver fibrosis[24].
A large number of activated SHH signaling pathway molecules in HHC tissue, suggesting SHH signaling pathway may participate in the occurrence of liver cancer, and targeted therapy for SHH signaling pathway may become a new way to prevent and cure of liver cancer.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Cheng WT, Hu ZW, Tian DY. The expression and significance of Hedgehog signaling pathway and Snail in liver cancer cell lines. J Huazhong Univ Science Technol (Med) 2010; 39(2): 212-215.
[2] Liu JP, Zhang DW, Chen T, Ye YB, Chen J, Wei JX, et al. Theexpression and significance of Hedgehogsignaling pathway in human liver cancer cell lines. J Lingnan Modern Clin Surg 2011; 11(6): 420-423.
[3] Wang F, Xu L, Guo CY. The role of Hedgehog signaling pathway in liver cancer. J Tongji Univ (Med Ed) 2013; 34(2): 120-124.
[4] Omenetti A, Choi S, MiChelotti G, Diehl AM. Hedgehog signaling in the liver. Hepatol 2011; 54(2): 366-373.
[5] Zhu DJ, Chen XW, Ju YL, Kang KF, Chen JP, Lu GS, et al. The expression and significance of Sm and Gli1 protein in colorectal cancer tissue. Guangdong Med 2009; 30(5): 757-759.
[6] Caro I, Low JA. The role of the Hedgehog Signaling pathway in the development of basal cell carcinoma and opportunities for treatment. Clin Cancer Res 2010; 16(13): 3335-3339.
[7] Merchant JL. Hedgehog signaling in gut development, physiology and cancer. Physiol 2012; 590(Pt3): 421-432.
[8] WAlter K, OmurA N, Hong SM, Griffith M, Goggins M. Overexpression of smoothened activates the Sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin cancer Res 2010; 16(6): 1781-1789.
[9] Wang D, Wu YJ, Li XY, Bai XY, Feng GY, Li JH. Changes of Shh, Ptch, Gli1 and CyclinB1-CDK1 in development of liver cancer. Chin J Clin Phys 2011; 5(17): 4997-5002.
[10] Wang D, Li XY, Li HX, Bai XY. Changes of vascular endothelial growth molecules with their receptors and Cy Clin E-CDK2 during the development of liver cancer. Chin J Histochem Cytochem 2011; 20(4): 310-313.
[11] Hui CC, Angers S. Gli proteins in development and disease. Ann Rev Cell Dev Biol 2011; 27(32): 513-537.
[12] Teglund S, Toftgad R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2010; 1805(2): 181-208.
[13] Dai JT, Yi KX. Research progress of Hedgehog signaling pathway in pancreatic cancer. J Modern Cancer Med 2009; 27(3): 588-590.
[14] Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog -signaling pathway in human cancer and the clinical implications. Oncogene 2010; 29(4): 469-481.
[15] Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical Hedgehog signaling. Vitam Horm 2012; 88: 55-72 . [16] Barginear MF, Leung M, Budman DR. The hedgehog pathway as a therapeutic target for treatment of breast cancer. Breast Cancer Res Treat 2009; 116(2): 239-246.
[17] Wang QL, Su L, Liu XG. Hedgehog signaling pathway and tumors. Chem Life 2011; 31(1): 21-26.
[18] Pan D, Li Y. Hedgehog-Gli signaling pathway and tumors. J Modern Cancer Med 2011; 19(3): 591-595.
[19] Cheng SY, Yue S. Role and regulation of human tumor suppressor SUFU in Hedgehog signaling. Adv Cancer Res 2008; 101: 29-43.
[20] Chen JW, Tang QQ. Hedgehog signaling pathway and fat cells development. Chin J Biochem Molecular Boil 2011; 27(1): 6-10.
[21] Gu GB, Li JK. Research progress of relationship between Hedgehog signaling pathway and tumor. J Modern Cancer Med 2011; 19(4): 808-811.
[22] Kagawa H, Shino Y, Kobayashi D, Demizu S, Shimada M, Ariga H, et al. A novel signaling pathway mediated by the nuclear targeting of C-terminal fragments of mam-malian Patched 1. PLoS One 2011; 6(4): e18638.
[23] Chinchilla P, Xiao L, Kazanietz MG. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non -canonical signaling pathways. Cell Cycle 2010; 9(3): 570-579
[24] Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, et al. Sonic hedgehog induces an-giogenesisvia Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol 2010; 49(3): 490-498.
[25] Page JM, Harrison SA. NASH and HCC. Clin liver Dis 2009; 13(4): 631-647.
[26] Zhang DW, Cao LQ, Lu HW, Song XD, Liu SH, Xue P. The expression and significance of Hedgehog signaling pathway transcription molecules Gli2 in liver cancer tissue. J Chin Arch Gneral Surg 2013; 7(1): 12-16.
ment heading
10.1016/S1995-7645(14)60126-7
*Corresponding author: Pei Liu, Chief Physician, Professor, MD, First Hospital Affiliated to Chinese Medical University, Shenyang 110001, China.
Tel: 13604218300
E-mail: goulytg@163.com
Foundation project: It is supported by Natural Science Foundation Project of Liaoning Province, No. 2013022005.
Sonic Hedgehog signaling pathway
Expression
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
- Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
